The degree of hydraulic vulnerability segmentation at the individual level is related to stomata sensitivity in seedlings of three oak species
Abstract
It is hypothesized that ‘hydraulic vulnerability segmentation’ (the vulnerability of expendable organs is higher compared to the vulnerability of nonexpendable organs) can enhance the drought-tolerance of trees. However, prediction of positive segmentation (leaf minus branch) did not gain undisputed support in field observations. Further, the main organs of trees (leaf/trunk/root) are vital to test the hypothesis at the individual level. Unfortunately, most verifications of the hypothesis focus on the hydraulic vulnerabilities between leaves and terminal branches of mature trees. In this study, we grew three deciduous oak (Quercus mongolica, Q. variabilis and Q. aliena) seedlings (6 months old), and measured the hydraulic vulnerabilities of leaves (by rehydration method), main stems and main roots (by bench dehydration method). The sensitivity of stomata to water potential was obtained by monitoring the stomata conductance response to drought stress. The xylem anatomy of main stem and main root was also measured. It was found that the leaves of all three oak species exhibited greater vulnerability than the main stems and main roots. The leaf-stem segmentation was more pronounced than the stem-root one, and the stem-root in Q. aliena even showed a reversed segmentation. Further, we found that higher stomatal sensitivity was associated with higher P50,leaf (water potential at 50% loss of leaf hydraulic conductivity), and narrower segmentation between leaf and the most embolism-resistant organ. Collectively, our results support the ‘hydraulic vulnerability segmentation’ hypothesis at the individual level, and highlight the importance of stomatal regulation in hydraulic vulnerability segmentation.
1 INTRODUCTION
With the unrelenting pace of global warming, drought-induced tree mortality is all the more frequent (Allen et al., 2010; Choat et al., 2012). Current studies suggest that hydraulic failure is the main factor inducing plant mortality during drought stress (Allen et al. 2010; Brodribb et al. 2020). It is found that water flows upward through xylem under tension, driven by gradients in water potential from root to leaf (Zimmermann, 2013). Such metastable water column is therefore vulnerable to air bubbles' entry, xylem then forms emboli when tension develops, and eventually, hydraulic failure (irreversible loss of hydraulic conductivity) will occur as tension becomes much higher (Tyree and Sperry, 1989). As such, the vulnerability to embolism of a species plays a pivotal role in determining its drought tolerance. The vulnerability to embolism is commonly quantified by P50 value, the water potential at which cavitation causes a 50% loss of conductivity (Anderegg et al. 2016; Choat et al. 2018). A safer water transport system means a lower P50, with a lower probability of hydraulic failure, but other benefits are potentially compromised (Pratt and Jacobsen, 2017).
Studies have proposed that hydraulic vulnerability segmentation (HVS) is an important strategy for plants to resist drought stress and affects their drought tolerance (Tyree & Ewers, 1991). HVS states that distal organs such as leaves and fine roots will be at a higher risk of embolism (higher P50) than basal organs such as the trunk. With the increase of drought stress, the renewable distal organs can be hydraulically dysfunctional and sacrificed to protect the irreplaceable basal organs from catastrophic hydraulic failure. Studies on the difference of P50 between terminal branches and leaves (Bucci et al., 2012; Bouche et al., 2016; Klepsch et al., 2018; Skelton et al., 2019; Levionnois et al., 2020) have shown that leaves can act as ‘hydraulic fuses’ to protect branches from embolism under drought stress. Further, some studies found that lateral roots are more susceptible to hydraulic failure than the trunk, in support of HVS (Maherali et al., 2006; Bucci et al., 2013; Johnson et al., 2014; Johnson et al., 2016). However, some studies also showed that there is no segmentation between branches and leaves (Chen et al., 2009; Skelton et al., 2018; Klepsch et al., 2018), or even reversed HVS in some species (Klepsch et al., 2018; Levionnois et al., 2020). As such, there exists no consensus on the significance of HVS in drought tolerance, and the underlying mechanism(s) is unclear.
This uncertainty could be ascribed to two factors seldom addressed in the past. Since most of the current studies involved less main stems and main roots, the verification of the HVSH (HVS hypothesis) is less conclusive. The main stem and main root largely determine the integrity of the hydraulic system and are vital components of the Soil–Plant-Atmosphere Continuum (Maherali et al., 2006; Bucci et al., 2013; Johnson et al., 2016). Unlike fine roots/leaves, which can be discarded in large numbers, once the main roots/trunk are irreversibly embolized, the water transport of the whole plant would be completely blocked, leading to drought-induced mortality of trees. Therefore, probing the HVS based on the main organs of plants (at the individual level) would be beneficial to refine the HVSH and contribute to a more comprehensive understanding of the hydraulic strategies of plants under drought stress.
The second factor is the regulation of stomata in hydraulics. Stomates are the critical control of gas exchange and water interactions between plants and the atmosphere (Salleo et al., 2000; Hochberg et al., 2017; Chen et al., 2019; Creek et al., 2020). The sensitivity and regulation of stomata are regarded as a key strategies for plants to resist drought stress (Martin Stpaul et al., 2017; Wu et al., 2022). However, there are some debates in the literature as to the coordination between stomatal regulation and hydraulics. Some studies have demonstrated that the loss of leaf hydraulic conductivity due to xylem embolism may be a key signal inducing stomata closure (Nardini & Tyree, 1999; Sperry, 2000; Brodribb & Holbrook, 2003). It has also been demonstrated that stomatal behavior is coordinated with stem hydraulic safety (Pivovaroff et al., 2018). Further, some studies have shown that stomata respond to root embolism (Alder et al., 1996). As such, the regulation of stomata aiming to guarantee organ's hydraulic safety remains controversial.
Based on the observed tight associations between the water potential corresponding to the closure of stomata and the water potential corresponding to the decline of xylem hydraulic conductivity among organs (Alder et al., 1996; Nardini & Tyree, 1999; Sperry, 2000; Brodribb & Holbrook, 2003; Pivovaroff et al., 2018), we can deduce three scenarios (see Figure 1). Scenario A: The degree of hydraulic vulnerability segmentation (HVS) is positively related to stomatal sensitivity. For three co-occurring species with similar drought resistance, a more sensitive stomata is related to a narrower HVS (note the reversed vulnerability segmentation appears for a more sensitive stomata species). Scenario B: HVS has no relation with stomatal sensitivity (note the reversed vulnerability segmentation is absent). Scenario C: HVS is negatively related to stomatal sensitivity. A more sensitive stomata is related to a wider HVS (note the reversed vulnerability segmentation appears for a less sensitive stomata species). As such, HVS seems to be a consequence of relative sensitivities of stomata to leaf and to the most embolism-resistant organ across species. Considering there are reports on the reversed HVS (Skelton et al., 2018; Levionnois et al., 2020), scenario A and C seem more possible, but which one is the case remains unknown. It is worth noting that the stomata sensitivity to water potential can also be evaluated by the “isohydric” or “anisohydric” behaviors, which can be identified by the slope (α) between pre-dawn water potential and mid-day water potential (Meinzer, et al., 2016). A more sensitive stomata tends to be closer to “isohydric” behavior with a smaller α, compared with a more sluggish one.
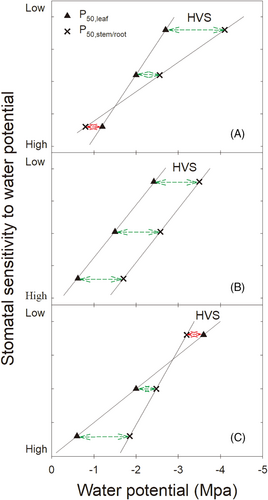
Northern China is one of the regions with the fastest global warming rate (IPCC, 2018). The intensification of climate change has led to significant changes in the structure and function of temperate forests in the region due to regional and staged droughts (Zhang et al. 2014; Yuan et al. 2021). In the present study, we grew 6-month-old seedlings of three deciduous oak species co-occurring in the temperate forest ecosystem and investigated the HVS of their main roots, main stems and leaves. Simulated drought stress experiment was also conducted to obtain the stomata responses to water potential. Quercus mongolica, Q. variabilis and Q. aliena are three deciduous oak species that are widely distributed in semi-arid areas and represent the dominant species of deciduous broad-leaved forests in the northern temperate zone of China (Menitsky, 2005). Most of the oak species are ring-porous wood, which are prone to embolism (Gil-Pelegrín et al., 2018). It is generally acknowledged that compared to adult trees, seedlings are more susceptible to surface soil water deficit, as their shallower and smaller root systems (Matzner et al., 2003) run a greater risk of mortality (van Mantgem et al., 2009; Allen et al., 2010). Studies have shown that water plays a critical role in the growth and survival of seedlings (Matzner et al., 2003; Mahall et al., 2009). The present intensification of drought stress is also accompanied by the intensification of forest decline, and the adaptation of seedlings is the cornerstone of forest renewal and stability. Therefore, conducting hydrodynamic studies on seedlings will help to understand and predict the future dynamics of forest ecosystem structure and function, species turnover and biodiversity maintenance mechanisms. We aimed to test the following two hypotheses: (1) Segmentation exists between leaves, main stems and main roots, leaves are more vulnerable than main stems, while main stems are more vulnerable than main roots; and (2) Stomatal sensitivity to water potential is coordinated with HVS degree, which may conform to scenario A or C in Figure 1.
2 MATERIALS AND METHODS
2.1 Study site and species
Experiments of the hydraulic measurement section were carried out in August and September 2021 on 6-month-old saplings of three deciduous oak species (Quercus variabilis, Q. mongolica, Q. aliena). To obtain the best growth conditions, the seeds were sown in large-scale containers (diameter: 110 mm; height: 40 cm). Then the plants were placed under Beijing Hot Spring Nursery (40°05′N, 116°17′E) outdoor awning and constantly irrigated to field capacity (every 4–5 days). The average monthly temperatures in August and September are 20–24°C (Data from China Meteorological Administration). At measuring time, the saplings of Q. variabilis were, in average, 56.47 cm tall with a stem diameter of 8.83 mm, the saplings of Q. mongolica were 32.94 cm with a stem diameter of 8.49 mm, and the saplings of Q. aliena were 35.24 cm with a stem diameter of 8.33 mm.
2.2 Water potentials
For the tree species in the study, all water potentials were monitored with a Scholander pressure chamber (Model 1505D; PMS Instrument Co.). The turgor loss point and water capacitance were estimated from pressure–volume (P-V) curves (Tyree & Hammel, 1972) taken in August 2021 using the methods described by Brodribb & Holbrook (2003).The night before, two saplings of each tree species were watered to achieve complete hydration and covered with black bags. At 8 a.m. of the next day, the fully expanded leaf in the same position of seedlings was selected for the test. The middle leaves were picked for P-V curve measurement. The leaves were separated from the stems and dried slowly on the laboratory bench. During this process, the leaf weight (with accuracy of 0.0001 g) and water potential were regularly measured to obtain a plot of leaf water potential versus the inverse of relative water content. The corresponding turgor loss point (TLP, MPa) was estimated as the inflection point of the TLP versus the relative water content plot. The slope of the curve before and after TLP represents the water capacitance (C; CFT, CTLP) lost before and after TLP (Brodribb & Holbrook, 2003). Leaf area was determined using a handheld laser leaf area meter (CID, CI-203) to standardize the water capacitance. One mature leaf exposed to the sun was taken from each seedling and 12 seedlings of each tree species were measured to construct pressure-volume curves and estimate the TLP, CFT, CTLP, etc. for the three deciduous oak species.
Leaf water potential measurements were performed at pre-dawn and midday on fully sun-exposed leaves. To measure the pre-dawn leaf water potential(ψpd), we covered the seedlings with a black plastic bag before dawn and then measured at pre-dawn (8:00 am). The midday leaf water potential(ψmid) was measured at 14:00–15:00. Two opposing and adjacent (paired) leaves of each plant were used to measure ψpd and ψmid, and 16–20 pairs of leaves were measured for each tree species. The stomata sensitivity to water potential can be evaluated by the “isohydric” or “anisohydric” behaviors, which can be identified by the slope (α) between pre-dawn water potential and mid-day water potential. We used the method described by Meinzer et al. (2016) to calculate α. Firstly, the very mild values where ψmid likely varied with irradiance only, independently of ψpd (vertical portions of trajectories) were excluded. Then, the remaining water potential at pre-dawn and midday was used for linear regression fitting, where α is the slope obtained by linear fitting.
2.3 Leaf embolism resistance
2.4 Stem and root embolism resistance
Whole seedlings were removed from the container and dried naturally without shearing to assess their vulnerability to embolism (Cochard et al., 2013). During the whole drying process, we did not cut the plants to ensure that there were no open vessels. To achieve a wide range of xylem tensions, the whole plant separated from the soil was placed on the test bench and air dried under lab lights for 0.5–72 h, bagged for a minimum of 1 h to allow xylem pressure to equilibrate across the whole plant (for each individual, water potential did not vary by more than 10%). When the water potential was balanced, the water potential of the top leaves of the plant was measured using the pressure chamber. According to the scheme of Wheeler et al. (2013), the sample was double cut in water to relax the residual tension, and the cutting length was more than twice the maximum vessel length. And according to the research on seedlings (Losso et al. 2018), the sample quilt was cut into 30-35-cm segments under water for relaxing, and trimmed several times with a sharp carving knife to gradually release tension, remove micro-bubbles and minimize eventual artefacts due to xylem refilling under rehydration (Trifilò et al., 2014; Johnson et al., 2016). During pruning, sharp pruning shears were used to avoid the crushing effect and to ensure that no air bubbles were attached to the pruning shears. The main stem (diameter 6.04 ± 0.81 mm) and main root (diameter 8.25 ± 0.62 mm) segments were placed in water to relax for about 0.5-2 h (Figure S2; Melcher et al., 2012; Wheeler et al., 2013; Hochberg, Herrera, et al., 2016b). The root/stem segments were reduced to about 10 cm before measurement (Cochard et al., 2013), and the xylem hydraulic conductivity (Kh) and maximum hydraulic conductivity (Kmax) were measured with a XYL'EM apparatus (Bronkhorst), which in turn yielded the value of hydraulic conductivity loss (Sperry & Tyree, 1988; Melcher et al., 2012). Degassed distilled water was used in the measurement process along with 10 mmol/L KCl filtered through a filter with a pore size of 0.22 μm (Blackman et al., 2019). The Kh value was measured at low pressures of 4–5 kPa for 5 min (De Baerdemaeker et al., 2019), while the Kmax value was measured at a pressure of about 150 kPa at 2-min intervals until the value was no longer elevated when measured at low pressure.
Finally, the sample size of Q. mongolica, Q. variabilis and Q. aliena used to determine the hydraulic conductivity of stems and roots was 34, 30, 34, respectively. And the sample size of Q. mongolica, Q. variabilis and Q. aliena used to determine the hydraulic conductivity of roots was 37, 30, 39, respectively. The water potential at 50% loss of hydraulic conductivity was recorded as P50 (P50,leaf, P50,stem, P50,root, MPa), and the water potential at 88% loss of hydraulic conductivity was recorded as P88 (P88,leaf, P88,stem, P88,root, MPa). Previous studies have suggested that P50 represents the water potential associated with significant damage and P88 is considered as the embolism threshold leading to irreversible drought damage (Domec & Gartner, 2001; Urli et al., 2013; Choat et al., 2018).
2.5 Stomatal conductance measurements and safety margin calculation
2.6 Xylem anatomy
Two samples were intercepted from the main root and main stem of three trees of each species at almost the same position as the assay PLC. Sections of about 8 μm were cut out with a frozen slicer (Leica CM1950) and colored with FASGA (safranin + Alcian blue) staining (Tolivia & Tolivia, 1987; van der Werf et al., 2007) before observation under a biological microscope (DM2500, Leica). Xylem vessel diameter (VD) and vessel frequency (VF) were calculated from micrograph measurements using ImageJ 1.49 V (National Institute of Health). More than 200 vessel diameters were determined for each tree species to compare the differences in vessel diameter between the main roots and stems.
2.7 Statistical analysis
Inter-specific differences in xylem anatomy and parameters of hydraulic conductivity were examined separately using one-way ANOVA. Differences between means were assessed using Tukey's HSD tests. The vulnerability data as well as the stomatal-water potential data were fitted using the Sigmoid function in Sigmaplot (version 14.0, Systat Software Inc. (Johnson et al., 2016), and 95% confidence intervals (ICs) were calculated. If the 95% ICs did not overlap, significant differences were considered to occur between species.
3 RESULTS
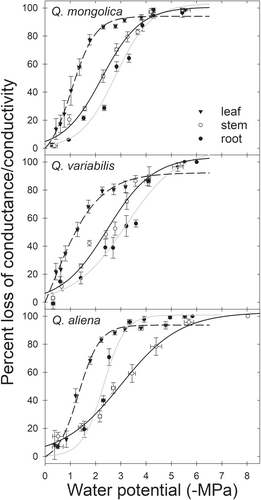
Overall, the leaves of all three deciduous oak species displayed more vulnerability than their stems (i.e., positive segmentation in the main stem and leaves) (P50, leaf-P50, stem of Q. mongolica, Q. variabilis and Q. aliena were 1.03 MPa, 1.19 MPa, and 1.53 MPa, respectively, with non-overlapping 95% CIs). Except for Q. aliena, which had a significant reversed segmentation on main stems and main roots, the other two species showed a significant positive segmentation (P50,stem-P50,root of Q. mongolica, Q. variabilis and Q. aliena were 0.50, 0.63, and − 0.65 MPa, respectively, and non-overlapping 95% CIs; Figure 2, Table 1). For P88, a significant degree of positive segmentation was found between the main stems and leaves of the other two species except for a small overlap in the 95% CI for Q. variabilis main stems and leaves (1.35 MPa for Q. mongolica, 0.51 MPa for Q. variabilis and 2.59 MPa for Q. aliena; Table 1). No significant difference was found in the P88,stem and P88,root of Q. mongolica and Q. variabilis, except for Q. aliena, which exhibited some degree of reversed segmentation.
| species | P50,root (MPa) | P50,stem (MPa) | P50,leaf (MPa) | P88,root (MPa) | P88,stem (MPa) | P88,leaf (MPa) |
|---|---|---|---|---|---|---|
| Q. mongolica | −2.75 | −2.25 | −1.21 | −4.01 | −3.72 | −2.37 |
| (−2.51, −3.00) | (−2.09, −2.43) | (−1.13, −1.29) | (−3.63, −4.92) | (−3.44,4.17) | (−2.13, −2.67) | |
| Q. variabilis | −3.02 | −2.39 | −1.20 | −4.66 | −4.02 | −3.51 |
| (−2.71, −3.36) | (−2.18, −2.64) | (−1.04, −1.39) | (−4.18, −5.39) | (−3.63, −5.61) | (−2.77, −4.29) | |
| Q. aliena | −2.30 | −2.95 | −1.40 | −3.12 | −5.09 | −2.50 |
| (−2.00, −2.51) | (−2.73, −3.20) | (−1.27, −1.59) | (−2.70, −4.29) | (−4.65, −5.12) | (−2.11, −3.70) |
In terms of vulnerability, there was no significant difference in P50,stem and P50,root for Q. mongolica and Q. variabilis species because the 95% CIs overlapped. Also, the P50,stem for Q. variabilis and Q. mongolica were significantly higher than that of Q. aliena, while the P50,root were significantly lower than that of Q. aliena (P50,stem of Q. mongolica, Q. variabilis and Q. aliena were − 2.25, −2.39, and − 3.95 MPa, respectively; P50,root of Q. mongolica, Q. variabilis and Q. aliena were − 2.75, −3.02, and − 2.30 MPa, respectively; with non-overlapping 95% CIs). And in Q. mongolica and Q. variabilis, the main root is the most embolism-resistant organ, while in Q. aliena, the main stem is the most embolism resistant organ (Figure 2, Table 1). Although there was no difference among the leaves of the three tree species when reaching P50,leaf, the water potential value of Q. variabilis when reaching P88,leaf was significantly lower than that of the other two tree species (−2.37 MPa for Q. mongolica, −3.51 MPa for Q. variabilis and − 2.50 MPa for Q. aliena; Figure 2; Table 1).
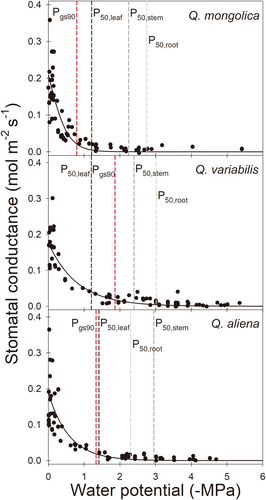
Stomatal conductance (gs) declined in response to increasing desiccation (decreasing water potential) in all three species (Figure 3). The Pgs90 was significantly higher in Q. mongolica than in Q. variabilis and Q. aliena (−0.79 MPa for Q. mongolica, −1.86 MPa for Q. variabilis and − 1.33 MPa for Q. aliena; Table 2), meaning that the stomatal sensitivity to water potential was from high to low: Q. mongolica, Q. aliena and Q. variabilis, respectively. This was also reflected in the relationship between the pre-dawn and midday water potentials of leaves, with Q. mongolica more inclined than the other two species to isohydric (Figure 4). In addition, the order of the degree of segmentation between leaves and the most embolism-resistant organs was the opposite of the stomatal sensitivity to water potential (1.54 MPa for leaf and main roots of Q. mongolica, 1.55 MPa for leaf and main stems of Q. aliena, 1.82 MPa for leaf and main roots of Q. variabilis; Table 2).
| species | Pgs90 (MPa) | P50,leaf-P50,stem (MPa) | P50,stem-P50,root (MPa) | P50,leaf-P50,root (MPa) |
|---|---|---|---|---|
| Q. mongolica | −0.79 | 1.03 | 0.50 | 1.54 |
| Q. variabilis | −1.86 | 1.19 | 0.63 | 1.82 |
| Q. aliena | −1.33 | 1.55 | −0.65 | 0.88 |
In the three tree species, the greater the VD (vessel diameter), the lower the VF (vessel frequency; Figure S1). Within the same species, main roots generally had larger vessel diameters and smaller vessel densities than main stems. Our research showed that the correlation between P50 of the main stems and main roots of the three deciduous oak seedlings and their corresponding VD and VF was not significant (P = 0.66, 0.84; Figure 6 A, B). The correlation between the hydraulic conductivity (Kmax) and the fourth power of a single VD was weak (P = 0.65), while there was a strong positive correlation between the Kmax and the product of VD4 and VF (P = 0.034; Figure 6 C, D).
4 DISCUSSION
4.1 Vulnerability segmentation at the individual level
The P50,leaf of the three species of oak seedlings was approximately −1.27 MPa, which is higher than the reported average P50,leaf of adult oaks (−1.70 ~ −3.74 MPa; Skelton et al., 2018), suggesting that seedlings may be less drought tolerant than adults. In addition, the P50,stem values in this study (−2.25 ~ −2.95 MPa) are within the 75% Quantile range of P50,branch values reported for Quercus trees around the world [−0.50 ~ −6.96 MPa, data collected by Gil-Pelegrín et al. (2013); −0.18 ~ −5.50 MPa, (Choat et al., 2015); −4.43 ~ −7.13 MPa, (Lobo et al. 2018); −2.74 ~ −6.27 MPa, (Skelton et al. 2018)]. This supports the statement that seedlings have a greater risk of mortality than their mature individuals under drought stress, if HVS is the case in the field (van Mantgem et al., 2009; Allen et al., 2010).
Our leaf vulnerability curve trends are similar to previous research results using the rehydration method (Hao et al., 2008; Brodribb et al., 2009; Blackman et al., 2010; Johnson et al., 2011; Scoffoni et al., 2012; Johnson et al., 2016). The leaf vulnerability measured in our study was the loss of hydraulic conductance of the entire leaf, including the xylem and outside-xylem components (Scoffoni et al., 2017). As stated by Bouche et al. (2016), the vulnerability segmentation hypothesis can be tested based on the whole leaf level (including extra-xylary pathways) because the water of a whole leaf is mainly supplied with branch/stem xylem vessels. Actually, the vulnerability of leaves should include two components: xylem and outside-xylem. Scoffoni et al. (2017) pointed out that the main cause of the decrease in the leaf hydraulic conductance comes from the outside-xylem component, hence the leaf-branch/stem vulnerability segmentation mainly comes from the difference of safety from the outside-xylem of leaf and that from the xylem of stem. If measuring the leaf vulnerability within the leaf xylem, such as either Micro-CT/NMR, or optical method, the whole leaf vulnerability may not be represented reliably. Further, the leaf xylem may experience elastic shrinkage (Wolfe et al., 2023; Zhang et al., 2023), which will further increase the measuring error of the leaf vulnerability curve. In our results, the leaves of three deciduous oak seedlings showed greater vulnerability than the main roots and stems (the P50 and P88 of leaves were higher than those of the roots and stems), supporting the HVSH. This result is an important extension to previous reports on adult trees and large seedlings where the leaves can act as ‘hydraulic fuses’ to protect terminal branch parts from embolism (Johnson et al., 2011; Hochberg, Albuquerque, et al., 2016a; Wolfe et al., 2016; Jin et al., 2019; Levionnois et al., 2020). The average HVS degree of leaf-stem segmentation for the three oak seedlings was approximately 1.26 MPa, which is in the range of HVS degree of leaf-terminal branch segmentation of the adult oak trees globally (1.82 to 0.15 MPa; Skelton et al., 2018), indicating that young oaks have similar vulnerability segmentation protection to adult trees. Interestingly, under the P88 threshold, the average degree of HVS of main stems and leaves increased to 1.48 MPa (wider HVS), indicating that the safety margin by HVS mechanism increases under severe drought stress. Many studies showed that P88 was considered as the embolism threshold beyond which irreversible hydraulic failure would occur in angiosperms (Domec & Gartner, 2001; Urli et al., 2013; Choat et al., 2018; Scholz et al., 2014).
However, stem-leaf segmentation is only one aspect of HVS, while leaf-root or stem-root hydraulic segmentation may also play a critical role in plant drought tolerance. In the case of HVS between the main root and main stem, both Q. mongolica and Q. variabilis showed a positive segmentation (stem P50 minus main root P50), meaning the main stem was more vulnerable than main root. This conclusion differs from previous ones where the root system was found to be more vulnerable than stem (Matzner et al., 2001; Martínez-Vilalta et al., 2002; Bucci et al., 2013; Johnson et al., 2016), but is consistent with the results measured for the main root which was more resistant than the stem (Rodriguez-Dominguez et al., 2018). This may be due to the fact that in the former studies (Matzner et al., 2001; Martínez-Vilalta et al., 2002; Bucci et al., 2013; Cuneo et al., 2016; Johnson et al., 2016), the selection of roots mostly included lateral roots or fine roots, while in the later study, as well as in the present one, the measured roots were the main root. As such, for Q. mongolica and Q. variabilis, the main root is more important to guarantee the integrity of the plant's hydraulic system than the stem, and critical to the survival of seedlings. Interestingly, compared to its relatives, Q. aliena showed reversed segmentation (negative HVS) between the main stem and main root, similarly to some studies (Creek et al., 2018; Losso et al., 2018; Rodriguez-Dominguez et al., 2018), demonstrating that stem-root segmentation can vary considerably across oak species. In the field, the main stem usually has a more negative water potential than the main root (Jackson et al., 2000). For Q. aliena, the water potential difference between the main stem and main root may be large in field conditions. As such, in the field, the main stem of Q. aliena may lose similar hydraulic conductivity to the main root due to its more negative water potential compared to the main root, leading to a positive segmentation. In addition, the reverse segmentation in Q. aliena can be attributed to its different life history strategies. For example, both a stronger hydraulic redistribution ability (Liu et al. 2021) and a greater segmentation between fine roots and main roots (Maherali et al., 2006; Bucci et al., 2013) may secure the hydraulic safety of the main roots to a greater extent in this species. Further research efforts in native PLC measurements of the main stem and main root of this species in field are warranted.
4.2 Stomatal regulation of the degree of HVS in oak seedlings
Our study found that the ranking of stomatal sensitivity to water potential (Pgs90) was the same as the ranking of P88,leaf and opposite to the ranking of the degree of hydraulic vulnerability segmentation between leaf and the most embolism-resistant organ (HVS: P50, leaf - P50, stem/root). In other words, the ranking of Pgs90 of Q. mongolica, Q. aliena, and Q. variabilis seedlings was the same as the ranking of P88,leaf of all three, and opposite to the ranking of the HVS of leaf-root of Q. mongolica, leaf-stem of Q. aliena, and leaf-root of Q. variabilis. Thus, the Pgs90 and HVS of the three oak species with similar drought tolerance in this study were consistent with scenario A (Figure 1); that is, the lower the Pgs90, the greater the HVS. As such, HVS seems to be the consequence of the relative stomatal sensitivity to leaf hydraulic safety and the most embolism-resistant organ's safety across the three oak species. For species with similar drought tolerance, a higher Pgs90 species leads to a lower degree of HVS (Figure 5), the trend can explain why positive segmentation (Johnson et al., 2011; Bouche et al., 2016; Hochberg, Albuquerque, et al., 2016a; Wolfe et al., 2016), no segmentation and even reversed segmentation (Skelton et al., 2018; Levionnois et al., 2020) were observed in previous studies. Further, it is postulated that when species have similar Pgs90 values (stomatal sensitivity to water potential), the greater the HVS a species has, the longer the dehydration time under drought conditions (Blackman et al., 2019), and the greater the drought tolerance it owns. Therefore, we postulated that stomatal sensitivity not only is coordinated with the embolism resistance of organs but also plays a role in the degree of HVS. The stomata sensitivity to water potential can also be evaluated by the “isohydric” or “anisohydric” behaviors, which can be identified by the slope (α) between pre-dawn water potential and mid-day water potential. A more sensitive stomata with a smaller α corresponded to a lower degree of HVS (Figure 5), consistent with the Pgs90 results. We also found the slope between stomata sensitivity (based on Pgs90 and α metrics) and leaf hydraulic safety is steeper than that between stomata sensitivity and the most embolism-resistant organ's safety, indicating stomata behavior is more sensitive to leaf hydraulic signal than other organ's hydraulic signal. However, it must be acknowledged that more measurements on other oak species with similar drought tolerance are needed to further confirm such an interesting pattern regarding the regulation of stomata on HVS across species.
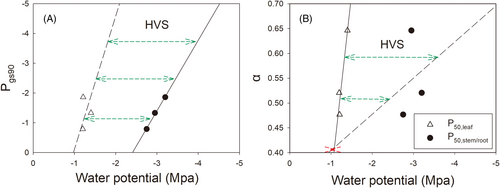

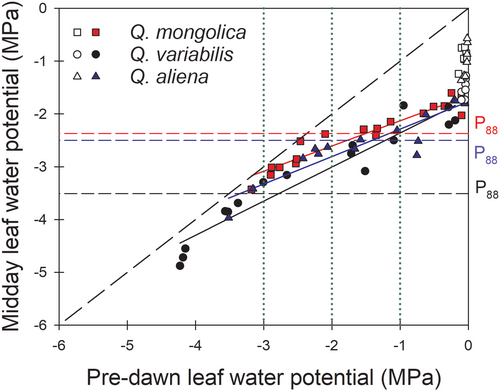
Interestingly, different tree species may adopt different strategies under different drought stress levels. As shown in Figure 4, under mild drought stress (−1 MPa), the leaves of all three tree species did not reach P88,leaf; as such, none of them activated the vulnerability segmentation mechanism during this time. Under moderate drought stress (−2 MPa), the leaves of two tree species (Q. mongolica, Q. aliena) with a low degree of hydraulic segmentation reached P88,leaf, the leaf hydraulic dysfunction, implying that these two species might initialize the strategy of hydraulic segmentation, i.e., fallen leaves may appear. In contrast, the leaves of Q. variabilis did not reach the hydraulic failure threshold under moderate drought stress, mainly due to the higher resistance to xylem cavitation than its counterparts (Figure S3). Under severe drought stress (−3 MPa), the leaves of Q. variabilis exceeded the water potential point P88,leaf. At this time, the HVS strategy played a role and leaves began to fall off. Obviously, species with similar drought tolerance can adopt different strategies (i.e., resistance to cavitation and HVS), depending on the severity of drought. Further, the stomatal sensitivity participates in the transition of strategies across the three studied species, as discussed in the previous paragraph.
4.3 Vulnerability and xylem anatomy
The vulnerability of the stems and roots of the three oak species may be related to xylem anatomy. In our study, there was no difference in P50 between the main roots and stems of the three tree species (paired t-test, P > 0.05); on the contrary, the main roots exhibited a larger vessel diameter than that of stems (paired t-test, P < 0.05). This is similar to the study by Wason et al. (2018), where organs with different vessel diameters exhibited similar safety. On average, the main root has a larger vessel diameter and a higher safety, while the main stem has a smaller vessel diameter and a lower safety. We speculate that such differences between the main stem and root may be related to the structural difference of pit between root and stem, such as the average inter-vessel pit area per vessel (Wheeler et al. 2005), the pit membrane thickness (Jansen et al. 2009; Trueba et al. 2019), the pit borders vestures (Zimmermann, 2013), and the wettability of the inter vessel wall (Lens et al., 2022). In contrast, our results show that, compared with hydraulic safety (P50), the coupling relationship is closer between VD, VF and xylem efficiency (Kmax). According to the modified Hagen–Poiseuille equation proposed by Tombesi et al. (2010), the theoretical hydraulic conductivity of a single vessel is positively correlated with the fourth power of the VD (Tyree & Ewers, 1991; Schuldt et al., 2013) under the condition that angiosperms have a relatively simple striatal structure (Choat et al., 2008). Moreover, we assumed that the hydraulic resistance (R) of each vessel in a certain cross-section was the same; the total hydraulic resistance, according to Ohm's principle, was R/VF. Therefore, theoretically, the Kmax was positively correlated with the reciprocal of the total hydraulic resistance (VF/R). In turn, we advanced the notion that the Kmax on a certain cross-section should be in direct proportion to the fourth power of the VD and the product of the VF (VD4VF). This also explains why, in our results, Kmax is positively correlated with the product of VD4 and VF, but not with VD4. This could also be a reason for some discrepancies between the theoretical Kmax obtained by Losso et al. (2018) using the Hagen-Poiseuille equation and the Kmax measured using the hydraulic method. Therefore, we believe that the influence of VF should be taken into account in the calculation of theoretical Kmax, which may be beneficial for the optimization of future theoretical Kmax calculations.
5 CONCLUSION
In summary, the work we conducted has served to further verify the phenomenon of vulnerability segmentation in plants. Unlike the usual studies on leaves and subtending branches, we conducted a systematic study on whether vulnerability segmentation exists among leaves, main stems, and main roots. Our results show that leaves, the most peripheral organs, display greater vulnerability and act as hydraulic fuses to protect main roots or stems. Both positive and reversed segmentation may take place between the main stem and the main root, which might be attributed to the different life history responses adopted by different plants. In addition, we found that stomatal sensitivity not only coordinated with the P88,leaf, but also played a role in regulating HVS. This coordination was related to the corresponding strategies adopted by different tree species under different drought stress levels. Through a comprehensive analysis of the relationship between the specific conductance and safety of the main stems and main roots of three tree species and the diameter and frequency of their vessels, we found that the diameter and density of the vessels could not explain the hydraulic safety of the main roots and main stems, but could well explain the specific conductance of the two. Future research should obtain more detailed anatomical structure features related to pits to explore the causes of vulnerability segmentation among different organs.
AUTHOR CONTRIBUTIONS
Conceptualization: Yinning Li, Chenrui Huo; Methodology: Yining Li, Jiaxi Wang, Zexia Dong, Xiaoqian Meng; Formal analysis: Yining Li, Xu Wang; Investigation: Yining Li, Chenrui Huo; Writing - original draft: Yining Li; Writing - review & editing: Dayong Fan, Guolei Li; Supervision: Guolei Li, Dayong Fan; Project administration: Guolei Li; Funding Acquisition: Guolei Li, Jiaxi Wang.
ACKNOWLEDGMENTS
The study was jointly funded by two National Natural Science Foundation of China (32171764, 32101503).
FUNDING INFORMATION
The study was jointly funded by two National Natural Science Foundation of China (32171764, 32101503).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon request.




