Bioinformatic and Expression Analyses of the Wall-Associated Kinase Genes Under High-Temperature Stress in Sorghum
Abstract
Sorghum (Sorghum bicolor L. Moench) is the world's fifth most-produced cereal. The wall-associated kinases (WAKs) gene family plays a crucial regulatory role in various aspects of plant biology, including the response to environmental stress and pathogens. Therefore, we aimed to characterize the members of the WAK gene family in sorghum using bioinformatics tools and to determine their functional roles in heat stress by detecting transcript levels using RT-qPCR. A total of 98 SbWAK/SbWAKL proteins were identified and classified into six phylogenetic groups. The SbWAKs/SbWAKL genes were unevenly distributed across ten chromosomes, and 33 duplications were observed on nine chromosomes. The number of amino acids and molecular weight of SbWAKs/SbWAKLs ranged from 496 to 1149 aa and 55.38 to 124.89 kDa, respectively. Forty-eight SbWAK/SbWAKL were unstable, with an instability index greater than 40. The synteny analyses revealed sixteen SbWAK/SbWAKL genes similar in foxtail millet, thirteen in maize, and seven in the rice genome. Additionally, 107 miRNAs, including cell wall-related miRNAs, targeted 85 SbWAK/SbWAKL genes. The cis-acting elements in SbWAK/SbWAKL genes pointed out that these genes may be associated with light, hormone, development, and environmental stress responses. RT-qPCR analysis of 13 SbWAK/SbWAKL genes revealed a relatively high transcript fold change in 6 SbWAK/SbWAKL under high-temperature conditions. In conclusion, cis-acting elements, protein–protein and miRNA interactions, and higher gene expression levels at high temperatures may indicate the existence of candidate SbWAKs/SbWAKLs genes with functions in abiotic and biotic stress response and their usage in future gene editing for breeding purposes.
1 INTRODUCTION
The sorghum crop is a crucial staple and a nutritious food source in many developing countries, particularly in drier and more remote regions of the semi-tropics. Foods made from sorghum are processed using various techniques, such as fermentation, mainly used in Africa (Afify et al., 2011; Elkhalifa et al., 2005). The nutritional value of sorghum grain is 95 to 98% of that of maize. While the vitamin contents of these cereal grains are equal, sorghum's mineral content is higher than that of maize (Balota, 2012). In addition, sorghum is an essential source of fiber and starch worldwide. Also, grain sorghum is one of the best energy crops in the United States (Staggenborg, 2019).
The cell membrane is the location of the transmembrane receptor-like kinases (RLKs). With more than 1131 RLKs in rice and 600 in Arabidopsis, RLKs are a large family of genes found in many plants and have been thoroughly researched across various species (Shiu et al., 2004). Receptor-like kinases are responsible for transferring signals inside and outside a cell (Walker, 1994). These RLKs are also essential in controlling disease resistance, stress responses, and plant developmental processes (Ou et al., 2021; Tang et al., 2017; Ye et al., 2017). The RLKs may be categorized into 15 subfamilies based on the differences and diversity in the extracellular regions (Diévart et al., 2020; Lehti-Shiu et al., 2012). A subfamily of RLKs known as wall-associated kinases (WAKs) has an extracellular domain directly connected to the cell wall and several epidermal growth factor-like repeat (EGFs), a transmembrane domain, and one intracellular serine/threonine kinase domain (Anderson et al., 2001; Kohorn, 2001). Immunological examination using the Arabidopsis WAK1 antibody has shown that some proteins in maize, tobacco and peas are similar to WAK (He et al., 1996). The Epidermal Growth Factor (EGF)-like repeats are also present in all WAK proteins and situated next to the transmembrane domains on the amino-terminal side. Arabidopsis was shown to have different forms of EGF repeats, such as EGF-2 and EGF-Ca2+. It was discovered that several WAKL genes have somewhat defective EGF-like domains, and it is currently unknown how this degeneration would affect the WAKL proteins' potential functionality. However, WAKLs remain firmly linked to the cell wall (Verica & He 2002; Kanneganti & Gupta 2008).
As an oligogalacturonide receptor, WAKs' extracellular domain can detect external environmental signal molecules and, through a series of intracellular mechanisms, activate the physiological and biochemical responses accordingly (Brutus, 2010; Wagner & Kohorn, 2001). When a pathogen infects a cell, it produces oligogalacturonic acids (OGs) recognized by WAKs (Kohorn & Kohorn, 2012). According to earlier research, heavy metal stress and injury can all cause WAKs to express themselves (Delteil et al., 2016; Kohorn, 2001; Lally et al., 2001). He et al. (1998) showed that WAK1 expression in Arabidopsis during pathogen stress was increased. Zuo et al. (2015) also discovered ZmWAK as a crucial gene for maize's resistance to head smut. According to Li et al. (2009), the overexpression of OsWAK1 in rice improved resistance to rice blast, which offered a fresh understanding of the connection between the OsWAK1 gene and disease resistance in rice. Additionally, reduced root length was observed in the wak1 mutant (TNP11) in barley with a single Ds transposon insertion (Tripathi et al., 2021).
In recent years, there has been an increase in the identification and characterization of various plant gene families, including the wall-associated kinase gene family. This study aimed to characterize in detail the members of the wall-associated kinase gene family in sorghum by analyzing phylogenetic relationships, chromosome mapping, synteny, gene structure, cis-acting elements, protein–protein interaction, and miRNA interaction. Also, gene expression analyses were carried out to detect the potential roles of WAK/WAKL genes under high temperatures. The results of this extensive research of the sorghum WAK gene family may provide a basis for future research, such as gene regulation, focusing on understanding the roles of these genes in stress response and development.
2 MATERIALS AND METHODS
2.1 Identification of SbWAK/SbWAKL genes and phylogenetic analysis
In identifying candidate SbWAK/SbWAKL genes, corresponding AtWAK/AtWAKL protein sequences (from https,//www.ncbi.nlm.nih.gov/, accessed on 24th July 2022) were used as queries in BLASTp and genomic (NCBI) searches against the sorghum genome database with a (1e−5) E-value. To identify the potential sorghum WAK/WAKLs, the protein domains of all candidate genes were checked. Protein sequences containing conserved domains (Wall-associated receptor kinase galacturonan-binding domain, Calcium-binding EGF or EGF domains, and Protein Kinase domain/protein tyrosine kinase domains) were considered as WAK proteins using Pfam 35.0 online software (http,//pfam.xfam.org/, accessed on 28th July 2022) (Mistry et al., 2021). However, sequences that lacked the GUB WAK or EGF/EGF CA domains were identified as WAKL proteins. Finally, the signal peptide and the transmembrane domains were examined using the HMMER v2.43 online program (https,//www.ebi.ac.uk/Tools/hmmer/, accessed on 20th October 2022). The redundant sequences were removed.
The phylogenetic tree was constructed using the Neighbor-joining method (Saitou & Nei, 1987) and WAKs/WAKLs from S. bicolor, Arabidopsis thaliana, one Z. mays, and six O. sativa with a bootstrap consensus tree inferred from 1000 replicates (Felsenstein, 1985). Evolutionary analyses were conducted in MEGA11 (Tamura et al., 2021). ClustalW was employed to align multiple sequences.
2.2 Physicochemical properties and subcellular prediction of SbWAKs/SbWAKLs
Physicochemical characteristics of the detected SbWAK/SbWAKL proteins were predicted using the online tool Expasy (https,//web.expasy.org/protparam/, accessed on 26th July 2022). All identified SbWAK/SbWAKL proteins' subcellular localizations were predicted using Wolf PSORT (https,//wolfpsort.hgc.jp/, accessed on 31st July 2022). TBtools software was used to create the HeatMaps (Chen et al., 2020).
2.3 Chromosome localization, synteny analysis, and Ka/Ks calculation
Sorghum WAK and WAKL genes were named and numbered as SbWAK1 to SbWAK31 and SbWAKL1 to SbWAKL67 according to their chromosomal locations. Genome data from S. bicolor, Setaria italica, Zea mays, and Oryza sativa were used for the synteny analysis. TBtools was used to calculate the Ka/Ks ratio and visualize the syntenic relationships and the chromosomal map (Chen et al., 2020).
2.4 Conserved motif and gene structure analyses of SbWAKs/SbWAKLs
The conserved motifs of SbWAK/SbWAKL proteins were analyzed using MEME 5.4.1 (https,//meme-suite.org/meme/tools/meme, accessed on 10th August 2022), with the default settings changed to: the maximum number of motifs to find 10, a maximum width of 50, and a minimum width of 6. Lastly, motif names were identified through the website https,//www.genome.jp/tools/motif/ (accessed on 1st December 2022). The online Gene Structure Display server (http,//gsds.gao-lab.org/, accessed on 3rd August 2022) was used to conduct the gene structure study.
2.5 Cis-acting elements prediction
Selected SbWAKs/SbWAKLs had their 2000 bp upstream promoter sequences manually retrieved from the NCBI website (https,//www.ncbi.nlm.nih.gov/, accessed on 3rd August 2022). Using the online program PlantCARE (http,//bioinformatics.psb.ugent.be/webtools/plantcare/html/, accessed on 3rd August 2022) (Lescot et al., 2002), cis-acting elements in the promotors were predicted. The values of collected cis-acting elements were manually entered into a draft cis-acting element sheet obtained from Zhang et al. (2015). Light-responsive elements, hormone-responsive elements, environmental stress-related, and development elements were the four main groups into which the elements were classified. TBtools program V1.098769 was used to create HeatMaps (Chen et al., 2020).
2.6 Protein–protein interaction and miRNA target site identification
The investigation of protein–protein interactions was done using the online STRING platform V11.5 (https,//string-db.org/, accessed on 28th October 2022) (Szklarczyk et al., 2019) using a medium confidence parameter of 0.400 and no more than 10 interactions to display, disconnected nodes in the network were hidden. By utilizing the default parameters on psRNATarget (https,//www.zhaolab.org/psRNATarget/analysis?function=2, accessed on 31st October 2022) (Dai et al., 2018) and the CDS sequences of all SbWAK/SbWAKL genes for select published S. bicolor miRNAs, it was possible to predict the genes that miRNAs target. The miRNA interaction network was visualized using Cytoscape 3.9.1 (Shannon et al., 2003).
2.7 Planting material and high-temperature treatment
The sorghum cv. Theis seeds were obtained from the GAP Agricultural Research Institute of the Ministry of Agriculture and Forestry in Türkiye. Five seeds were planted in each plastic pot at room temperature and watered to prevent water stress. After germination, three plants were left in each pot. The plants were grown in a Nukleon manufacturer growth chamber with a condition of 30°C, 65% relative humidity, 370 μmol m−2 s−1 and 16/8 h day/night photoperiod. The temperature was increased from 30°C to 42°C by 2°C every 30 minutes. The temperature at 42°C was kept for four hours, and the top leaf samples (leaves of 3 plants grown in the same pot were taken together) were transferred into liquid nitrogen and kept frozen at −80°C. Control plant stayed at 30°C during the whole experiment.
2.8 RNA isolation, cDNA, and RT-qPCR gene expression analysis
Plant leaf tissue was collected and immediately frozen in liquid nitrogen. RNA was isolated from the leaves using the EZ-10 Spin Column Plant RNA Mini-Preps Kit (Bio Basic Inc.). The OneScript Plus cDNA Synthesis Kit (Applied Biological Materials Inc.) was used to synthesize cDNA from RNA. The qPCR analysis was carried out using 2X qPCR SYBR-Green MasterMix (A.B.T.™) with slight modifications according to the manufacturer's guidelines. The internal reference gene PP2A (Serine/threonine-protein, XM-002453490) was used (Sudhakar Reddy et al., 2016). The Applied Biosystems 7500 Real-time PCR system was used for real-time qPCR. An initial holding at 50°C C for 2 mins, followed by 40 cycles (95°C for 5 mins, 95°C for 15 sec, 49°C for 30 sec, and 72°C for 10 sec). Then, a melting curve of 95°C for 15 sec, 60°C for 60 sec, and 95°C for 30 sec. The 13 SbWAK/SbWAKL gene primers were designed using https://www.ncbi.nlm.nih.gov/tools/primer-blast/ (accessed on 24th October 2022) (Table S1). Primer Tm ranged from 57–60°C, primer length 18–22, GC% 50–55, and amplicon size of 80 to 140 bp. The primer properties were cross-checked again on https://www.bioinformatics.org/sms2/pcr_primer_stats.html (accessed on 24th October 2022). The 2−ΔΔCT approach (Livak & Schmittgen 2001) was used to examine the relative gene expression levels. The experiments consisted of three biological replicates and two technical replicates.
3 RESULTS
3.1 Identification and phylogenetic analysis of SbWAKs/SbWAKLs in S. bicolor
A total of 98 Sorghum WAK/WAKL proteins were predicted using Pfam identifiers to include Wall-associated receptor kinase galacturonan-binding domain, Calcium-binding EGF or EGF domains, and Protein Kinase/protein tyrosine kinase domains after scrutinizing and eliminating redundant sequences. Thirty-one proteins were characterized as WAKs containing (GUB-WAK, EGF/EGF-Ca, and Pkinase) and sixty-seven were characterized as WAKLs (containing at least a Pkinase domain and a Calcium-binding EGF/EGF, GUB-WAK or WAK domains) (Figure S1). The proteins were then numbered from SbWAK1 to SbWAK31 and SbWAKL1 to SbWAKL67 (Table S2).
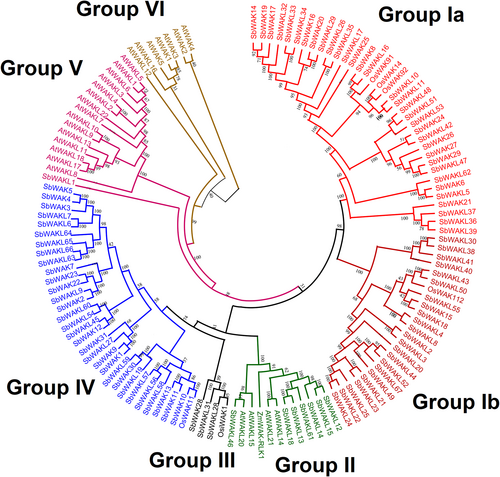
To investigate the evolutionary relationship among sorghum, Arabidopsis, rice, and maize WAKs/WAKLs, a phylogenetic tree was created using the Neighbor-joining method with 1000 replicates (Figures 1 and S2, S3). The phylogenetic tree was grouped into six main groups (Groups I to VI). Group I, the largest group, was further sub-grouped into Group Ia and Group Ib, consisting of 56 SbWAK/SbWAKL and 4 OsWAK proteins. These OsWAKs play roles in plant defense response against pathogens. The second largest group, Group IV, contained 31 SbWAK/SbWAKL proteins and OsWAK11. The majority of AtWAK/AtWAKL proteins were found in Groups V and VI, a species-specific group. It is also interesting to note that SbWAKL1 was found to be the outgroup of Group V. Apparently, Group II consisted of 7 SbWAKLs, 4 AtWAKLs, and ZmWAK-RLK1 protein. Three SbWAKL proteins and OsiWAK1 represented the smallest group (Group III). The closeness of some SbWAKs and SbWAKLs with different domain compositions in the tree is attributed to the similarities in variable sequences other than the conserved domain regions.
3.2 Physicochemical properties and subcellular prediction analysis of SbWAKs/SbWAKLs
An overview of the distinctive features of the SbWAK/SbWAKL proteins is given in Table S2. The amino acid length ranged from 496 aa (SbWAKL32) to 1149 aa (SbWAK19) and an average of 769 aa. The protein with the smallest and largest molecular weight (kDa) was SbWAKL32 (55.38 kDa) and SbWAK19 (124.89 kDa), respectively, with an average of 84.55 kDa. Regarding the gravy index score, 0.018 and 0.006 were recorded in SbWAKL5 and SbWAKL47, respectively, indicating the hydrophobic nature of these proteins. The remaining proteins were, however, hydrophilic. The aliphatic index ranged from 75.36 (SbWAK9) to 95.47 (SbWAKL35). Also, the isoelectric point (pI) values ranged from 4.96 to 8.58. Out of the 98 SbWAKs/SbWAKLs, 48 were unstable, with an instability index greater than 40.
According to the subcellular prediction analysis, SbWAK/SbWAKL proteins were widely distributed across the extracellular membrane, cytoskeleton peroxisome, Golgi_plasma, Golgi body, plasma membrane, cytoskeleton_plasma membrane, cytoskeleton_nucleus, endoplasmic reticulum, chloroplast, vacuole, mitochondrion, cytoplasm, and nucleus (Table S3). Predictably, the plasma membrane was where the majority of SbWAK/SbWAKLs were found.
3.3 Chromosomal distribution, synteny analysis, and selective pressure analysis
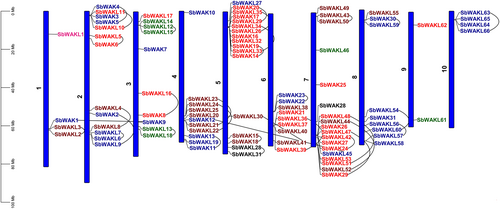
The SbWAK/SbWAKL genes were randomly distributed on all ten chromosomes (Figure 2). The highest number of genes were found on chromosomes 7 and 5, with 18 and 17 genes, respectively, while chromosome 9 contained two genes, representing the least number of genes on a chromosome. A total of 33 duplication events were observed.
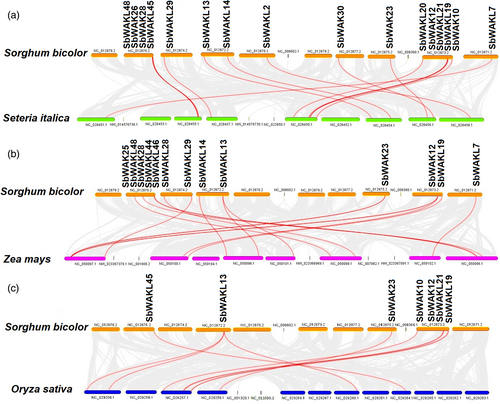
In the evolutionary relationships of WAK/WAKL genes among plant species, the genomic data of S. bicolor, Setaria italica, Z. mays, and O. sativa were used to construct three comparative syntenic maps (Figure 3a-c). Sixteen SbWAK/SbWAKL genes were found to have syntenic relationships with Setaria italica (Figure 3a). Thirteen and seven SbWAK/SbWAKL genes were found between Z. mays (Figure 3b) and O. sativa, respectively (Figure 3c). Furthermore, the Ka/Ks ratios of 19 linked gene pairs were calculated to understand better the evolutionary relationship of the SbWAK/SbWAKL gene family (Table S4). Genes for Ka/Ks were selected based on the aligned homologous SbWAK/SbWAKL sequence pairs. Positive selection is denoted by Ka/Ks >1, neutral selection by Ka/Ks =1, and purifying selection is represented by 0 < Ka/Ks <1 (Yadav et al., 2015). All but one of the 19 linked gene pairs were observed to be less than 1, indicating selection by purification. However, SbWAKL13 ~ SbWAKL18 (1.095193) represents a Ka/Ks >1, showing positive selection. Both evolutionary patterns significantly impacted preserving this gene family's existence (Table S4).
3.4 Motif identification and gene structure analysis of SbWAKs/SbWAKLs
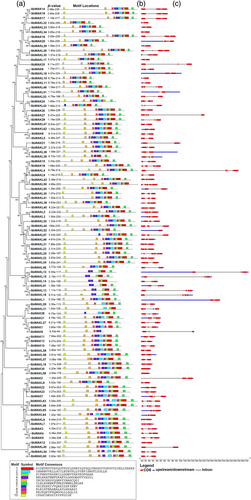
A total of ten conserved motifs on all SbWAK/SbWAKL proteins were identified (Figure 4a-c). Motifs 1 and 6 were present in all SbWAK/SbWAKL proteins. All but 34 of the SbWAK/WAKL proteins contained all ten motifs. The least number of motifs were found in SbWAK9, SbWAKL31, and SbWAKL61, containing 5 motifs each. Gene structure analysis was performed to obtain insight into the structure and intron/exon distribution of SbWAK/SbWAKL genes. The results showed that the number of exons and the gene length varied from 1 to 7 and 2 kb to 27 kb, respectively (Figure 4a-c). Genes SbWAKL50 and SbWAKL25 contained the highest number of exons (7). The remaining exons ranged from 2 to 6 amongst SbWAK/SbWAKLs. However, SbWAK30, SbWAKL59, and SbWAKL52 contained only one exon each. Additionally, all but 19 of the SbWAK/SbWAKL genes lacked a UTR region. The longest and shortest gene length was observed in SbWAKL12 and SbWAKL17, respectively.
3.5 Analysis of cis-acting elements in the promoter region of SbWAK/SbWAKL genes
Cis-regulatory elements in the promoter control the physiological functions of the gene to some extent (Shi et al., 2022). In this study, these elements were categorized into four main groups: light-responsive elements, environmental stress-responsive elements, hormone-responsive elements, and development-related elements. For light-responsive elements, 32 significant elements were found (Table S5). Furthermore, all but 16 SbWAKs/SbWAKLs contained the G-box motif, making it the most abundant light response WAK/WAKL element in sorghum. Thirteen cis-elements were associated with the development and environmental stress response elements (Table S5). As-1, MYB, MYC, STRE, ARE, W box, and WRE were the most predominant amongst the SbWAKs/SbWAKLs. SbWAK12 showed the lack of any of the 13 identified development response elements. Finally, 14 cis-acting elements were also identified as hormone-responsive (Table S5). ABRE was the most abundant hormone response element. Only SbWAKL30 lacked all the 14 expected elements. These results suggest the significance of SbWAKs/SbWAKLs in the overall growth and development of sorghum.
3.6 Protein–protein interaction and miRNA target prediction
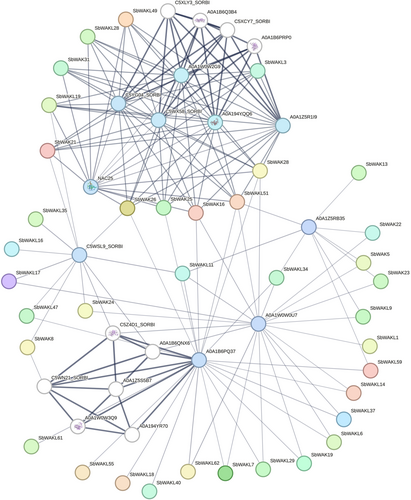
For the interactions of proteins, all query SbWAK/SbWAKL sequences were associated with a unique stringId (Table S6). Thirty-seven SbWAK/SbWAKL were seen to have interacted with various S. bicolor proteins (Figure 5). Six WAKs and five WAKLs interacted with A0A1Z5R1I9, A0A194YQQ6, C5YG04_SORBI (uncharacterized protein), C5WX58_SORBI and A0A1W0W2G9 (DNA (cytosine-5)-methyltransferase), NAC25 (transcription factor), which also had a strong link with A0A1B6Q3B4 and A0A1B6PRP0, involved in S-adenosylmethionine synthase and Adenosylhomocysteinase. Also, from the interaction network, most SbWAK/SbWAKLs interacted with C5WSL9_SORBI, A0A1W0W0U7, A0A1B6PQ37 and A0A1Z5RB35 (protein kinase domain-containing proteins). Two MITOGEN-ACTIVATED PROTEIN KINASE proteins (C5Z4D1_SORBI and A0A1B6QNX6) triggered by stress signals strongly interacted with the kinase domain-containing protein. In this interaction network, there were 175 edges, 86 nodes, and disconnected nodes were concealed. According to this finding, this network contains many more interactions than was predicted, evidenced by the PPI enrichment value of <1.0e-16, suggesting that these proteins are partially biologically related in nature.
Furthermore, the potential interactions between microRNAs (miRNAs) and their SbWAK/SbWAKL targets were also estimated (Table S7). Of the 98 SbWAK/SbWAKLs, 85 genes interacted with 107 miRNAs. While the same miRNA may constrain the regulation of multiple SbWAK/SbWAKL genes, one SbWAK/SbWAKL gene may be the target of multiple miRNAs. The three miRNAs that interacted most with our target genes for miRNA count were sb-miR5386, sb-miR6228-6p, and sb-miR6231-3p, recording 12 counts each. Thirty-seven miRNAs had single counts, while the remaining ranged from 2 to 9. SbWAK28, SbWAKL58, and SbWAKL64 were however specifically targeted by sb-miR6233-3p, sbi-miR2118-3p, and sbi-miR5389, respectively. The modes of inhibition were translation and cleavage.
3.7 Expression analysis of some SbWAK/SbWAKL genes under heat stress
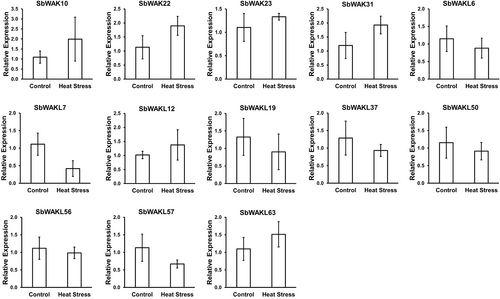
The functional role of SbWAKs/SbWAKLs under high temperature was detected by the expression analyses of 13 SbWAK/SbWAKL, reported to be expressed in the leaf in the Phytozome database and had the highest number of environmental stress-related cis-elements (STRE, MYB, MYC and WRE3). Under high-temperature treatment, when compared to control plants, six of the 13 SbWAK/SbWAKL (SbWAK10, SbWAK22, SbWAK23, SbWAK31, SbWAKL12 and SbWAKL63) had higher fold changes while the rest had lower fold changes. The highest fold change was observed in SbWAK10 with a 2.0-fold change, followed by SbWAK22 and SbWAK31 with a 1.9-fold change. The lowest expression level was in SbWAKL7, with a 0.4-fold change. Thus, six SbWAK/SbWAKL genes, which were relatively highly expressed during heat stress, may be related to the overall heat stress response. There were, however, no statistically significant differences (p > 0.05) in the medians of the treatment and control samples.
4 DISCUSSION
A special set of RLKs called WAKs and WAKLs are firmly attached to the cell walls of plants. These WAKs/WAKLs detect alterations in the cell wall, convey extracellular signals over the plasma membrane to the cytoplasm, and initiate a response to external stimuli (Kanneganti & Gupta, 2008). They play a role in plant growth and development, heavy metal stress tolerance, pathogenic bacterial resistance, and hormone response (Lally et al., 2001; Verica & He, 2002). Also, Sobic.007G214300, characterized in this research as SbWAKL44, was identified to regulate red leaves in sorghum (Lv et al., 2022).
In this study, a total of 98 SbWAK/SbWAKL proteins were identified in sorghum compared to 100 in maize (Zuo et al., 2015), 125 in rice (de Oliveira et al., 2014), 115 in Brachypodium (Wu et al., 2020), 91 in barley (Tripathi et al., 2021), 68 in rose (Liu et al., 2021), 53 in cannabis (Sipahi et al., 2022), 44 in apple (Zuo et al., 2019), 26 in Arabidopsis (Verica & He, 2002), 29 in tomato (Sun et al., 2020), 58, 68 and 99 in cotton (Gossypium arboreum, G. raimondii, and G. hirsutum, respectively) (Zhang et al., 2021), and 29 in potato (Yu et al., 2022).
A phylogenetic study utilizing the Neighbor-joining method was designed to look at the evolutionary relationship of SbWAK/SbWAKL proteins (Figure 1). Pathogen defense response (OsWAK112, OsWAK91, OsWAK92, OsWAK14 and ZmWAK-RLK1) (Delteil et al., 2016; Hurni et al., 2015), defense against copper (OsWAK11) and root development response (OsiWAK1) (Xia et al., 2018) proteins from O. sativa and Z. mays were also incorporated in this phylogenetic analysis. The fact that the known pathogen defense response WAKs identified in rice and maize were found in Groups I and II, respectively, might suggest the similarities in fundamental roles played by members of that group. The majority of Arabidopsis WAKs did not mingle with sorghum WAKs, which is consistent with the findings in tomatoes (Sun et al., 2020), barley (Tripathi et al., 2021), and cotton (Zhang et al., 2021). The outgroup of Group V (SbWAKL1) could be attributed to its unique gene structure and position on the chromosome. Furthermore, OsWAK11, which functions in copper detoxification, formed a sub-group in Group IV. The members of this subgroup all contained cis-acting elements involved in defense and stress response, suggesting similarity in function. In Group III, OsiWAK1 was isolated with SbWAK28, SbWAKL28 and SbWAKL31. Kanneganti and Gupta (2011) identified the importance of OsiWAK1 in root development after RNAi-mediated gene silencing.
The high number of SbWAK/SbWAKL in sorghum compared to A. thaliana suggests the role of gene duplication during speciation. Segmental duplication is often one of the main reasons for the expansion of the cotton gene family due to the polyploid nature of the species (Zhang et al., 2021). It is then hypothesized that tandem duplication may be one of the common sources of growth in the WAK/WAKL gene family in various species. Tandem duplication is a significant single-gene duplication method because these genes are tightly spaced on the same chromosome (Freeling, 2009). Duplicated genes share a similar DNA sequence and common purpose, but duplicated genes can also acquire different specialized functions over evolution. The vast number of WAK gene family members may provide the ability to detect and react to various ligands. Therefore, it is important to understand and define the functions of WAK gene family members involved in growth, development, or stress resistance in sorghum.
Neofunctionalization and subfunctionalization are just two of many possibilities for duplicate gene pairs. Positive selection is applied to neofunctionalized gene pairs (Ka/Ks value >1), whereas purified selection is used for subfunctionalized gene pairs (Ka/Ks value <1) (Roulin et al., 2013). A large majority of the SbWAK/SbWAKL homologous pairings in this analysis underwent purifying selection, while one underwent a positive selection, indicating that subfunctionalization in S. bicolor may have been the evolutionary fate of most WAK/WAKL members in maintaining the original gene function. This result is in agreement with the findings of Li et al. (2022) in common walnuts and Yu et al. (2022) in potatoes.
A comparative syntenic map was drawn to determine the orthologous relationships of the SbWAK/SbWAKL genes between sorghum, foxtail millet, maize, and rice (Figure 3). Sixteen, thirteen, and seven WAK homologs were found in foxtail millet, maize, and rice, respectively. In general, SbWAK/SbWAKL genes are more closely linked to the homologs of foxtail millet and maize than that of rice. This could be attributed to sorghum being an allied species of maize and foxtail millet (Hermuth et al., 2016; Shi et al., 2022).
Intriguingly, members of SbWAKs/SbWAKLs in the same phylogenetic groups (Figure 4a) shared a similar motif distribution pattern. Diversity exists among groups with different compositions of conserved motives. Motifs 1–3 were found to be associated with the Protein Kinase domain. Motifs 4 and 8 were identified as the catalytic domain for the Protein Tyrosine Kinase and Epidermal Growth factor receptor. Motif 5 was associated with complement clr-like EGF-like, EGF-like domain, and EGF domain. The Pfam description prediction of motifs 6, 7, and 9 was, however, not found. Motif 10 was associated with the Wall-associated receptor kinase galacturonan-binding domain. The conserved motifs identified in this study are in tandem with previous WAK-related research.
Gene structure analysis revealed that most genes within the same clade were highly conserved (Figure 4c). Several SbWAKs/SbWAKLs contained more than three exons. SbWAK25, SbWAK27, SbWAKL4, SbWAKL13, SbWAKL15, SbWAK9 and SbWAK2 contained longer introns (Figure 4c). This phenomenon was recently seen in J. regia and J. mandshurica WAKs/WAKLs (Li et al., 2022). Increasing intron length and number may be more advantageous to plants than harmful. Introns can shield genes from mutation interference with their non-coding regions, hence preserving the gene's function (Jo & Choi, 2015; Mukherjee et al., 2018). The UTR region of SbWAKL1 was much longer than the remaining SbWAKs/SbWAKLs. The UTRs are known to be significant genetic elements that regulate post-transcriptional processes. UTRs can also boost the genome's coding capacity due to having many variations from the same gene. This gives the gene expression system flexibility and enables plants to respond to changes in their environment (Srivastava et al., 2018).
The SbWAK/SbWAKL promoters varied in quantity and type of cis-acting elements, suggesting the probable role in various stress reactions and development via signaling pathways. In response to environmental challenges, promotors control gene expression through unique cis-regulatory elements by enhancing or repressing transcription efficiency. The characterization of many cis-acting elements, including ABA-responsive element (ABRE) in response to ABA and drought-responsive element (DRE/CRT) sensitive to salinity, low temperature, and dehydration, is observed in several plant species (Yamaguchi-Shinozaki & Shinozaki, 2005). The ABREs, also known as G-boxes or G-box hybrids, have been identified as promotors of ABA-responsive genes using molecular dissection investigations (Hattori et al., 2002). Evidence of the varying number of these elements is found in the promoter regions of SbWAKs/SbWAKLs (Table S5).
A protein–protein interaction study was carried out to forecast the probable role of the SbWAKs/SbWAKLs (Table S6, Figure 5). The Protein kinase domain-containing proteins are involved in phosphorylation, cellular processes, and transferase activities. Tyrosine and dual-specific phosphatases play essential roles in the cell cycle, cell proliferation, and cell signaling (Yakura, 1998). The MAPK (MITOGEN-ACTIVATED PROTEIN KINASE PROTEIN) pathways are usually triggered by mitogenic and stress signals (Keyse 1995; Sun and Tonks 1994); hence, C5Z4D1_SORBI and A0A1B6QNX6 were seen to have strong interactions (Figure 5). Anderson et al. (2001) indicated that the interaction of AtWAK1 to a cytoplasmic 2C protein phosphatase (KAPP, KINASE-ASSOCIATED PROTEIN PHOSPHATASE) also suggests the close association of plant phosphatase to WAKs. These protein–protein interactions observed indicate the possible comprehensive functions of SbWAK/SbWAKL proteins. The more interactions there are, the more transcription factors, family enzymes, and intrinsically disordered proteins can be included in the interactions (Dunker et al., 2005).
Post-transcriptional gene silencing (PTGS) and transcriptional gene silencing (TGS) are the two major ways microRNAs (miRNAs) control the expression of genes (Katiyar et al., 2012). Plant growth, nutritional balance, and abiotic and biotic stress are all significantly influenced by miRNAs (Zhang et al., 2008; Zheng et al., 2011). In response to drought stress, miR5386 precursor transcripts increased by more than two folds (Ghate et al., 2019), an interaction is seen with several SbWAKs/SbWAKLs. According to Xue et al. (2017), no known targets of miR6231 were predicted in sorghum. Our results, however, predict the targets of miR6231 to be SbWAKs/SbWAKLs. Also, the predicted target gene family of miR2118 was not stated by Zhang et al. (2015). We suggest possible targets in WAKs based on our findings.
Several miRNA families, including miR169, miR156, and miR398, were proposed as one of the likely reasons for sorghum's response to abiotic stress (Katiyar et al., 2015; Paterson, 2009). In this study, interactions were detected among varying numbers of miRNAs (especially miR169a-q) and some SbWAK/SbWAKL, indicating the roles of SbWAK/SbWAKL in the abiotic stress response (Table S7). Previous studies suggesting the involvement of miR156, miR160, miR399, and miR164 in changes in the environment and plant growth in plants (Katiyar et al., 2015; Unver & Budak, 2009; Xie et al., 2011), were also confirmed by this research. A legume-conserved disease-resistant regulated miRNA (miR2118) and its variant miR2118-3p only interacted with SbWAKL58 (Jagadeeswaran et al., 2009). Simon et al. (2015) described the miR5389 as related to some critical agronomical traits. This miR5389 only interacted with SbWAKL64. Additionally, the six different miRNA families (miR156, miR164, miR397, miR528, miR5566, and miR6230) known to target cell wall-related genes (Rai et al., 2016) were confirmed in this research.
Yang et al. (2006) indicated that thermotolerance in plants may be influenced by several genes associated with the cell walls. Similarly, He et al. (1999) explained that several WAK genes have been differentially expressed in different organs and various biotic and abiotic stress conditions. Also, it was demonstrated that heat stress caused relatively high expression levels of some WAK genes (Bra025882 and Bra025883) in Chinese cabbage leaves (Zhang et al., 2020). In agreement with these researches, in this study, high-temperature treatment upregulated the transcript levels of 6 SbWAK/SbWAKL genes (Figure 6), suggesting they had relationships with heat stress. In contrast, seven SbWAK/SbWAKL genes were repressed by high temperature. Further research needs to be done to elucidate the roles of WAK genes in response to heat stress.
AUTHOR CONTRIBUTIONS
Hülya Sipahi designed and supervised the research; Terik Djabeng Whyte performed the bioinformatics data analyses and created the figures; Hülya Sipahi and Terik Djabeng Whyte performed qPCR and wrote the manuscript; All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
This research was supported by Eskişehir Osmangazi University Scientific Research Projects Coordination Unit within the scope of the project number FYL-2022-2613.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created in this study.




