Chloroplast K+/H+ EXCHANGE ANTIPORTER 3 modulates abscisic acid-induced reactive oxygen species generation in guard cells
Abstract
Reactive oxygen species (ROS) are important signaling molecules in stomatal closure. In a previous report, we demonstrated that ROS generated through photosynthetic electron transport (PET) act as signaling molecules in abscisic acid (ABA)-induced stomatal closure. However, the mechanism by which ABA induces ROS generation through PET remains unclear. Here, we assessed the possibility that chloroplast K+/H+ EXCHANGE ANTIPORTER 3 (KEA3) functions in ABA-induced ROS generation in guard cells, resulting in stomatal closure. KEA3 localizes to a thylakoid membrane and allows proton efflux from the thylakoid lumen by K+/H+ antiport, regulating photosynthesis by proton motive force. KEA3 loss-of-function mutants (kea3-1 and kea3-2) were impaired in ABA-induced ROS generation of guard cells and stomatal closure. The small molecule electroneutral K+/H+ antiporter nigericin induced ROS generation in guard cells and stomatal closures in the kea3 mutants. This study demonstrates that KEA3 is an important factor for ABA-induced ROS generation in guard cells and stomatal closure.
1 INTRODUCTION
Stomata serve as channels that enable the entry of CO2 into leaves from the atmosphere and the exit of water from plants, playing a vital role in maintaining plant life. Consequently, their opening and closing are precisely regulated by various environmental stimuli, including light, air humidity and soil moisture levels (Schroeder et al., 2001; Shimazaki et al., 2007). When plants are subjected to drought conditions, the hormone abscisic acid (ABA) is produced, which induces stomata closure. In ABA guard cell signaling, numerous molecules act as signaling molecules, with reactive oxygen species (ROS) being particularly significant (Pei et al., 2000; Yan et al., 2007). ROS are highly reactive forms of oxygen that have been considered toxic, but are now recognized as important molecules for signal transduction when present at low levels (Mittler, 2017). It has long been believed that NADPH oxidase plays a crucial role in the ABA-induced ROS generation in guard cells (Kwack et al., 2003; Song et al., 2014). However, our previous study showed that photosynthetic electron transport (PET) mediates ABA-induced ROS generation in guard cells and subsequent stomatal closure (Iwai et al., 2019). Nevertheless, it remains unclear which component of PET is regulated by ABA.
Photosynthetic electron transport is the process by which photosynthetic organisms convert light energy into chemical energy in the form of ATP and NADPH, which are used to drive carbon dioxide fixation. This process occurs in the thylakoid membranes of the chloroplasts and involves a sequence of redox reactions that transfer electrons from water molecules to NADP+. Electrons derived from splitting water in photosystem II (PSII) are transported to photosystem I (PSI) via plastoquinone, the cytochrome b6f complex, and plastocyanin. PSI reduces ferredoxin, which donates electrons to NADP+. These processes are coupled to proton translocation across the thylakoid membrane into the thylakoid lumen via the quinone cycle in the cytochrome b6f complex and by water splitting in PSII, generating proton motive force (PMF). PMF is energetically composed of two components: a proton gradient across the thylakoid membrane (ΔpH) and a membrane potential across the thylakoid membranes (ΔΨ). Both components drive the synthesis of ATP (Yamori & Shikanai, 2016).
Under suboptimal conditions, such as chilling and fluctuating light, excess electrons flow into PET, which results in its over-reduction (Sonoike, 2011; Armbruster et al., 2016). The accumulated electrons increase the probability of producing ROS from electron carriers in PSI, causing photoinhibition of PSI (Sonoike et al., 1995; Sonoike, 2011; Sejima et al., 2014). Lumenal proton concentration serves an important regulatory function in avoiding ROS generation and maintaining photosynthetic activity. A high proton concentration in the thylakoid lumen activates non-photochemical quenching (NPQ), a mechanism that dissipates absorbed light energy as heat (Müller et al., 2001). A low pH in the thylakoid lumen also slows down electron transfer from PSII to PSI by downregulation of the cytochrome b6f complex in a process called photosynthetic control (Nishio & Whitmarsh, 1993). The PMF is regulated via ion channels/transporters localized to the thylakoid membrane, including VOLTAGE-DEPENDENT CL− CHANNEL 1 (VCCN1) and chloroplast K+/H+ EXCHANGE ANTIPORTER 3 (KEA3). Arabidopsis VCCN1 is involved in light-triggered voltage-dependent influxes of Cl− into the thylakoid lumen, which contributes to counterbalancing the pumping of H+ into the lumen and elevating ΔpH to accelerate NPQ (Herdean et al., 2016). Arabidopsis thylakoid K+/H+ antiporter KEA3 localizes to the chloroplast thylakoid membrane and allows proton efflux from the thylakoid lumen to stroma with a counter influx of K+ (Armbruster et al., 2014; Kunz et al., 2014). KEA3 dissipates ΔpH, leading to the recovery of PET and reducing NPQ (Armbruster et al., 2014, 2016). Thus, these ion channels/transporters fine-tune photosynthetic activity and optimize photosynthesis under adverse environmental conditions, controlling ROS generation and photodamage of photosystems (Spetea et al., 2017).
Ogawa et al. (1982) investigated the kinetics of chlorophyll a fluorescence in guard cells of Vicia faba. The rate of fluorescence decline from the peak (P) to the quasi-stationary level (S) is slower in guard cells than in mesophyll cells. The decline in fluorescence is suppressed by ABA and the uncoupler carbonyl cyanide m-chlorophenyl hydrazone (CCCP) in guard cells. This fluorescence decline is also suppressed by the CCCP analogue FCCP (carbonyl cyanide 4-trifluoromethoxyphenylhydrazone), which dissipates ΔpH (Krause, 1974). Since this fluorescence decline reflects the increase of lumenal proton concentration (Briantais et al., 1979), ABA and CCCP are considered to decrease proton concentration in the thylakoid lumen of guard cell chloroplasts. Interestingly, CCCP has the same effect as ABA in inducing stomatal closure (Ishikawa et al., 1983). These results suggest that ABA dissipates ΔpH through an ion channel/transporter to elicit ROS, which in turn induces stomatal closure.
The two thylakoid membrane channels/transporters apparently have opposite roles: KEA3 dissipates ΔpH (Armbruster et al., 2014), whereas VCCN1 elevates ΔpH (Herdean et al., 2016). The possibility that VCCN1 is involved in ABA-induced stomatal closure can be ruled out because VCCN1 counters the effect of ABA. Another antiporter, KEA3, is a candidate for modulating ABA-induced stomatal closure. We used KEA3 knockout mutants (kea3-1 and kea3-2) to determine the role of thylakoid membrane ion transporter/channel on stomatal movement.
Here, we assessed the possibility that KEA3 is involved in ABA-induced ROS generation and stomatal closure. KEA3 knockout mutants were impaired in ROS generation and stomatal closure in response to ABA. This study demonstrates that KEA3 is pivotal in ABA-induced ROS generation in guard cells and stomatal closure.
2 MATERIALS and METHODS
2.1 Plant Materials
Seeds of Arabidopsis mutant kea 3–1 and kea 3–2 (Armbruster et al., 2014) were kindly provided by Dr. Ute Armbruster (Max Planck Institute of Molecular Plant Physiology, Germany). Seeds of Arabidopsis thaliana ecotype Col-0 and Ws were obtained from the Arabidopsis Biological Research Center (Columbus, OH, USA). The plants were grown in soil for 1–2 months at a temperature of 20–25°C under an 11-hour light/13-hour dark photoperiod using a fluorescent lamp (PLANTLUX, Toshiba Lighting & Technology Corp.) providing a photosynthetic photon flux density of 50 μmol m−2 s−1.
2.2 Stomatal Aperture Assays
Stomatal apertures were measured as described previously (Joudoi et al., 2013). Briefly, the epidermal strips were floated on the opening medium (Joudoi et al., 2013) under a photosynthetic photon flux density of 50 μmol m−2 s−1 for 3 h to induce stomatal opening. Subsequently, the epidermal strips were transferred to fresh opening medium containing various reagents (10, 30 μM ABA, 100 μM hydrogen peroxide, 2 mM CaCl2, 10 μM 3-(3, 4-dichlorophenyl)-1, 1-dimethylurea [DCMU], 0.2 μM 2, 5-dibromo-3-methyl-6-isopropyl-p-benzoquinone [DBMIB], 0.1–10 μM nigericin) and incubated under light for 2 h. Images of the stomata were acquired using a digital camera (LCD; KENIS Ltd) connected to a microscope (Olympus BH2). Stomatal aperture was measured using a digital analyzer (Japan Polaroid Digital Products). ABA was dissolved in dimethyl sulfoxide (DMSO), while DCMU, DBMIB and nigericin were dissolved in ethanol, and then diluted with the opening medium to the predetermined concentrations. The final concentrations of DMSO and ethanol in assay medium were less than 0.01% and 0.1%, respectively. At these concentrations, neither DMSO nor ethanol affected stomatal aperture in our experimental conditions (Joudoi et al., 2013). ABA, DCMU, DBMIB and nigericin were from Sigma-Aldrich. Other reagents were from FUJIFILM Wako Pure Chemical Corporation. The data are presented as mean ± standard error of the mean of three independent experiments. Statistical analysis was performed using one-way analysis of variance (one-way ANOVA) followed by Tukey's test.
2.3 ROS Measurement in Guard Cells
ROS were measured as previously described (Iwai et al., 2019). Briefly, the epidermal strips were placed in the opening medium and exposed to light (50 μmol m−2 s−1) for 3 h. Subsequently, they were treated with the opening medium containing ABA or nigericin for 5 min under light. Epidermal strips were incubated with 10 μM 2, 7-dichlorofluorescin diacetate (H2DCFDA) in the dark for 20 min. For experiments in Figure 2, fluorescence images stained with H2DCFDA were captured using a laser scanning confocal microscope (EZ-C1, Nikon Corp.). For experiments in Figure 5, the dye-loaded epidermal strips were observed with a fluorescence microscope (UplanFl; Olympus Corp.) equipped with a CCD camera (DS-Fi3; Nikon Corp). At least 60 guard cells were measured for each experiment. Fluorescence intensity was measured using EZ-C1 FreeViewer Version 3.70 software (Nikon Corp), and the fluorescence intensity per guard cell was expressed as the ratio of treated cells to untreated cells. The data are presented as mean ± standard error of the mean of three independent experiments, with individual experimental results also plotted on the graph. The statistical significance between the two groups was determined by Student's t-test.
2.4 Drought Tolerance Assays
Wild-type and mutant seeds were germinated and allowed to grow for one month. Seedlings were subjected to drought stress by withholding water for 25 days and then rewatered for 2 days. Leaves were removed and immediately weighed to obtain leaf fresh weight (FW), then dried at 105°C for 1 h and reweighed to obtain leaf dry weight (DW). Leaf relative water content (RWC) was calculated as (FW-DW)/FWx100. Data are presented using boxplots, and six individual data points are also shown on the graph. Statistical analysis was performed using one-way ANOVA followed by Tukey's test.
2.5 Stomatal Density
Stomatal density was expressed as the number of stomata per unit leaf area (mm2, Radoglou & Jarvis, 1990). Epidermal strips were peeled from the abaxial surface of fully expanded leaves and photographed with a digital camera (DS-Fi3; Nikon Corp.) connected to a microscope (UplanFl; Olympus Corp.). The number of stomata was counted using eight digital images from each epidermal strip. The data are presented as mean ± standard error of the mean of five independent experiments, with individual experimental results also plotted on the graph. The statistical significance between the two groups was determined by Student's t-test.
3 RESULTS
3.1 The kea3 mutants are impaired in ABA-induced stomatal closure and ABA-induced ROS generation
To test the involvement of KEA3 in ABA-induced stomatal closure, we performed stomatal aperture assays using Arabidopsis knockout mutants of KEA3, kea3-1 and kea3-2. We used an assay method with epidermal strips to accurately investigate the function of guard cell chloroplasts. Since previous studies have shown that photosynthesis in mesophyll cells influences stomatal movement (Mott et al., 2008), it is essential to eliminate the effect of mesophyll cell chloroplasts. The isolated epidermal strips do not contain mesophyll cells or vascular bundle sheath cells, which allows us to exclude the influence of these cells. Epidermal strips were treated with ABA for 2 h in the light and ABA induced stomatal closure in ecotype Col-0 (wild-type of kea3-1) and Ws (wild-type of kea3-2) at concentrations of 10 and 30 μM (Figure 1A,B). However, ABA-induced stomatal closure was not observed in the kea3-1 and kea3-2 mutants at either 10 μM or 30 μM (Figure 1A,B, Table S1A,B), demonstrating that KEA3 is involved in ABA-stomatal closure. Calcium and hydrogen peroxide are ABA signaling components in guard cells (Pei et al., 2000). We therefore applied hydrogen peroxide and CaCl2 to epidermal strips from Arabidopsis wild-type, the kea3-1 and kea3-2 mutants (Figure 1C,D, Table S2A,B). These compounds, unlike ABA, induced stomatal closure in mutants kea3-1 and kea3-2, indicating that the processes downstream of ROS and calcium were functional but were impaired upstream of ROS and calcium in ABA-induced stomatal closure in kea3-1 and kea3-2.
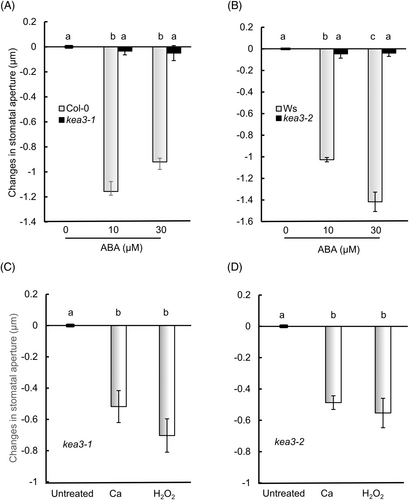
(A) kea3-1. (B) kea3-2. (C, D) Calcium-induced and H2O2-induced stomatal closure in kea3-1 and kea3-2. Epidermal strips containing pre-opened guard cells were incubated with 0–30 μM ABA, 100 μM H2O2 or 2 mM CaCl2 for 2 h. Error bars represent standard error of the mean (n = 3 biological replicates, each being 100 stomata counted on 4 epidermal strips from four individuals). Different letters indicate significant differences at P < 0.01.
We measured ABA-induced ROS generation using H2DCFDA. Weak fluorescent background signals were detected in untreated guard cells (Figure 2A). In wild-type, epidermal guard cells treated with ABA showed strong fluorescence in chloroplasts, as also shown in a previous report (Iwai et al., 2019). However, in mutants kea3-1 and kea3-2, epidermal guard cells treated with ABA showed weak signals. ABA induced a 210% increase in ROS generation in wild-type (Col-0) guard cells and a 170% increase in wild-type (Ws) guard cells (Figure 2B,C). In contrast, ABA did not induce ROS generation in the guard cells of mutants kea3-1 and kea3-2, consistent with previous observations in mutants abi1 (Murata et al., 2001; Joudoi et al., 2013) and ost1 (Mustilli et al., 2002). These results indicate that KEA3 acts upstream of ABA-induced ROS generation in guard cell chloroplasts.
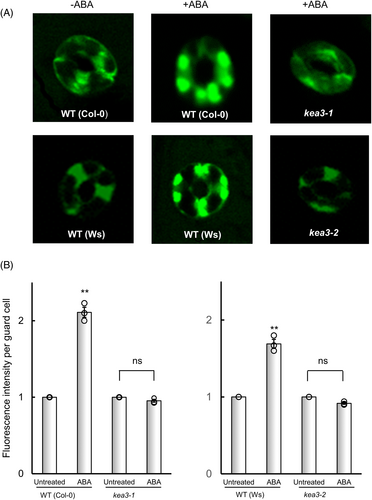
(A) Confocal micrographs of guard cells showing fluorescent signals from H2DCFDA. (B) Relative fluorescent intensity derived from H2DCFDA in guard cells. Epidermal strips containing pre-opened guard cells were incubated with 10 μM ABA for 5 min. ROS were measured by fluorescence from guard cells stained with H2DCFDA. Fluorescence intensity per guard cell was expressed as the ratio of treated cells to untreated cells. Error bars represent standard error of the mean (n = 3 biological replicates, each being 60 guard cells counted on 4 epidermal strips from four individuals). Double asterisk (**) indicates a significant difference (P < 0.01) compared with the value for untreated cells. “ns” indicate no significant compared with the value for untreated cells.
3.2 The kea3 mutants are susceptible to drought
Given that ABA-induced stomatal closure is impaired in the kea3 mutants, they should be susceptible to drought. The kea3 mutant and wild-type plants were grown in pots under the same conditions, and then water was withheld for 25 days. The mutants kea3-1 and kea3-2 exhibited more severe wilting symptoms than the wild-type plants. (Figure 3A). The leaf relative water content (RWC) of wild-type plants was 89% after watering was stopped for 25 days, whereas the RWC of kea3-1 and kea3-2 was 70% and 69%, respectively (Figure 3B,C). Stomatal density was almost the same between wild-type, mutants kea3-1 and kea3-2 (Figure 3D). These results indicate that these mutants are more susceptible to drought than the wild-type.
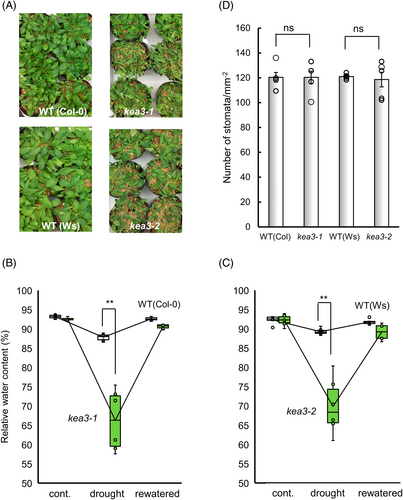
(A) Photographs of drought-stressed WT (Col-0 and Ws) kea3-1 and kea3-2. (B) RWC of WT (Col-0) and kea3-1. (C) RWC of WT (Ws) and kea3-2. (D) Stomatal density of WT, kea3-1 and kea3-2. (A, B, C) Seedlings were subjected to drought stress by withholding water for 25 days (drought) and then rewatered for 2 days (rewatered). Open boxes indicate WT and green boxes indicate mutants (n = 6 biological replicates, each being one leaf from an individual). Double asterisks (**) indicate significant differences at P < 0.01. (D) Stomatal density was expressed as the number of stomata per unit leaf area (mm2). Error bars represent standard error of the mean (n = 5 biological replicates,each being one epidermal srips from an individual). “ns” indicate no significant differences between three genotypes at P < 0.01.
3.3 Nigericin compensates for the loss-of-function of KEA3
Nigericin is an antibiotic derived from Streptomyces hygroscopicus that acts as an ionophore promoting K+/H+ exchange across chloroplast thylakoid membranes, resulting in the dissipation of ΔpH across thylakoid membranes (Giersch, 1983), and thus functioning as a mimic of KEA3 (Armbruster et al., 2014). To verify the observation that KEA3 functions in ABA-induced stomatal closure, we measured the effect of nigericin on stomatal movement. Nigericin did not affect stomatal aperture at 0.1 μM, but slightly closed the stomata at 1 μM, and significantly closed the stomata at 10 μM (Figure 4A, Table S3A). That is, nigericin induces stomatal closure in a dose-dependent manner in wild-type plants. The photosynthetic electron transport inhibitors, DCMU and DBMIB, suppressed nigericin-induced stomatal closure in wild-type (Figure 4B, Table S3B), suggesting that nigericin-induced stomatal closure is mediated by PET, and that nigericin acts as an ionophore across thylakoid but not plasma membranes. In mutants kea3-1 and kea3-2, nigericin did induce closure at 10 μM as well as in wild-type plants (Figure 4C,D, Table S4A,B).
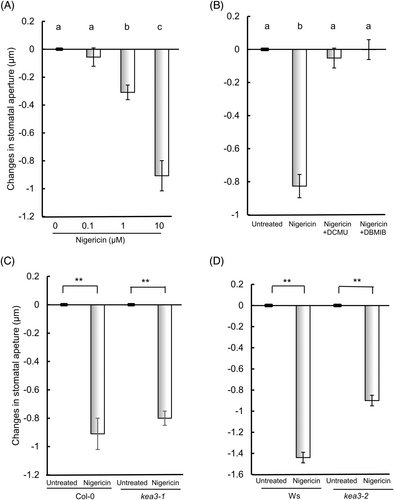
(A) Wild-type (Col-0). (B) Effect of photosynthetic inhibitors on nigericin-induced stomatal closure in wild-type (Col-0). (C) kea3-1 (D) kea3-2.
(A-B) Epidermal strips containing pre-opened guard cells were treated with (A) 0–10 μM nigericin for 2 h or (B) with 10 μM nigericin, 10 μM DCMU and/or 0.2 μM DBMIB for 2 h. (C, D) Epidermal strips containing pre-opened guard cells were incubated with 10 μM nigericin for 2 h. Error bars represent standard error of the mean (n = 3 biological replicates, each being 100 stomata counted on 4 epidermal strips from four individuals)). Different letters indicate significant differences at P < 0.05. Double asterisk (**) indicates a significant difference (P < 0.01) compared with the value for untreated cells.
At a concentration of 10 μM, nigericin induced a 200% increase in ROS generation in wild-type guard cells (Figure 5), suggesting that dissipation of the ΔpH triggers ROS generation in these cells. Similarly, in the kea3-1 mutant, 10 μM nigericin induced a 150% increase in ROS generation in guard cells, indicating that nigericin compensates for the loss of KEA3 function, thereby supporting the observation that KEA3 is involved in ABA-induced ROS generation and subsequent stomatal closure.

Epidermal strips containing pre-opened guard cells were incubated with 10 μM nigericin for 5 min. ROS were measured by fluorescence from guard cells stained with H2DCFDA. Fluorescence intensity per guard cell was expressed as the ratio of treated cells to untreated cells. Error bars represent standard error of the mean (n = 3 biological replicates, each being 60 guard cells counted on 4 epidermal strips from four individuals). Double asterisks (**) indicate significant differences (P < 0.01) compared with the value for untreated cells.
4 DISCUSSION
In a previous report, we demonstrated that PET plays a crucial role in ABA-induced ROS generation in guard cell chloroplasts and stomatal closure (Iwai et al., 2019). Based on results using the PET inhibitors DCMU and DBMIB, we concluded that ROS are derived from PSI. However, which factor is regulated by ABA remains to be determined. In this report, we present genetic and pharmaceutical evidence that KEA3 functions in ABA-induced ROS generation in guard cell chloroplasts and ABA-induced stomatal closure.
Ogawa et al. (1982) showed that ABA decreases proton concentrations in the thylakoid lumen of guard cells and dissipates ΔpH in guard cell chloroplasts. Proton concentrations in thylakoid lumen are regulated through ion transporters/channels localized to the thylakoid membrane, as well as by chloroplast ATP synthase (Spetea et al., 2017). KEA3 allows proton efflux from the thylakoid lumen to the stroma with a counter influx of K+ and dissipates ΔpH across thylakoid membranes (Armbruster et al., 2014; Wang et al., 2017). In the present report, we examined the role of KEA3 in ABA-induced ROS generation by guard cell chloroplasts. In knockout mutants of KEA3, kea3-1 and kea3-2, ABA did not induce ROS generation in guard cells, nor did it induce stomatal closure (Figures 1 and 2), indicating that KEA3 is pivotal in ABA-induced ROS generation and stomatal closure. On the other hand, in the kea3-1 and kea3-2 mutants, stomata closed in response to calcium, which acts downstream of ROS (Pei et al., 2000), indicating that KEA3 functions upstream of ROS but not downstream of calcium in guard cell signaling. The small molecule electroneutral K+/H+ antiporter nigericin increases the rate of H+ efflux-linked K+ influx across the thylakoid membrane and rescues the NPQ phenotype of kea3-1 and kea3-2 mutants (Armbruster et al., 2014). We demonstrated that nigericin induced ROS generation in guard cells of wild type, which induced stomatal closure (Figures 4C,D and 5). These results confirm the role of H+/K+ antiporter KEA3 in ABA-induced ROS generation and ABA-induced stomatal closure. The mechanism(s) by which ABA regulates ROS requires further study.
AUTHOR CONTRIBUTIONS
Naotaka Yamada and Sumio Iwai performed the experiments and contributed to the writing of the article with Ken-ichiro Shimazaki and Kintake Sonoike. Naotaka Yamada, Sumio Iwai, Michio Onjo and Ken-ichiro Shimazaki designed the research and analyzed data. All authors discussed the results and commented on the manuscript.
ACKNOWLEDGMENTS
We thank Dr. Ute Armbruster (Max Planck Institute of Molecular Plant Physiology, Germany) for kindly providing seeds of kea3-1 and kea3-2. We also thank NAI, Inc. (https://www.nai.co.jp/) for editing our manuscript. This work was supported by Grants-in-Aid for Scientific Research (17K07698 to Naotake Yamada, 18K06292 to Ken-ichiro Shimazaki and 22H02651 to Kintake Sonoike) from the Ministry of Education, Sciences, Sports and Technology, Japan (MEXT).
Open Research
DATA AVAILABILITY STATEMENT
All relevant data can be found within the article and its Supporting Information.




