Evolutionary studies of the basic helix–loop–helix (bHLH) IVc gene family in plants and the role of AtILR3 in Arabidopsis response to ABA stress
Abstract
The basic helix–loop–helix (bHLH) IVc transcription factors (TFs) play central roles in controlling iron (Fe) homeostasis and biotic stress responses. However, their evolutions and functions in other abiotic stresses are poorly understood. In this study, the IAA-LEUCINE RESISTANT3 (ILR3) homologs were traced roughly back to before the early origin of land plants and divided into six main clades (Clade A-F). Further analysis found that the ILR3 orthologs were angiosperm-specific, suffering from motif-acquisition events and loose purifying selection. Synteny analysis displayed that the whole genome duplications (WGDs) contributed to the establishment of the IRON DEFICIENCY TOLERANT1 (IDT1, also called bHLH34)/bHLH104 lineage prior to the divergence of angiosperms. Sequence analysis revealed that the ILR3 homologs had some novel and conserved motifs except bHLH and leucine zipper (ZIP) domains. Particularly, Arabidopsis thaliana ILR3 (AtILR3) was a nuclear protein and greatly activated by ABA and CdCl2 stresses. Simultaneously, the molecular and genetic analyses suggested that the AtILR3 acted as a positive regulator in the ABA stress response through enhancing the ability of reactive oxygen species (ROS)-scavenging, such as the activities of superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT). The inductively coupled plasma mass spectrometry (ICP-MS) analyses exhibited that the AtILR3 affected the absorption of nutrient elements, especially iron elements, under ABA stress. Collectively, our findings could shed deep light on the origin and evolution of the plant ILR3s, as well as the functions of the AtILR3 under ABA stress.
1 INTRODUCTION
Iron (Fe) is involved in multiple metabolic processes, such as intracellular redox reaction and electron transfer, and is an essential trace element for the growth and development of all organisms (Hansch & Mendel 2009; Kobayashi & Nishizawa 2012). Fe-deficiency (−Fe) can affect the normal growth and development of plants, while excessive Fe is prone to the Fenton reaction, producing harmful superoxides that cause damage to cells (Dixon & Stockwell 2014; Briat et al., 2015). Therefore, the rigorous maintenance of iron homeostasis, such as its acquisition, transportation, utilization and storage, is the key to plant growth and development.
Although the earth's crust is very rich in iron, the iron exists mainly in the form of insoluble ferric oxide and hydroxides and are, thus, difficult for plants to directly absorb and utilize, so −Fe stress is widely present in plants. When plants respond to this stress, a series of basic helix–loop–helix (bHLH) transcription factors (TFs) are activated (Gao & Dubos 2021; Liang 2022). For example, the FER-LIKE IRON DEFICIENCY-INDUCED TRANSCRIPTION FACTOR (FIT)/bHLH29 can regulate the Fe3+ reductase FERRIC REDUCTION OXIDASE 2 (FRO2) (Robinson et al., 1999) and the Fe2+ transporter IRON-REGULATED TRANSPORTER 1 (IRT1) (Henriques et al., 2002; Vert et al., 2002) in a non-transcriptional regulation manner (Schwarz & Bauer 2020). The FIT also interacts with four bHLH Ib genes (bHLH38, bHLH39, bHLH100 and bHLH101) which can be induced by −Fe stress and constitutively activate the expression of IRT1 and FRO2 (Yuan et al., 2008; Wang et al., 2013). While the four bHLH IVc proteins including bHLH34/IRON DEFICIENCY TOLERANT1 (IDT1), bHLH104, bHLH105/IAA-LEUCINE RESISTANT3 (ILR3) and bHLH115 can directly bind to the promoters of the four bHLH Ib genes, thereby promoting their expressions (Zhang et al., 2015; Li et al., 2016; Liang et al., 2017). Notably, bHLH IVc members respond to iron deficiency not at the transcriptional level but at the post-translational level regulated by Fe sensor BRUTUS (BTS), a putative E3 ligase protein that promotes its protein degradation (Selote et al., 2015; Xing et al., 2021). Under −Fe conditions, IRONMANs suppress the interaction between BTS and ILR3/bHLH115, causing the elevation of the bHLH IVc proteins, ultimately increasing the plant's absorption of iron (Li et al., 2021b).
The Arabidopsis thaliana ILR3 (AtILR3) is characterized by a leucine zipper (ZIP) domain following the bHLH domain, which plays a vital role in controlling iron homeostasis (Heim et al., 2003; Toledo-Ortiz et al., 2003). The ZIP domain is necessary for protein dimerization (Bresnick & Felsenfeld 1994), and the bHLH domain contains a basic region associated with DNA binding and an HLH region involved in the formation of homo- or hetero-dimers (Murre et al., 1989; Massari & Murre 2000). AtILR3 is expressed in all organs throughout the plant life cycle and likely modulates the expression of Vacuolar Iron Transporter-Like1 (AtVTL1), AtVTL2 and AtVTL5 genes (Gollhofer et al., 2014), as well as AtNEET gene containing an iron-binding zinc finger CDGSH-type domain (Nechushtai et al., 2012), and three A. thaliana vacuolar iron transporter homologues (VITh) (Rampey et al., 2006). The AtILR3 interacts with POPEYE (PYE)/bHLH47 which directly inhibits the expressions of three Fe homeostasis-related genes, NA SYNTHASE4 (NAS4), FRO3, and ZINC-INDUCED FACILITATOR1 (ZIF1), acting as a transcriptional repressor in responding to Fe-deficiency in A. thaliana (Long et al., 2010; Tissot et al., 2019). In addition to iron homeostasis, the AtILR3 is also involved in biotic stress responses. For instance, the AtILR3 directly interacts with the coat protein (CP) of alpha-mosaic virus (AMV), resulting in the down-regulation of the host factor NEET, which activates the plant hormone responses (Aparicio & Pallas 2017). In the absence of iron, AtILR3 and AtPYE play a role in the regulatory network that controls pathogen response to plant root damage by regulating the biosynthesis of aliphatic glucosinolates (GLS) (Samira et al., 2018). The effector protein AvrRps4, delivered by Pseudomonas syringae, interacts with and targets BTS, alleviating the BTS-mediated degradation of AtILR3 and AtbHLH115, thereby promoting iron uptake in A. thaliana and pathogen colonization (Xing et al., 2021).
Except for A. thaliana, few ILR3 genes have been annotated in plants. For example, Oryza sativa POSITIVE REGULATOR OF IRON HOMEOSTASIS 1 (OsPRI1) /OsbHLH060, an ortholog of AtILR3, as well as OsPRI2/OsbHLH058 and OsPRI3/OsbHLH059, has been shown to have the capacity to bind and activate the promoter of iron-related bHLH TF 2 (OsIRO2/OsbHLH056) belonging to the rice bHLH Ib gene family member (Zhang et al., 2017; Zhang et al., 2020). OsPRI1/2/3 can positively regulate the response of the OsFIT/OsbHLH156, an ortholog of AtFIT, to Fe-deficient stress (Liang, 2022). OsbHLH057, designated as OsPRI4, another member of the OsbHLH IVc subgroup, also positively function in regulating Fe homeostasis (Wang et al., 2022), suggesting that the ILR3s conservatively regulate iron homeostasis in monocotyledon and dicotyledon plants. However, whether it is a conserved function in gymnosperms or even non-seed plants is unknown. The evolutionary history analysis of plant gene families provides clues about domain organization, expansion mechanism, functional evolution, and so on (Mohanta et al., 2015; Jiang et al., 2023). Since gene duplication is considered to be an important mechanism of functional evolution besides mutation, it offers novel chances and fundamental sources for evolutionary success (Van de Peer et al., 2009), of which whole genome duplications (WGDs) are a crucial evolutionary characteristic (Jiao et al., 2011; Qiao et al., 2019). Therefore, further tackling the evolutionary history of ILR3s could help to a better understanding of the origins and evolutions of their functions.
Abscisic acid (ABA) is considered a hormone in response to stress (Yu et al., 2020). It is one of the fastest synthesized hormones in plant responses to abiotic stresses and plays an important role in modulating plant abiotic stress response, thereby causing osmotic adjustment, senescence, antioxidant defence and stomatal closure (Chen et al., 2020b). ABA regulates the expression of ABA-induced genes that are involved in osmoregulation, ion transport, and stress signalling pathways and can also interact with other signalling molecules, such as reactive oxygen species (ROS) (Raza et al., 2023), to transmit their signals through mitogen-activated protein kinase (MAPK) pathway (de Zelicourt et al., 2016). ILR3 can promote salicylic acid (SA)-dependent defence response to Alfalfa mosaic virus and activate the ROS (Aparicio & Pallas 2017). However, the mechanism between ILR3 and ABA signalling pathway remains unclear.
In this study, we systematically investigated the taxonomic distribution, synteny, gene and domain organization, selection pressure, genetic distance and phylogenetic relationships throughout plant lineages of the ILR3 homologs using bioinformatics methods. The expression profiles of AtILR3 were dissected under ABA, iron deficiency and CdCl2 treatments, and its subcellular localization was also analyzed and observed. Under ABA treatment, the phenotypes and fresh weights (FWs) in ilr3, ilr3 with ILR3P: ILR3 and 35S: ILR3 plants were analyzed, and the activities of catalase (CAT), peroxidase (POD) and superoxide dismutase (SOD) were also measured. The accumulation patterns of a cluster of macronutrients including Phosphorus (P), Calcium (Ca), Potassium (K), Magnesium (Mg), and micronutrients including Sodium (Na), Zinc (Zn), Iron (Fe), Cadmium (Cd) and Manganese (Mn) in leaves and roots of ilr3 and 35S: ILR3 plants after exogenous ABA treatment were studied by using inductively coupled plasma mass spectrometry (ICP-MS), aiming at providing insights into the origin and evolution of the plant ILR3s, and underlying the function dissection of AtILR3 in responding to ABA stress.
2 MATERIALS AND METHODS
2.1 Plant materials, growth conditions and treatments
A. thaliana wild type (WT), ilr3, functional complementation of ilr3 mutant (ilr3 with ILR3P: ILR3) and ILR3-overexpressing (35S: ILR3) A. thaliana plants were cultivated in 12-cm pots with a soil mix containing matrix and vermiculite (3:1, v/v) and placed in an environment with a photoperiod of 16/8 h, 25/20°C light/dark cycle and 70% room humidity. Nicotiana benthamiana plants were placed in an environment with a 16/8 h light/dark, at 26/22°C. For stress treatments, two-week-old WT seedlings were treated in liquid 1/2 Murashige and Skoog (MS) medium supplemented with 25 μM ABA, iron deficiency or 200 μM CdCl2, respectively. The iron-deficient medium was the liquid 1/2 MS medium with 300 mM ferrozine as an iron chelator instead of iron sulfate. Distilled water was used as control (CK) in the liquid 1/2 MS medium. The samples were harvested for expression analyses at 0, 3, 6, 12, and 24 hours after treatments, respectively.
The T0 transgenic A. thaliana seeds were filtrated by solid 1/2 MS medium containing 25 mg L−1 hygromycin. Then, the Hyg-resistant lines were potted in soil containing matrix and vermiculite (3:1, v/v) and transferred to a growth cabinet with a photoperiod of 16/8 h and temperature 25/20°C. This method propagated for two generations until homozygous lines were obtained. Under ABA conditions, the T3 homozygous lines of ilr3, transgenic (ilr3 with ILR3P: ILR3 and 35S: ILR3) and the WT A. thaliana plants were first grown in solid 1/2 MS medium for 10 d and then transferred into solid 1/2 MS medium supplied with 2.5 μM ABA for 5 d as described previously (Huong et al., 2022). Under CK conditions, plants were cultured on solid 1/2 MS medium 15 d. After stress, samples were harvested for phenotypic observation, fresh weight (FW) and biochemical determination and stored in the refrigerator at −80°C until use. For ion content analysis, the 10-d-old T3 homozygous lines of ilr3, 35S: ILR3 and WT A. thaliana plants were also treated with 2.5 μM ABA for 5 d, respectively, as described above. Leaf and root samples for Prussian blue staining and ion content measurements were separately collected at 5 d after treatments, respectively, and stored in the refrigerator at −80°C until use. The specific process of the experiment is shown in Figure S1.
2.2 Genome-wide identification and phylogenetic analyses of the ILR3 homologs in plants
The genetic data of ILR3 homologs were retrieved from the database PLAZA (http://bioinformatics.psb.ugent.be/plaza/) (Van Bel et al., 2018). We searched 65 representative plant genomes from the charophytes to the angiosperms A. thaliana based on BLASTP (E-value threshold at 1e-5) and labelled the sequence similarity with the InterProScan (https://www.msi.umn.edu/sw/interproscan) (Quevillon et al., 2005). Then, the sequence harbouring “bHLH” domain (IPR011598) that belongs to “Transcription factor ILR3-like (IPR044818)” was only retained for further analysis. These alignments were performed by MAFFT v7.471 (Katoh & Standley 2013) and trimmed by trimAL v1.4.1 (Capella-Gutierrez et al., 2009) with the condition -gt = 0.7 as the threshold. The maximum likelihood (ML) phylogenetic tree was constructed by using the IQ-TREE software (Nguyen et al., 2015) with the best JTT + I + G models screened by the protetest3 (Darriba et al., 2011) with 1000 bootstraps.
2.3 Gene structure, synteny and genomic analyses of the plant ILR3s and their deduced proteins
The intron/exon patterns and splicing phases of the ILR3s were determined using the Gene Structure Display Server 2.0 (http://gsds.gao-lab.org/). The gene duplication events and synteny relationships of the ILR3s between five representative plant species (A. thaliana, Capsella rubella, Schrenkiella parvula, Populus trichocarpa and Amborella trichopoda) were analyzed by the Dual Synteny Plot for MCScanX software in TBtools (v1.120) (Chen et al., 2020a). The theoretical isoelectric point (pI) and molecular weight (Mw) of the ILR3s were obtained by using the online Compute pI/Mw tool (https://web.expasy.org/compute_pi/). The multiple sequences were aligned by the Clustal Omega tool (https://www.ebi.ac.uk/Tools/msa/clustalo/) with default settings, and the conserved domains were visualized by the WebLogo (https://weblogo.threeplusone.com/). The values of Ka and Ks and their ratios of the aligned cDNA sequences of the diverse ILR3 groups were analyzed by DNASP v5.10, and the genetic distances among the ILR3 groups and their overall mean distances were calculated by MEGA7.0 under 1000 bootstraps with Jones-Taylor-Thornton (JTT) model. The type and distribution of cis-acting elements in the 2 kb upstream of the AtILR3 promoter were investigated by the PLANTCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/).
2.4 Analyses of the lineage-specific domains/motifs and likelihood ratio tests of the plant ILR3s
The lineage-specific domains/motifs of the ILR3s were predicted by the online MEME program (https://meme-suite.org/meme/tools/meme/) (Bailey et al., 2015), and the parameters were set as default except for the selection of 20 motifs. Predicted motifs were identified as known conserved domains based on the sequences and their relative positions in the protein sequences, while others were defined as lineage-specific or unique motifs.
The CodeML program implemented in the PAML v4.8 package (Yang, 1997) was used to detect the substitution rate changes between the foreground branches and the background ones. Meanwhile, the Likelihood Ratio Tests (LRTs) were compared with the likelihood of the two branch-specific models. The first was the one ratio model, which supposed a fixed ώ (dN/dS = nonsynonymous/synonymous substitutions) along tree branches (ώo). The other was the two-ratio model premised on a designated clade (foreground = ώf) and had a different ratio relative to the remaining sequences (background = ώb). The ILR3 lineage was designated as a foreground branch. A chi-squared distribution was calculated through 2Δℓ with the difference between np2 and np1 as the freedom degree (difference between the parameter number of the one- and the two-ratio models) (Jeffares et al., 2015).
2.5 Gene cloning, vector construction and genetic transformation
The full-length open reading frame (ORF) of the AtILR3 was amplified using the double digests with the specific primers (Table S1). The PCR product was cloned into the linear overexpression vector pCAMBIA1300 that was digested by XbaI and SalI, yielding the plasmid 35S: AtILR3-p1300 for the transformation of A. thaliana. Meanwhile, the ORF of the AtILR3 without stop codon was amplified using AtILR3-GFP-F(SacI) and AtILR3-GFP-R(KpnI) primers (Table S1) and cloned into modified expression vector pCAMBIA1300-GFP at SacI/KpnI sites, generating the plasmid 35S: AtILR3-GFP, then expressed transiently in N. benthamiana leaves for subsequent subcellular localization analysis. Then, these vectors were mediated by Agrobacterium tumeficans strains EHA105 and transferred into the plant genome by floral dip method to obtain the corresponding transgenic A. thaliana plants.
2.6 Gene expression analysis
The expression patterns of AtILR3 in WT plants treated with 25 μM ABA, iron deficiency or 200 μM CdCl2, respectively, were performed by RT-qPCR. The RNA extraction was used by the TRIzol reagent (Invitrogen). First-strand cDNA was synthesized by the PrimeScript RT Master Mix Perfect Real-Time (TaKaRa). The 10 μL qPCR solution comprised 5 μL 2x SYBR green master mix, 0.4 μL primers, 4 μL first-stand cDNA (5–100 ng PCR products), and 0.2 μL ROX. The RT-qPCR amplification profile was for 95°C initial activation for 5 min, then 45 cycles of 95°C for 30 s and 60°C for 30 s. The relative expression levels of AtILR3 mRNA were calculated by the 2−ΔΔCt method with internal reference ACT2 (AT3G18780). Each individual experiment was tested with triple-biological replicates. The primer-sets information is listed in Table S1.
2.7 Subcellular localization analysis
The subcellular localization predictions of the ILR3 homologs were performed by the online website Plant-mSubP (bioinfo.usu.edu/Plant-mSubP) (Sahu et al., 2019) with the select prediction model N-Center-C terminal Composition (NCC), Pseudo Amino Acid Composition (PseAAC), and Dipeptide Composition (Dipep). In total, we could obtain three multi-location proteins, including cytoplasm-Golgi apparatus, cytoplasm-nucleus and mitochondrion-plastid and 11 single localizations such as endoplasmic reticulum (ER), nucleus, peroxisome, and so on. To confirm the accuracy of the above predictions, a subcellular localization assay was executed. The resulting Agrobacterium containing 35S: AtILR3-GFP or 35S: GFP were infiltrated into the epidermal cells of N. benthamiana leaves and grown in a greenhouse for 48 h. The GFP fluorescence was conducted through a high-resolution laser confocal microscope (Zeiss LSM 510 Meta) with a wavelength of 488 nm for excitation and a 500–530 nm wavelength for emission.
2.8 Phenotypic observation, FW and physiological measurements
For ABA stress assays, the 10-d-old plants were cultivated on the solid 1/2 MS medium (CK) or 1/2 MS medium supplied with 2.5 μM ABA, incubated in the standard greenhouse for 5 d and then photographed and collected for further experiment. For FW measurements, 10 seedlings were randomly collected for each measurement, and the independent biological assays were repeated three times for each individual treatment. For enzyme assays, the activities of the SOD, POD and CAT were detected by spectrophotometry as described previously (Li et al., 2021a). At least three independent biological replicates were conducted for each individual treatment, and each sample contained at least 20 seedlings for analysis. The data treatment was analyzed using the DPS 7.05 Data Processing System Software. In all analyses, the difference significance was indicated by p < 0.05, and the sample variability was denoted by the standard deviation (SD) of the mean.
2.9 Analyses of histochemical staining and ion content in roots and leaves
For Prussian blue staining assays, the plant samples, including the roots and leaves prepared above, were treated with equal volumes of 4% (w/v) K-ferrocyanide and 4% (v/v) HCl for 60 min. The staining experiment was conducted as previously reported (Roschzttardtz et al., 2009). The stained roots and leaves were then photographed and analyzed. For ion content analysis, the plant samples prepared above were washed with deionized double-distilled water (ddH2O) three times and dried for 3 d at 80°C in an oven. After cooling, the dried plants (5–10 mg) were digested in a muffle furnace at 220°C with 5 mL HNO3 for 1 h, then the digests were diluted 10-folds with sterile ddH2O and suffered from element measurements including K, Na, P, Ca, Mg, Zn, Fe, Mn and Cd by using ICP-MS (NexION 300D; PerkinElmer) followed the method described by Wang et al. (2019). The ion concentrations were calculated from the dry weights of the samples. Therefore, a blank sample was run before and after the plant samples to ensure the cleanliness of the inspection system. With indium as the standard reference, the recovery rate was between 99 and 105%. The sample variability was represented by the SD of the mean, and each individual experiment was performed with triple-biological replicates. Asterisks indicated the statistical significance in the Student's t-test, i.e., *p < 0.05 and **p < 0.01.
3 RESULTS
3.1 Identification of plant ILR3 homologs
To comprehensively investigate the origin and diversity of ILR3 protein architectures in green plants, we selected 65 plants from different lineages for homology search and domain prediction and finally identified a total of 275 non-redundant ILR3 genes (Figure 1). The ILR3s first occurred in the charophytes, and the number of the ILR3s gradually increased as the species evolved, ranging from one copy in Chlorokybus atmophyticus to 11 copies in Euryale ferox and tetraploid genome Glycine max. Compared with other plants, more ILR3 genes were found in monocotyledonous and dicotyledonous plants, among the top ten species with a higher proportion of ILR3 family genes, eight were eudicots, and two were monocots (Figure 1 and Table S2). In gymnosperms, the number of the ILR3s was no more than four copies and dominated with two copies, while the ones varied from one to four in bryophytes (Figure 1). These results suggested that the ILR3 genes were widely distributed in green plants and likely first appeared in the charophytes.
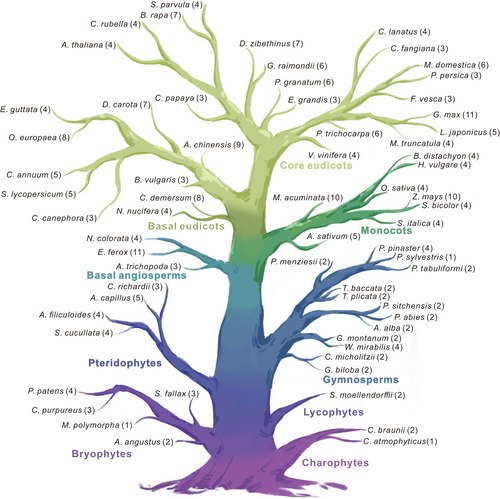
3.2 Phylogenetic and gene duplication analyses of plant ILR3 homologs
The phylogenetic tree was reconstructed by these ILR3 sequences, and the number of main ILR3 homologs and their relationships to each other could be distinctly noticeable from the tree (Figure 2A). In this tree, the ILR3 homologs fell into six major groups, i.e., clade A, clade B, clade C, clade D, clade E and clade F (Figure 2A). Because the ILR3 homologs existed in the charophyte genome but not in chlorophyte algae, they could be traced roughly back to the early origin of land plants (Figure 2B). We could conclude that the last common ancestor of the extant charophytes evidently contained at least one homologous ILR3. They had an ancient origin, but their characteristics were shaped by several lineage-specific duplication events. The first early duplication occurred in bryophytes before the divergence of lycophytes, leading to the formation of ILR3_L and extant main lineages. Subsequently, the second duplication took place in one of the members of the gymnosperms, establishing the ILR3, IDT1, and bHLH34_115 lineages (Figure 2B). Notably, ILR3_L lineage included only the sequences of ferns and gymnosperms, indicating that their evolutionary intermediates had not survived or that their evolution was blocked for some reason in angiosperm. However, the ILR3 orthologs were found only in flowering plants, originating from gene duplications and succeeding diversification of an ancestral gymnosperm gene (Figure 2B). On the other hand, the ILR3 homologs had highly conserved bHLH and ZIP domains. Simultaneously, seven new highly conserved motifs (motifs 3, 5, 7, 8, 10, 11, and 12) were also discovered in the ILR3 homologs (Figure S2). Compared with their ancestral sequences, the ILR3 orthologs were subjected to motif gain events (Figure 2C and Figure S2). To further characterize the consensus sequences among phylogenetic groups, the Weblogo 3 online tool was used to analyze the sequence logo motifs, e.g., the bHLH and ZIP domains, of each group and clarify the sequences features of the functional domains (Figure S3). It was obvious that the sequence of the basic motifs of clade D was quite different from those of other phylogenetic groups (Figure S3).
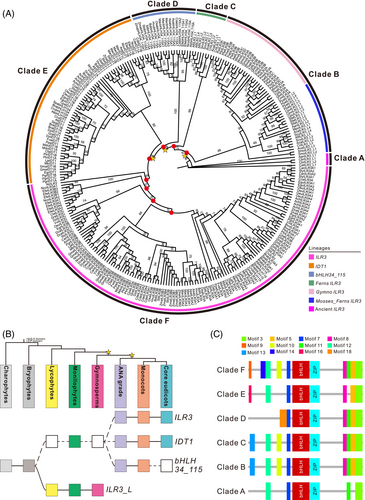
The main force of the evolution of different plants is derived from gene duplication, which leads to the generation of gene families. Compared with the diploid plant species, the polyploid plants were more likely to lead to duplicated ILR3 genes. Thus, G. max, Brassica rapa, Musa acuminate, and Zea mays possess more duplicated genes (Table S2). To investigate the form of these duplication events, the synteny analysis was executed by estimating the genomic regions that harboured the ILR3 homologs. We found that most duplicate gene pairs were presented in the corresponding syntenic genomic regions, suggesting that these multiple gene copies were caused by WGDs or segmental duplications (Figure 3). Particularly, the AtrIDT1 had a synteny relationship with the eudicots IDT1 lineages (Figure 3), indicating that the duplication leading to the establishment of the IDT1/bHLH104 lineage was involved with the WGDs occurring before the divergence of angiosperms.
3.3 Gene structure and genomic analyses of plant ILR3 homologs
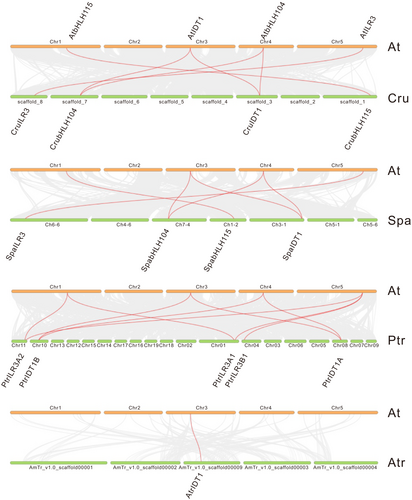
We examined the structure, exon position, and intron phases of each gene to determine the diversity of the ILR3s' structure. Our results disclosed that the ILR3s intron varied from 1 to 5. All selected ILR3s contained four introns except for the three introns in MdoIDT1A, five introns in MtrIDT1 of clade E, and one intron in CatILR3_L1 of clade A (Figure 4), indicating that the intron numbers in ILR3s remained constant after plant landing. Meanwhile, the intron phases in plant ILR3s also had little variable, with most genes having phase 2, while phase 1 usually occurred in non-flowering plants such as CriILR3_L3 (Figure 4). Additionally, most gene pairs shared the intron phase 2, 2, 2, 0 (Figure 4). The conserved exon/intron structure and intron phases in the ILR3 genes showed their close evolutionary relationship among all species.
The pIs of the ILR3 homologs ranged from 4.62 (AtbHLH104 and SpabHLH104) to 10.69 (GmaILR3B2), and, interestingly, the pIs varied from acidic to alkaline in each group. The Mws varied from 9.611 (PpaILR3_L2) to 51.293 (ZmaILR3B5) kDa (Table S2), which was consistent with their amino acid numbers varying from 86 (PpaILR3_L2) to 480 (ZmaILR3B5) (Table S2). The average composition of amino acids of the ILR3s ranged from 1.29 (tryptophan) to 9.00 (leucine) (Table S3). Conspicuously, the average abundance of the conserved configuration of amino acids (K-E-R in bHLH IVc members) which have been proven to bind to a variation in the E-box hexanucleotide sequence (E-box: CANNTG, variation: CACGTG) (Ledent & Vervoort 2001) in basic region including lysine (K), glutamic acid (E) and arginine (R) were 7.93, 7.82 and 5.75, respectively (Table S3). Compared with the average abundance of other amino acids, that of the hydrophobic amino acids such as leucine (L; 9.00), alanine (A; 8.98), valine (V; 4.86) and proline (P; 7.90) was higher (Table S3).
3.4 Genetic distance and evolutionary selection pressure analyses of plant ILR3 homologs
The genetic distance of the ILR3s between Clade B and F was 0.470, and the distances of other counterparts were the smallest (Table 1). Meanwhile, there was the maximal genetic distance difference between Clade A and E ILR3s, indicating the largest sequence divergence between them. Particularly, the distances of the combinations with respect to Clade A were higher than those of other combinations (Table 1), indicating that the ILR3 sequences of Clade A were much different from those of other clades. In total, the overall mean distance of the ILR3s was 0.510 (standard error 0.046), suggesting the sequence divergence of the ILR3s was not large during the evolution.
| A | B | C | D | E | |
|---|---|---|---|---|---|
| A | |||||
| B | 0.560 | ||||
| C | 0.661 | 0.586 | |||
| D | 0.657 | 0.494 | 0.583 | ||
| E | 0.726 | 0.603 | 0.641 | 0.626 | |
| F | 0.651 | 0.470 | 0.603 | 0.536 | 0.625 |
Simultaneously, the Ka and ώ values (ώ = 0.2241) of the Clade D ILR3s were the lowest among all clades, reflecting that the Clade D ILR3s had a strong purifying selection during evolution (Table 2). While the Clade F ILR3s had a greater than 1 of mean ώ values, suggesting that these ILR3s underwent positive selection (Table 2). Likewise, all ILR3 sequences had ω0 = 0.20405 values under one ratio model, implying all ILR3s had a strong purifying selection. The foreground value of the ILR3 lineage (ώf =0.211434) was larger under the two-ratio model compared with the background value of the remaining ILR3 sequences (ώb = 0.197198), indicating that the ILR3 lineage existed a relaxed purifying selection during their evolution process (Table 3). What's more, substitution rate tests revealed the differences in the substitution rates between ILR3 and other lineages were significant (p < 0.05) (Table 3).
| Clade | N | Ka | Ks | ώ | G + C content |
|---|---|---|---|---|---|
| A | 4 | 0.48240 | 0.56433 | 0.8548 | 0.555 |
| B | 50 | 0.30429 | 0.32450 | 0.9377 | 0.478 |
| C | 9 | 0.20821 | 0.71197 | 0.2924 | 0.472 |
| D | 16 | 0.16083 | 0.71770 | 0.2241 | 0.488 |
| E | 59 | 0.24847 | 0.68254 | 0.3640 | 0.479 |
| F | 126 | 0.31075 | 0.19454 | 1.5970 | 0.486 |
- N, number of sequences; Ka, the number of nonsynonymous substitutions per nonsynonymous site; Ks, the number of synonymous substitutions per synonymous site; ώ, Ka/Ks.
| Family | Model | Tree length | ώ (dN/dS) | lnL | 2Δℓ | df | p value |
|---|---|---|---|---|---|---|---|
| ILR3 | One Ratio | 157.56 | ώ0 = 0.20405 | −104410.2234 | 5.325574 | 1 | 0.02101 |
| Two Ratios | 158.05 | ώf = 0.211434 ώb = 0.197198 |
−104407.5606 |
3.5 AtILR3 is a conserved and canonical bHLH gene involved in stress responsiveness
The AtILR3 encoding 234 aa protein harboring bHLH and ZIP domains (Figure 5A). Multiple sequence alignment revealed that the AtILR3 protein sequence had high similarity to those of A. thaliana, O. sativa and Brachypodium distachyon (Figure 5A), indicating that the ILR3s were conserved in plants. The phylogenetic tree between AtILR3 and other A. thaliana bHLH proteins displayed that AtILR3 was most closely related to AtbHLH115 (Table S4 and Figure 5B), which was in accordance with the evolutionary relationship (Figure 2A). It was noted that these bHLH genes were usually involved in plant development and stress response (Figure 5B). Moreover, the promoter region (i.e., 2000 bp upstream of the start codon) of the AtILR3 gene harboured many cis-elements associated with stress response. For instance, in the responsiveness of the defence and stress (TC-rich repeats), two elements, Me-JA (TGACG-motif) and ABA (ABRE), were involved, respectively (Figure 5C and Table S5), indicating that the AtILR3 might be associated with the stress- and hormone-mediated signalling pathways in plants.
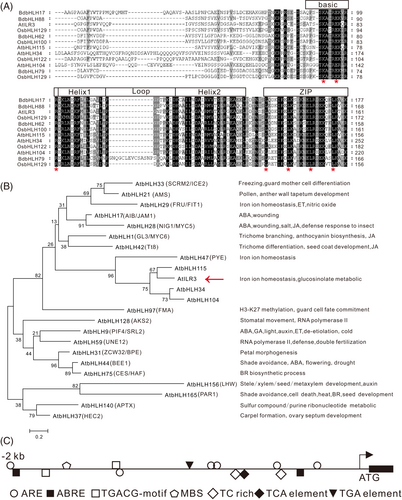
3.6 AtILR3 is localized in the nuclei
Subcellular localization prediction displayed that almost all ILR3 members were dominatingly distributed in the nuclei, while other members were distributed in the cell membrane (3), plastid (7), cytoplasm (1), ER (1) and mitochondrion (1) (Table S6). Particularly, the AtILR3 was present in the nucleus with a 92% probability at all predicted locations (Table S6). To confirm the subcellular localization of the AtILR3, a transient transformation assay of 35S: AtILR3-GFP constructs was executed in N. benthamiana leaves and green fluorescent signals were surveyed at 48 hours post-incubation (hpi) by confocal microscopy. The control cells expressing GFP alone were distributed throughout the entire cell without specific compartment, while the AtILR3-GFP green fluorescence signal was symmetrically observed in the nuclei (Figure 6A), suggesting that the AtILR3 could be a nucleus-localized protein, as in accordance with the predicted results (Table S6) and similar to the localization of other bHLH proteins in A. thaliana (Aparicio & Pallas 2017; Jiang et al., 2022).
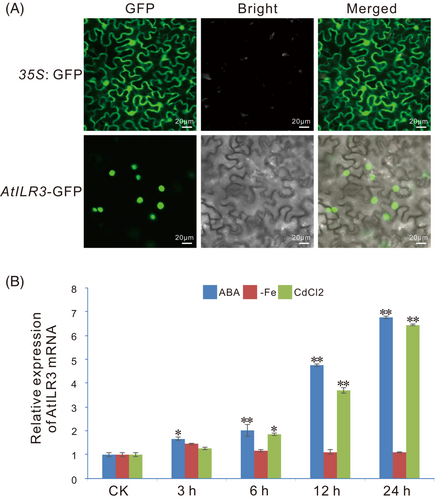
3.7 Expression profiles of the AtILR3 gene in various abiotic stresses
To analyze the effects of the AtILR3 in response to abiotic stress, we measured the consequences of ABA, iron deficiency, and CdCl2 stresses on AtILR3 gene expression in WT plants by RT-qPCR experiments and discovered that the AtILR3 expression was prominent induced by ABA and CdCl2 treatment, not in iron deficiency (Figure 6B). The expression level of AtILR3 was up-regulated after ABA stress, peaking at 24 h with up to 7-fold changes (Figure 6B). It is, therefore, probable that AtILR3 plays a crucial role in response to ABA stress and maintenance of cadmium ion homeostasis, while the response to iron deficiency is likely not at the transcriptional level but at the post-translational level.
3.8 AtILR3 enhances the A. thaliana tolerance to ABA stress
To elucidate the potential function of AtILR3 in response to ABA stress, the phenotypic analysis was performed by using the various genetic materials of AtILR3 under 2.5 μM ABA condition. Firstly, we investigated the expression levels of AtILR3 in WT, ilr3, ilr3 with ILR3P: ILR3 and 35S: ILR3 plants. RT-qPCR experiments showed that the expression of AtILR3 in complementation plants was approximately 80% of that of WT plants, up to 4 times in the ILR3-overexpressing plants and almost undetectable in ilr3 mutants (Figure S4). Subsequently, the ABA stress experiment results showed that the growth and chlorotic leaves of the ilr3 mutants were significantly weaker than those of the light-green and larger leaves of the WT plants. While there were no discernable differences between the mutants and the WT plants under normal (CK) environments (Figure 7A). As expected, the etiolated degrees of leaves in the functional complementation of the ilr3 mutants (ilr3 with ILR3P: ILR3) were partially relieved in comparison with the ilr3 mutants (Figure 7A). Under ABA stress, the ILR3-overexpressing A. thaliana plants were much stronger than the WT plants (Figure 7B).
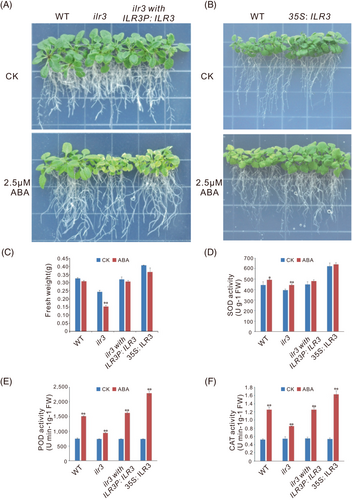
The biomass of plants under ABA stress conditions was generally lower. Thus, we also studied the FWs of the WT, ilr3, ilr3 with ILR3P: ILR3 and 35S: ILR3 plants. Under the ABA exposure conditions, the FW of ilr3 mutants were significantly lower than that under the CK condition, while no apparent change in the FW was observed in WT and different ecotypes of AtILR3 (Figure 7C), suggesting that AtILR3 was necessary for ABA tolerance.
To explore the mechanism of the AtILR3 in response to ABA stress, the activities of the SOD, POD and CAT of AtILR3 transgenic plants under the ABA condition were performed. The SOD activities in the WT and ilr3 plants were significantly higher after ABA treatment than that under the CK condition, while there were no obvious changes in functional complementation of the ilr3 mutants and ILR3-overexpressing plants (Figure 7D). Unlike the SOD, the POD and CAT activities displayed significant increases in all genetic materials of AtILR3 after ABA stress (Figure 7D and E). More importantly, the change trends of the SOD, POD and CAT activities were accordant both in normal and ABA conditions in all WT, ilr3, ilr3 with ILR3P: ILR3 and ILR3-overexpressing plants. Specifically, their activities were the lowest in the ilr3 mutants, the second in the WT and ilr3 with ILR3P: ILR3 lines, and the highest in ILR3-overexpressing plants (Figure 7D-F). In general, these results suggested that the AtILR3 likely enhanced plant stress tolerance by increasing the ability of ROS-scavenging and attenuating cellular oxidative damage caused by ROS.
3.9 ABA stress alters the nutrient uptake patterns in A. thaliana
To examine the effects of ABA stress on the nutrient absorptions in diverse genetic plants of AtILR3 in A. thaliana, the contents of a cluster of macronutrient elements including K, P, Ca and Mg, and micronutrients including Na, Fe, Zn, Mn, and Cd in both roots and leaves were determined by ICP-MS. Firstly, we stained ABA-treated samples with Prussian blue to qualitatively and visually observe the Fe in the roots or leaves of the WT, ilr3 and ILR3-overexpressing plants. The ilr3 mutants accumulated excessive Fe3+ in the roots, whereas the ILR3-overexpressing plants contained the lowest levels of Fe3+, and the WT plants had middle levels of Fe3+ in roots under both ABA and normal conditions (Figure 8A). As expected, the situation in leaves was in opposition to that in roots (Figure 8A). Subsequently, ICP-MS was conducted to detect the contents of various elements. Compared with the CK plants, the roots of the WT and ILR3-overexpressing plants showed obvious increases in Fe levels after ABA treatment, and the ilr3 mutants showed a significant decrease, whereas the Fe levels in the leaves were opposite in the CK and ABA treatments (Figure 8B). The elevation of Fe contents in the plants after ABA treatments thus seemed to require the participation of the AtILR3 gene. Similar to Fe, the accumulation patterns of Ca, Mg, Mn, and Zn displayed that the AtILR3 gene affected the uptake of these elements both under the CK and ABA conditions (Figure 8B).
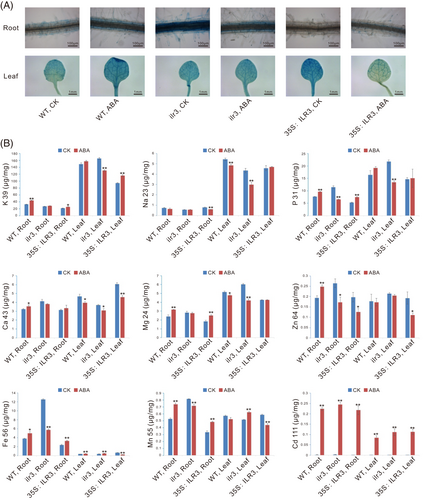
K and P are the two major macronutrients necessary for plant growth and development. Unsurprisingly, the two elements were significantly higher compared with other elements detected, i.e., Na, Fe, Zn, Mn, Ca, Mg, and Cd (Figure 8B). The ABA treatment also apparently elevated the P levels of the roots both in WT and ILR3-overexpressing plants. The pattern of increased P accumulation in plants after the ABA treatment was maintained in leaves, although the difference between the ABA and CK conditions was less significant than that in roots (Figure 8B). In contrast, the ABA treatment also reduced evidently the P levels of the roots and leaves, respectively, in ilr3 mutants. Interestingly, the ABA treatment also significantly increased the Cd levels of roots and leaves, respectively, in the WT, ilr3 and ILR3-overexpressing plants (Figure 8B), indicating that the Cd increase under the ABA treatment seemed to be unnecessary for the existence of AtILR3 gene.
4 DISCUSSION
4.1 Evolutionary history and function evolution of the ILR3 homologs in plant species
Our results showed that the ILR3 gene was first present in charophytes and even did not exist in the genome of subaerial Mesostigma viride (Figure 1, 2 and Table S2). Reconstruction of the phylogenetic tree revealed that the ILR3 homologs had been subjected to duplication-divergence events in evolution (Figure 2A) and, firstly, had undergone at least two ancient duplications in history. Following the duplication, the first early expansion happened before the divergence of lycophytes, resulting in the occurrence of Clade B (ILR3_L linage) and other Clades. It was noteworthy that the Clade B ILR3s only existed in pteridophyte and gymnosperm genome, implying that their evolutionary intermediates probably did not survive, or their evolution was intercepted due to some reasons in angiosperms, which was similar to the situations of C3HDZ genes (Jiang et al., 2023). Afterwards, the second expansion occurred before the divergence of angiosperms, resulting in the establishment of ILR3, IDT1, and bHLH34_115 lineages (Figure 2B and Figure 3), exposing distinct functional redundancy. For instance, bHLH34/IDT1, bHLH104, bHLH105/ILR3 and bHLH115 all take part in the regulation of iron homeostasis (Gao et al., 2020). The overexpression of each of the AtbHLH IVc genes increased the expression levels of the AtbHLH Ib genes and restored the phenotype of the bhlh115-1 mutant (Li et al., 2016; Liang et al., 2017). The function in modulating iron homeostasis was likely an ancient role dating back to their common ancestor. Particularly, the ILR3 orthologs were replicated only in flowering plants, predating radiation in extant angiosperms, dating back to the duplications and diversification of an ancestral gymnosperm gene (Figure 2B). Strictly speaking, the ILR3 orthologs were angiosperm-specific, which were similar to the evolutionary patterns of the WRKY66 and Tóxicos en Levadura 63 (TL63) gene families (Li et al., 2023; Zhang et al., 2023). As is well known, the WGDs, as well as other types of duplications such as tandem duplication, can provide rich genetic materials for the structural and functional evolutions of modern genes (Van de Peer et al., 2009; Jiao et al., 2011). The succeeding diversification of protein motifs/domains was surveyed in the paralogous genes of angiosperm genomes. The ILR3 homologs had structural variations among different clades, such as the ILR3 orthologs suffering from motif gain events than their paralogs (Figure 2C). Ever since a WGD event, because of the relaxed constraints on the genes formed, plants began to get rid of most redundant gene copies through mutation or exon shuffling to alter motifs/domains to adapt to the corresponding environmental changes (Maere et al., 2005; Jarvela & Hinman 2015). The ILR3 orthologs exhibited a relaxed purifying selection during the evolution (Table 3), meaning that the ILR3 orthologs might generate neo-functionalization or sub-functionalization. Specifically, the AtILR3 and its closest homologs both modulated the iron homeostasis, but the loss of function or gene silencing in any of their homologs in rice resulted in the down-regulation of OsIRO2 and increased sensitivity to iron deficiency (Kobayashi et al., 2019; Zhang et al., 2020). AtPYE and AtILR3 specifically mediated photoprotection during iron deficiency (Akmakjian et al., 2021). Hence, AtbHLH IVc proteins have not only overlapping functions but also independent functions in maintaining iron homeostasis, which is similar to the expression patterns of the HD-ZIP III genes during A. thaliana development (Prigge et al., 2005).
4.2 The impacts of ABA stress on ion accumulation levels in plants
Fourteen mineral elements essential for plant growth and development are absorbed by the roots, transferred to the buds, and then distributed to different tissues and organs as needed (Sasaki et al., 2016). The iron is present in very high levels in roots (Figure 8B), implying that it is likely to have other roles or simply be stored in roots for preparation for other physiological activities of the aboveground organs, such as the biosynthesis of chlorophyll and photosynthesis. The iron apoplastic storage in roots is a conservative mechanism by which plants respond to iron deficiency (Longnecker & Welch, 1990; Curie & Mari, 2017). The P content is also very high both in roots and leaves (Figure 8B), indicating that the impact of ABA stress on the plasma membrane of cells did not block the absorption of P that is usually mediated by plasma membrane-localized phosphate transporter (PHT1/PT) family (Raghothama 1999). Plant growth requires 50–100 mg kg−1 Mn, and even the accumulation of up to 5 000 mg kg-1 DW of Mn in rice will not produce toxicity symptoms (Sasaki et al., 2016). The Mn contents both in roots and leaves of ILR3-overexpressing plants were lower than those of the WT and ilr3 mutant plants, regardless of CK or ABA treatments, and their amounts far exceeded the Mn content required for plants' normal growth (Figure 8B), which became a partial reason for the better growth of ILR3-overexpressing plants (Figure 7B). Interestingly, knockout or overexpression of the AtILR3 gene did not alter the Cd accumulation, while the ABA treatment observably increased the Cd accumulation in the roots and leaves of all plants (Figure 8B), suggesting that there appeared to be an unidentified influx transporter for the Cd in roots that were associated with ABA signalling and completely unrelated to the AtILR3. ABA has a vital function in the Cd stress response, but the regulatory mechanism by which plants accumulate and uptake Cd remains unclear (Shen et al., 2022). Taken together, these ion accumulation patterns suggested that ABA treatment has a strong effect on ion homeostasis, implying that certain metal ions, such as Cd, play potential roles in ABA signalling pathways.
4.3 bHLH IVc proteins may be involved in responding to abiotic stress through ABA signalling pathway
Our predictions and experimental results showed that the AtILR3 was located in the nucleus (Table S6 and Figure 6A), which was in keeping with the localization of the AtbHLH IVc protein reported by Liang (2022). The bHLH IVc proteins facilitate the accumulation of the bHLH IVb subgroups, bHLH11 and bHLH121, in the nucleus, as well as another member PYE. This pattern of nuclear accumulation is essential for maintaining iron homeostasis (Pu & Liang 2023). The expression level of the AtILR3 did not change apparently under the iron deficiency (Figure 6B), which was consistent with the results previously described (Zhang et al., 2015; Li et al., 2016; Liang et al., 2017). The activity and abundance of bHLH IVc members might increase under the iron deficiency condition, promoting the protein degradation of BTS at the post-translational level (Sharma & Yeh 2020; Liang 2022). Moreover, ABA greatly induced the expression of the AtILR3 (Figure 6B), which was in accordance with the ABRE elements in the promoter (Figure 5C). Furthermore, the molecular and genetic evidence displayed that overexpressing AtILR3 improved the plant tolerance to ABA (Figure 7), implying that the AtILR3 content or activity might rise under ABA stress. In fact, ABA can regulate the abundance of the mRNA and protein of bHLHs. For example, ABA can greatly induce the protein degradation of a nuclear-localized atypical bHLH TF ATBS1-INTERACTING FACTOR 2 (AIF2) (Kim et al., 2017). The transcription of a strawberry PACLOBUTRAZOL RESISTANCE1 (PRE1), an atypical bHLH gene, could be activated by ABA (Medina-Puche et al., 2019). Collectively, the AtILR3 and even other bHLH IVc members are believed to participate in the abiotic stress responses except for iron deficiency through an ABA-mediated signalling pathway, although experimental evidence is still lacking.
5 CONCLUSIONS
The ILR3 homologs could be dated roughly back to charophytes and fell into six clades. Exactly speaking, the ILR3 orthologue genes were angiosperm-specific, had experienced motif gain events, and had relaxed purifying selection. The WGDs were conducive to the formation of various lineages of the ILR3 homologs, such as the IDT1/bHLH104 lineage, which probably dates from an ancestral gymnosperm gene. Meanwhile, the ILR3 homologs possessed highly conserved bHLH and ZIP domains and a high average abundance proportion of key residues. The AtILR3 was localized in the nuclei, and its expression was also strongly induced by ABA and CdCl2 stresses. Genetic analysis displayed that the AtILR3 positively regulated the plant response to ABA stress by improving the ability of ROS-scavenging. ICP-MS analysis revealed that the AtILR3 was associated with the impacts of ABA stress on the uptake of nutrient elements, especially iron. In general, this research deeply revealed the evolution of the ILR3 homologs in plants and provided clues for further investigations on the ILR3 functions in responding to ABA stress.
AUTHOR CONTRIBUTIONS
MJ and CZ conceived and designed the work; MJ and YN performed most of the experiments, analyzed the data; GW contributed to analysis and collection data; QG and GL executed the gene transcription level detection and reviewed the manuscript. MJ wrote the manuscript. The authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
FUNDING INFORMATION
This research was supported by the Shanghai Sailing Program (19YF1414800) to Min Jiang. The funding body had no role in study design, analysis, decision to publish or preparation of the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study is included in this published article and its supplementary information files. The dataset can be obtained from the corresponding author according to reasonable requirements.




