Transcriptomic and physiological shade avoidance responses in potato (Solanum tuberosum) plants
Abstract
Plants detect competitors in shaded environments by perceiving a reduction in photosynthetically active radiation (PAR) and the reduction between the red and far-red light (R:FR) ratio and blue photons. These light signals are detected by phytochromes and cryptochromes, which trigger shade avoidance responses such as shoot and petiole elongation and lead to increased susceptibility to pathogen attack. We studied morphological, anatomical, and photosynthesis differences in potato plants (Solanum tuberosum var. Spunta) exposed to sunlight or simulated shade in a greenhouse. We found that simulated shade strongly induced stem and internode elongation with a higher production of free auxin in stems and a lower production of tubers. The mesophyll thickness of the upper leaves of plants grown in simulated shade was lower, but the epidermis was wider compared with the leaves of plants cultivated in sunlight. In addition, the photosynthesis rate was lower in the upper leaves exposed to nonsaturated irradiances and higher in the basal leaves at saturated irradiances compared with control plants. RNA-seq analysis showed that 146 and 155 genes were up- and downregulated by shade, respectively. By quantitative reverse transcription polymerase chain reaction, we confirmed that FLOWERING LOCUS T (FT), WRKY-like, and PAR1b were induced, while FLAVONOL 4-SULFOTRANSFERASE was repressed under shade. In shaded plants, leaves and tubers were more susceptible to the necrotrophic fungus Botrytis cinerea attack. Overall, our work demonstrates configurational changes between growth and defense decisions in potato plants cultivated in simulated shade.
1 INTRODUCTION
The crop biomass at harvest and the magnitude of seed yield is closely associated with the capacity of plants to capture radiation for photosynthesis throughout the crop cycle. Light competition among individual plants is dynamic, and plants adjust their physiology and architecture to optimize the capture of light for photosynthesis (Sultan, 2000). In high plant density stands, the light environment shows a reduction in the photosynthetic photon flux density (PPFD), lowering red/far-red (R:FR) ratios, and decreasing blue photons. This phenomenon is the consequence of the optical and biochemical properties of leaves, which efficiently absorb red and blue photons and transmit or reflect far-red and green photons (Smith, 1982). The best-known photosensory mechanism for the detection of plant neighbors is the R:FR phytochrome system. Low R:FR ratios principally inactivate phytochrome B (phyB) photoreceptor and promote the shade avoidance syndrome (SAS), a set of physiological responses such as the elongation of stems and petioles (Morgan & Smith, 1976), the increase of the apical dominance and reduction of branching (Deregibus et al., 1983), acceleration of flowering and leaf senescence (Rousseaux et al., 2000), and lowering seed yields (Libenson et al., 2002). When plant density increases, the reduction of photosynthetic photon flux density (PPFD) transmitted to the crop and the lowering of blue photons are detected by cryptochrome 1 (cry1) photoreceptor, which reinforces the SAS (Keller et al., 2011).
In Arabidopsis thaliana, the SAS is promoted by PHYTOCHROME INTERACTING FACTORS (PIF) and coregulated in a feedback loop by PIF-interacting proteins that are typically also transcriptionally activated by PIFs, such as LONG HYPOCOTYL IN FAR-RED (HFR1), PHYTOCHROME RAPIDLY REGULATED1 (PAR1), and PAR2 (Roig-Villanova et al., 2006; Sessa et al., 2005). These are atypical HLH proteins that do not directly bind to DNA but interact with the DNA-binding domain of PIFs, thereby repressing PIF transcription factors (Galstyan et al., 2011; Hornitschek et al., 2009). DELLAs are another group of PIF-interacting proteins, which upon interaction, render PIFs inactive (De Lucas et al., 2008; Feng et al., 2008). In addition, B-BOX DOMAIN PROTEIN 24 (BBX24) protein can physically interact with DELLA proteins to sequester them, leading to an enhancement of PIF4 that promotes the SAS (Crocco et al., 2015). Enhanced PIF binding to G-boxes of auxin biosynthetic genes such as YUCCAs rapidly increases free indole-3-acetic acid (IAA) active auxin and the expression of other auxin-related genes (Devlin et al., 2003; Kohnen et al., 2016; Kozuka et al., 2010; Sessa et al., 2005). The accelerated transition to flowering by shade light is mediated by the cooperation of PIFs with the CONSTANS (CO) transcription factor to directly regulate the expression of FLOWERING LOCUS T (FT) and TWEEN SISTERS OF FT (TSF) (Galvao et al., 2019; Zhang et al., 2019).
In addition, pathogens and insects can spread very easily, supported by favorable microclimates, such as elevated humidity and shielding from weather extremes, generated as a consequence of the short interplant distances in dense vegetation (Ballaré & Pierik, 2017). Reduced resistance to herbivores and pathogens is often associated with reduced leaf thickness and strength, which are typical shade avoidance responses that maximize light interception per unit of carbon invested in the leaves (Gommers et al., 2013). Consequently, plants reduce the investment in defense-related specialized metabolites (Guo et al., 2018). In low R:FR ratio environments, the reduction of the active form of phyB decreases plant immunity toward pathogens (Ballaré & Pierik, 2017; Pieterse et al., 2014). It is well known that the inactivation of phyB attenuates the defense responses controlled by jasmonate (JA) phytohormone. The phyB inactivation regulates the JASMONATE ZIM DOMAIN (JAZ) and DELLA balance, as well as the abundance of MYC transcription factors to promote the SAS (Cerrudo et al., 2012; Chico et al., 2014).
Potato (Solanum tuberosum L.) has become the most important dicotyledonous crop worldwide, ranking after rice, maize, and wheat. Potato has a higher harvest index and more dry matter and protein per hectare than other major cereal crops (Dolničar, 2021; Hay, 1995). The increase in plant density per hectare is a common practice to improve crop yield. Earlier evidence showed that phyB controls tuberization in inductive photoperiodic conditions because antisense potato plants, in which the phyB expression is downregulated, accelerate the tuberization under long-day conditions (Jackson et al., 1996, 1998), whereas the PHYB overexpression increases tuber yield (Thiele et al., 1999). At field conditions, the PHYB overexpression produces higher maximum photosynthesis by improving leaf conductance in all strata of the canopy and thereby increases the production of tubers, particularly at very high densities (Boccalandro et al., 2003). In another study, the higher tuber productivity of PHYB transgenic plants cultivated under low irradiances has been associated with a lower carbon loss from respiration (Schittenhelm et al., 2004).
Although the SAS has been studied in the PHYB transgenic potato plants, the transcriptomic and physiological changes that naturally occur because of shading in wild-type plants of this species have been elusive. Moreover, it is unknown the effect of shade signals along the vertical profile of potato leaves. This work aimed to study the SAS in S. tuberosum var. Spunta plants. We performed experiments to evaluate morphological, anatomical, and photosynthesis differences between leaves located in the vertical profile of plants cultivated under sunlight and simulated shade in a greenhouse. In addition, we analyzed the transcriptome regulated by simulated shade and validated the expression of genes of interest by quantitative reverse transcription polymerase chain reaction (RT-qPCR) and the susceptibility to the attack of the necrotrophic fungus Botrytis cinerea that produces important economic losses (Tiwari et al., 2021) in leaves and tubers of plants exposed to shade.
2 MATERIALS AND METHODS
2.1 Plant material and experimental conditions
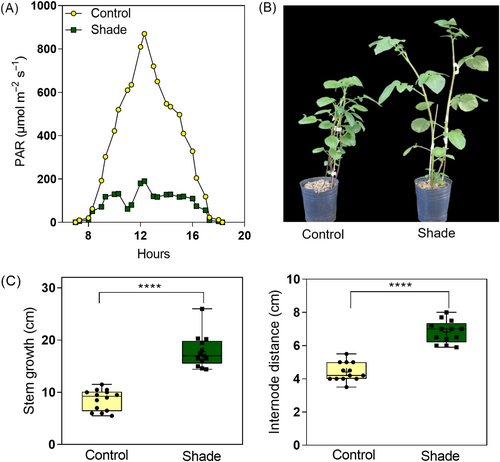
All experiments were carried out with S. tuberosum var. Spunta wild-type potato plants. The experiments were performed in a greenhouse located at the Faculty of Agronomy, University of Buenos Aires (34°35′S, 58°29′W). Two independent experiments were designed to study the effects of shade on vegetative growth and the tuber yield of potato plants. Sprouted tubers of similar size were sown in pots of 12 cm × 12 cm (1000 cm3) for the vegetative experiment and in pots of 16 cm × 22 cm (4000 cm3) for the yield experiment. The soil mix consisted of vermiculite (1/4), peat (1/4), and perlite (1/2). In the vegetative experiment, the plants were grown in two light conditions: sunlight for 42 days or simulated shade growing the plants for 28 days in sunlight +14 days faced to green filters (no. 89 LEE filters, https://leefilters.com/) located at both sides of the plants. The sunlight treatment established a maximum photosynthetically active radiation (PAR) at noon = 870 μmol m−2 s−1 and R:FR ratio = 1.14, while the simulated shade treatment established a maximum PAR at noon = 190 μmol m−2 s−1 and R:FR ratio = 0.5 (Figures 1A and S1). The plants were staked to ensure they grow vertically, as shown in Figure 1B. For photosynthesis measurements, we defined three vertical positions of the leaves: (1) upper corresponding to the youngest fully developed leaf, (2) middle corresponding to the leaf in the position estimated as the 0.5 of the total number of leaves, and (3) basal corresponding to the oldest true leaf of the plant (Figure S2). At the beginning of light treatments in 28-day-old plants, the middle and the basal leaves were fully developed, while the leaves of the upper position developed during the following 14 days under sunlight or simulated shade. At the end of the experiment, 42-day-old plants had between nine and 12 leaves, regardless of the light treatments. In the yield experiment, the control condition consisted of growing the plants in sunlight for 70 days, while the simulated shade condition consisted of growing the plants for 28 days in sunlight +14 days in simulated shade +28 days in sunlight until the tubers were harvested. We designed an additional experiment to investigate the susceptibility to B. cinerea infection in shade light. The experimental design consisted of growing the plants for 21 days in sunlight and then exposing them for additional 7 days to (1) sunlight, (2) simulated shade, or (3) sunlight supplemented with far-red light in which plants received equal irradiance than sunlight, but with a lower R:FR ratio = 0.45 (i.e., similar to the R:FR ratio of simulated shade treatment). In all experiments, the temperature in the greenhouse ranged between 15 and 25°C, regardless of light treatments (Figure S1). The experiments were carried out between March and October with a mean photoperiod of about 10.5/13.5 h day/night.
2.2 Morphological and anatomical determinations
To estimate stem growth, the height of the principal stem of plants was measured with a ruler, and the number of leaves was counted. To assess internode distance, the second and third internodes were measured for each plant with a ruler, and the largest was selected. Dry weight and the ratio of the shoot to root weight were measured using a digital balance. The material was enveloped into paper and incubated at 60°C for 96 h and then weighed again to obtain the dry weight. Tubers were harvested, counted, and weighed as indicated above.
2.3 Histological measurements of leaves
Upper leaves were harvested, diaphanized, and safranin-stained following the methodology indicated in Dizeo de Strittmatter (1973). Transverse sections of each leaf were embedded in paraffin and serially cut at 10–12 μm with a Minot-type rotary microtome. Sections were stained with safranin-fast green (Johansen, 1940), photographed with polarized light with a Zeiss Axioplan optical microscope, and analyzed with the Zeiss AxioCam ERc 5s software (Jena). Three biological samples with 10 technical replicates for each treatment were analyzed.
2.4 Photosynthesis determinations
Net photosynthesis rate, stomatal conductance (gs), transpiration rate, and CO2 concentration of stomatal cavity (Ci) were estimated in the basal, middle and upper leaves of four plants per treatment, using an open infrared gas analysis system (Li-Cor 6400, Li-Cor Inc). To compare quantum yields (i.e., the linear phase of the net photosynthesis rate curves), we estimated the best-fit linear regression for sunlight and simulated shade and compared statistically the slopes by Student's t-test. In addition, the maximum rate of Rubisco carboxylase activity (Vcmax) and the maximum rate of photosynthetic electron transport (Jmax) were estimated in the upper leaf. For detailed procedures, see Gómez-Ocampo et al. (2021). All measurements were done between 10:00 a.m. and 3:30 p.m. Chlorophyll determinations were performed in the basal, middle and upper leaves of 15–17 plants per treatment using a SPAD 502 (Minolta).
2.5 RNA-seq transcriptome: Library preparation, sequencing, and analysis
Three biological samples, each consisting of a pool of six upper leaves (the latest fully expanded) from different plants, were collected for each treatment, immediately immersed in liquid nitrogen, and then stored at −80°C. Total RNA was extracted by grounding each sample in a mortar with liquid nitrogen and then using the Spectrum™ Plant Total RNA kit (Sigma-Aldrich) according to the manufacturer's instructions and following the protocols described in Gómez-Ocampo et al. (2021). Libraries were prepared using the TruSeq Stranded mRNA Sample Prep Kit v2 (ref. RS-122-2101/2) according to the manufacturer's protocol. Briefly, 1 μg of total RNA was used for poly(A)-mRNA selection using oligo(dT) magnetic beads, which was subsequently fragmented to ~300 bp. cDNA was synthesized using reverse transcriptase (SuperScript II, ref. 18064-014; Invitrogen) and random primers. The second strand of the cDNA incorporated 2′-deoxyuridine 5′-triphosphate (dUTP) in place of 2′-deoxythymidine 5′-triphosphate (dTTP). Double-stranded DNA was further used for library preparation. dsDNA was subjected to A-tailing and ligation of the barcoded Truseq adapters. All purification steps were performed using AMPure XP Beads (ref. A63881). Library amplification was performed by PCR using the primer cocktail supplied in the kit. Final libraries were analyzed using Fragment Analyzer (DNF-473) to estimate the quantity and check size distribution and were then quantified by qPCR using the KAPA Library Quantification Kit (ref. KK4835; KapaBiosystems) before amplification with Illumina's cBot. Libraries were sequenced 1 × 50 on Illumina's HiSeq 2500.
From the whole RNA-seq, we identified genes significantly expressed in our experimental conditions (false discovery rate [FDR] < 0.05). Genes were considered statistically upregulated or downregulated by simulated shade when the fold-change (FC) between treatments (i.e., GWS vs. GWC) was >2 (log2FC > |1|, with adjusted p-value < 0.05). For homology assessment between genes of S. tuberosum and A. thaliana, we used the Spud DB Potato Genomic Resources (http://spuddb.uga.edu/). The Gene Ontology (GO) analysis was done with the PANTHER overrepresentation test and with the GO database DOI: https://doi.org/10.5281/zenodo.6799722 (http://pantherdb.org). To obtain the heatmap of the D-CHIP analysis, the z-score was calculated from the normalized counts of all transcripts for each sample, and then a dendrogram was generated to choose the clusters accumulating the largest number of genes, using pheatmap (Kolde, 2022), dendextend (Galili, 2015), ggplot2 (Wickham, 2016), and packages of R 4.2.1 (R Core Team, 2022).
2.6 RT-qPCR gene expression
To validate RNA-seq results by RT-qPCR, an independent experiment was conducted to gather material from three biological replicates per treatment. Each replicate consisted of a pool of leaves (the third leaf from the apex) from six plants that were sampled and stored at −80°C. RNA extraction was performed as explained for the RNA-seq assay. First-strand cDNA was synthesized from a 1.5 mg DNA-free RNA template using an oligo(dT) primer and M-MLV reverse transcriptase (http://www.promega.com). RT-qPCR reactions were performed on an optical 96-well plate using LightCycler 480 SYBR Green I Master mix (Roche, https://www.roche.com/) and a LightCycler 480 II real-time PCR system (https://www.roche.com/). The thermal conditions consisted of a first step of 5 min at 95°C, followed by 40 cycles of 15 s at 95°C and 1 min at 60°C. Specific primer pairs for each gene were designed using NCBI Primer-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) and are listed in Table S1. The expression of each gene was normalized to actin (ACT) and standardized to the expression in the control treatment, following the methodology cited in Crocco et al. (2018).
2.7 Phytohormone determinations
The content of IAA and GA3 was measured in the stem and in the latest fully expanded leaf. The stem samples were obtained by cutting the upper 2 cm. Four biological replicates were harvested from a pool of six plants each. The samples were harvested at 11 am and immediately immersed in nitrogen liquid until lyophilization. For phytohormone determinations, the samples were ground to powder with a mortar and pestle and weighed (100–200 mg per sample). The identification and quantification of phytohormones were carried out using liquid chromatography coupled with tandem mass spectrometry (Waters and Micromass, respectively) following the protocols indicated in Gómez-Ocampo et al. (2021) and Cassán et al. (2009). Phytohormone determinations were performed at the Plant Physiology Laboratory, Universidad Nacional de Río Cuarto, Córdoba, Argentina.
2.8 Botrytis cinerea infection assays
Botrytis cinerea (strain B05) was grown and maintained on potato dextrose agar (1.5% agar, 2% potato extract, 2% dextrose). Conidia were collected from agar plates with distilled water and a glass rod, filtered, and resuspended in a 0.1 M sucrose/0.07 M KH2PO4 solution to induce germination (Elad, 1991). To investigate leaf susceptibility, the suspension of B. cinerea spores was used to inoculate the first fully expanded leaf of 28-day-old plants. After 72 h, infected leaves (one per plant) were collected and photographed.
To analyze the defense ability of potato tubers harvested from plants cultivated under sunlight control or shade conditions, tubers were first cleaned in water and then soaked in 75% ethanol for 30 s to avoid contamination before treatment. Afterwards, the tubers were placed at room temperature and air-dried. Then, two protocols were assayed. In the first one, tubers were cut in half and immediately infected with 5 μL of B. cinerea spores (3 × 103 spores μL-1). In the second one, we made a bit hole of 0.4 cm3 using an awl and infected it with a superficial drop of 5 μL of B. cinerea spores (3 x 103 spores μL-1). Following inoculation, the tubers were stored for 5 days at 28°C in a dark and damp environment. Lesion areas were measured on digital photographs using ImageJ software (Schneider et al., 2012).
2.9 Experimental design and statistical analysis
A completely randomized block design was used for the experiments. The experiments were replicated at least three times. Statistical analyses were performed by Student's t-test. Analyses were carried out using Prism (GraphPad Software, San Diego, CA).
3 RESULTS
3.1 Simulated shade triggers shade avoidance responses
We studied morphological shade avoidance responses in adult potato plants (S. tuberosum var. Spunta). Potato plants of 28-day-old were exposed to sunlight or simulated shade for 14 days until the end of the experiment (Figures 1A and S1). The plants exposed to simulated shade were visibly taller, with significantly longer stems than control (17.81 ± 3.15 vs. 8.57 ± 1.99 cm, respectively). In addition, shaded plants had higher internode distance than control (6.82 ± 0.67 vs. 4.41 ± 0.56 cm, respectively; Figure 1B,C). These results show that potato plants displayed strong shade avoidance responses in our experimental conditions.
3.2 Simulated shade produces thinner leaves
The SAS can induce anatomical and morphological changes in leaves (Kim et al., 2005). To study the effect of shade on the leaf anatomy, we evaluated the leaf, mesophyll, and epidermis thickness of the upper leaf, which emerged and developed in sunlight or simulated shade between 28 and 42 days. The leaf blades were thinner in simulated shade than in sunlight due to the reduction of mesophyll cells (Figure 2). However, the thickness of epidermis cells was greater for both abaxial and adaxial faces in plants cultivated in simulated shade compared with control (Figure 2). Together, these results demonstrate that simulated shade produces changes in the morphology and anatomy of potato leaves.

3.3 Simulated shade produces lower photosynthesis
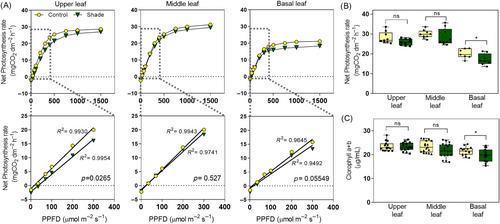
The light intercepted for photosynthesis depends on canopy architecture and plant density (Casal & Fankhauser, 2023). We evaluated the photosynthesis parameters in potato plants cultivated under control and simulated shade conditions in leaves at the upper, middle, and basal positions of the same plant. We measured net photosynthesis rate, stomatal conductance, and CO2 concentration in the stomatal cavity between 0 and 2000 μmol m−2 s−1. We found significant differences in net photosynthesis rate, stomatal conductance, and CO2 concentration in the stomatal cavity between light conditions (Figures 3 and S3). In simulated shade, the leaves located in the upper position had similar photosynthesis rates under high irradiances (Figure 3A,B) but produced lower photochemical quantum yields than control plants (Figure 3A, p = 0.02), suggesting that they were less efficient in using the photons for photosynthesis. The leaves located in the middle position showed similar photosynthetic rates to the upper leaves without significant differences in photochemical quantum yields between light treatments (Figure 3A,B). In contrast, basal leaves of plants exposed to simulated shade had lower photosynthetic rates at high irradiances (Figure 3A,B), probably associated with a less chlorophyll content than control plants (Figure 3C). On average, the leaves exposed to simulated shade had higher stomatal conductance (gs) and CO2 concentration in the stomatal cavity (Ci) than those from the same stratum of plants cultivated in sunlight (Figure S3). Independent of the light treatment, the upper leaves showed similar chlorophyll content (Figure 3C), net photosynthesis rate as a function of Ci, maximum rate of Rubisco carboxylase activity (Vcmax), and maximum capacity of electron capacity (Jmax), suggesting that these biochemical parameters of photosynthesis are not altered by shade (Figure S4).
3.4 Simulated shade reduces tuber yield
To have a better comprehension of the shade avoidance effects in potato plants, we measured total dry weight, total fresh weight, dry/fresh weight ratio, and shoot/root weight ratio in 42-day-old plants cultivated in sunlight and simulated shade conditions. Although the dry and fresh weights were higher, the ratio of dry/fresh weight was lower in shaded plants than in control, suggesting that the water content per unit of mass is higher in shaded plants. In addition, the shoot/root weight was higher in plants exposed to simulated shade than control (Figures 4A and S5).
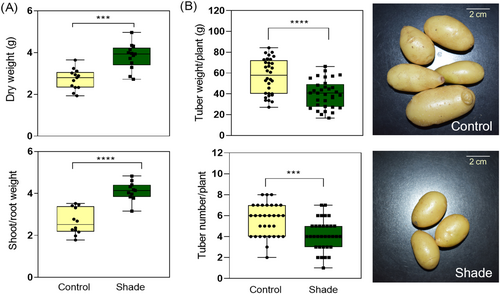
As the yield of potatoes is deeply affected by light (Boccalandro et al., 2003; Jackson et al., 1996, 1998; Thiele et al., 1999), we performed an independent experiment to evaluate the tuber production in 70-day-old plants. The simulated shade condition consisted in growing plants for 28 days in sunlight, followed by 14 days in simulated shade, and finally, 28 days in sunlight until harvest. The control plants were grown for 70 days in sunlight. Tuber number and tuber weight per plant were lower in shaded than in control plants (Figure 4B). These results show that simulated shade reduces the yield of tubers in potato plants.
3.5 Potato transcriptome regulated by simulated shade
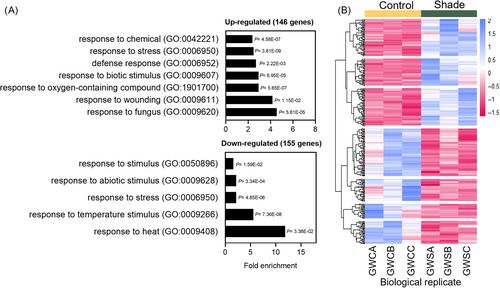
To have a better understanding of the molecular events associated with the SAS in potato plants, we performed RNA-seq analysis to identify genes regulated by shade. For this, we prepared three pools of samples, each consisting of upper leaves collected from six 42-day-old plants cultivated under control and simulated shade conditions. We identified 790 genes whose expression levels differed significantly between our experimental conditions (FDR < 0.05). Then, we selected genes up- and downregulated by simulated shade with a fold-change >2 (log2FC > |1|) between control and simulated shade conditions (i.e., GWS vs. GWC). We found 146 and 155 genes up- and downregulated by simulated shade, respectively (Table S2). Among those genes whose expression was higher under simulated shade, we found an auxin-regulated gene (AuxRP; PGSC0003DMT400077929), FT (PGSC0003DMT400041726), MYB (PGSC0003DMT400014383), PAR-1b (PGSC0003DMT400077162), WRKY-type (PGSC0003DMT400056352), a dormancy gene (PGSC0003DMT400033385), and pathogenesis-related genes (PGSC0003DMT400003364, PGSC0003DMT400013094, PGSC0003DMT400007904). Genes downregulated by simulated shade included FLAVONOL 4’-SULFOTRANSFERASE (PGSC0003DMT400072853), EXTENSIN class 1 (PGSC0003DMT400011908), heat shock proteins (PGSC0003DMT400050441, PGSC0003DMT400036856, PGSC0003DMT400077357, PGSC0003DMT400073713), chaperones (PGSC0003DMT400081406), genes related with the biosynthesis of terpenes (PGSC0003DMT400017206) and raffinose (PGSC0003DMT400046636). We performed a GO analysis to find the enriched terms for up- and downregulated genes in the shade. Upregulated genes were associated with response to chemicals, response to stress, defense response, and response to fungus, among other terms (Figure 5A). Downregulated genes were associated with response to abiotic stimulus, response to stress, response to heat, among others (Figure 5A). The heatmap analysis clustered the shade-regulated genes into seven groups, of which three were upregulated, and four were downregulated (Figure 5B and Table S3). Because the potato genomics database is incomplete, we searched for the A. thaliana homologs among 315 shade-regulated genes. We identified some known genes previously documented in seed dormancy, shade avoidance, flowering and defense processes such as SOK5 (AT5G59790), FT (AT1G65480), MYB62 (AT1G68320), PAR1 (AT5G52390), WRKY75 (AT5G13080), DRM1 dormancy gene (AT1G28330), SULFOTRANSFERASE 1, SOT1/SOT12 (AT2G03760), and EXTENSIN19 (AT1G26240; Table S3).
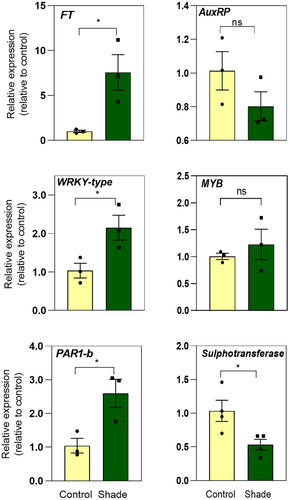
Then, we designed a new experiment to validate the expression of five upregulated (AuxRP, FT, MYB, PAR-1b, and WRKY-type) and one downregulated (FLAVONOL 4’-SULFOTRANSFERASE) genes by RT-qPCR analysis. We confirmed the expression patterns of FT, PAR-1b, WRKY-type, and FLAVONOL 4'-SULFOTRANSFERASE transcripts. FT expression was induced 7-fold, while PAR-1b and WRKY-type were expressed between 2- and 3-fold higher in simulated shade than sunlight (Figure 6). The levels of FLAVONOL 4'-SULFOTRANSFERASE transcripts were significantly reduced in simulated shade compared with control (Figure 6). AuxRP and MYB transcripts were not significantly different between light treatments (Figure 6). These results prove the robustness and reliability of our transcriptome data to identify shade-regulated genes in potato plants, as well as the repeatability of our experimental design.
3.6 Simulated shade induces a higher content of IAA in stems
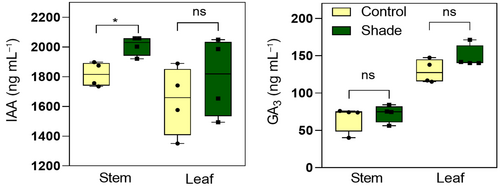
Among the genes regulated by simulated shade, we found some related to hormonal metabolism (Table S2). For example, we found CYTOKININ OXIDASE/DEHYDROGENASE 2 (homolog to AtCKX3, PGSC0003DMT400000883) that catalyzes the degradation of cytokinins, GIBBERELLIN 2-OXIDASE (homolog to AtGA2OX2; PGSC0003DMT400071043) that inactivates GAs, and 9-CIS-EPOXYCAROTENOID DIOXYGENASE (homolog to AtNCED1; PGSC0003DMT400011006) which is involved in the synthesis pathway of abscisic acid. Regarding that auxins and gibberellins are important hormones for the promotion of growth (Casal & Fankhauser, 2023), we measured the content of the auxin IAA and the gibberellin GA3 in the stem apex and in the upper leaf of 42-day-old potato plants cultivated in sunlight and simulated shade. We found that plants exposed to shade contain higher amounts of IAA in stems but not in leaves and that both tissues produce similar levels of GA3 between treatments (Figure 7). These results suggest that free auxin in potato stems contribute to elongation responses in simulated shade.
3.7 Simulated shade increases susceptibility of B. cinerea in leaves and tubers
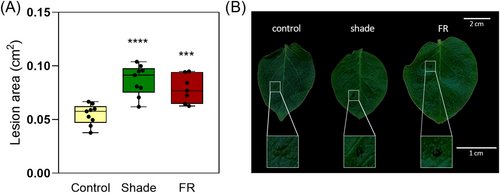
One of the most studied effects of shade exposure in plants is enhanced susceptibility to pathogens (Ballaré & Pierik, 2017). To test if simulated shade increases susceptibility to pathogens, we infected leaves treated for 7 days with shade, sunlight supplemented with far-red, or sunlight (control) with B. cinerea spores. We found that simulated shade increased 1.6-fold the size of necrotic lesions generated by B. cinerea, and the affected area was darker. The same lesion effects were produced in leaves of plants exposed to sunlight supplemented with far-red (Figure 8A,B). These results suggest that the susceptibility response to the attack of fungus in potato is mediated by the phytochrome, which perceives changes in the R:FR ratio, as it was previously documented (Cerrudo et al., 2012; Fernández-Milmanda et al., 2020).
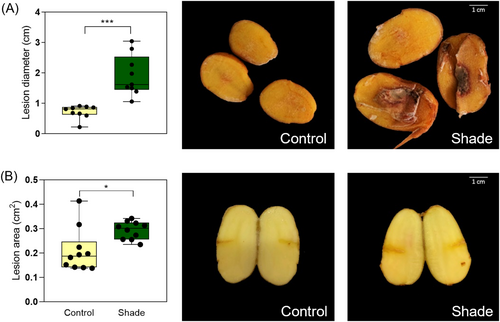
We also investigated the susceptibility to B. cinerea infection in tubers collected from plants that were exposed to simulated shade or sunlight (Figure 9). The infection was carried out in two ways. In the first one, we did a transversal cut and infected in the same way that the leaves (Figure 9A). In the second approach, we performed a tiny hole into the tuber and infected with B. cinerea just around the lesion (Figure 9B). In both experiments, the tubers from plants exposed to simulated shade were more susceptible to B. cinerea than control (Figure 9). Together, these results demonstrate that simulated shade increased the susceptibility to pathogen infections in leaves and tubers of potato plants.
4 DISCUSSION
There are several works showing that shade light induces SAS as a resource reallocation strategy to elongate vegetative structures and redefine the plant architecture to compete for photosynthesis light. The PHYB overexpression, the principal phytochrome responsible for the SAS, increased photosynthesis, reduced photoinhibition, retarded senescence, and produced more tuber yield in potato plants cultivated in a greenhouse (Schittenhelm et al., 2004; Thiele et al., 1999) and in the field (Boccalandro et al., 2003). However, a recent field experiment with plants cultivated at three different irradiances using neutral nets suggests that potato is a shade-tolerant crop (Schulz et al., 2019). To have a better understanding of SAS in potato, here we evaluated the SAS in plants exposed to simulated shade for 2 weeks during the initiation of tuber development (Ewing & Struik, 1992). The results confirm that potato plants var. spunta trigger strong responses to shade light, such as stem and internode growth promoted by auxins and the reduction of leaf thickness and tuber yield. Simulated shade promotes lower photochemical quantum yields in upper leaves and reduced photosynthesis rate in basal leaves. In addition, shade light enhances plant susceptibility to the attack of the necrotrophic fungus B. cinerea in leaves and tubers. Overall, our work clearly demonstrates configurational changes between growth and defense decisions in potato plants cultivated in simulated shade.
At the transcriptional level, PAR1b, FT, and WRKY-like genes were significantly promoted by simulated shade in potato plants (Figure 6 and Table S3). Shade light rapidly promotes the gene expression of PAR1, and its protein product forms a negative feed-forward loop with PIFs to avoid exaggerated elongation responses in Arabidopsis seedlings (Crocco et al., 2010; Hornitschek et al., 2009; Roig-Villanova et al., 2007). Low R:FR ratios can also accelerate flowering and increase FT expression in Arabidopsis (Adams et al., 2009; Kasulin et al., 2017). Interestingly, PIFs collaborate with CO to promote the expression of FT and its paralog TSF in Arabidopsis (Galvāo et al., 2019; Zhang et al., 2019). Furthermore, AtWRKY75 (AT5G13080), the homolog of WRKY-type of potato (PGSC0003DMT400056352), pinpoints as a potential regulator of plant growth and flowering in shade. The overexpression of WRKY75 accelerates flowering by binding to the promoter of FT (Zhang et al., 2018), and WRKY75 is induced in low R:FR ratios to restrict root growth in Arabidopsis seedlings (Rosado et al., 2022).
We found that both simulated shade and sunlight supplemented with far-red increased the size of necrotic lesions generated by B. cinerea in leaves and tubers, suggesting that phytochrome signaling is involved in the response. Notably, the set of genes that were upregulated in simulated shade was enriched in defense-related terms even when the plants were not exposed to the pathogen attack. We found that FLAVONOL 4’-SULFOTRANSFERASE expression is significantly reduced by simulated shade in potato plants (Figure 6). The closest homolog of FLAVONOL 4’-SULFOTRANSFERASE in Arabidopsis is SOT12 (At2g03760) which sulfonates SA increasing the production of active SA and the resistance to the pathogen Pseudomonas syringae (Baek et al., 2010). These results are in agreement with previous reports showing that SA synthesis and response are inhibited in plants exposed to shade light (De Wit et al., 2013; Griebel & Zeier, 2008). More experiments are needed to decipher the molecular mechanism associated with the increased susceptibility to pathogen infections in potato plants grown under shade.
The leaves can integrate information about past and current light exposure to trigger acclimation responses to adjust structural, biochemical, and physiological traits (Niinemets et al., 2015). The results of this work support the idea that shade light produces photosynthesis changes among leaves located at different positions in the vertical gradient of the plant (Figure 3). The shaded leaves of the upper strata produce lower photochemical quantum yield (p = 0.0265), suggesting a lower efficiency in the use of photons (Figure 3), as a consequence of the increased resistance to CO2 diffusion for photosynthesis. Interestingly, the leaves of the upper stratum are significantly thinner, but notably, the thickness of the epidermis was greater than those leaves grown in sunlight (Figure 2). On the other hand, the basal leaves of potato plants cultivated in simulated shade produced less photosynthesis and lower content of chlorophylls (Figure 3), probably related to the earlier senescence induced by shade as it was previously documented (Li et al., 2023). In a study with tobacco leaves, Clarke et al. (2021) found that older leaves had thicker mesophyll cell walls and lower mesophyll conductance that correlate with a lower CO2 assimilation in basal leaves.
A current strategy to increase the yield of crops is to cultivate a higher number of plants per unit of soil area (Fan et al., 2012). When the canopy increases, the light environment surrounding plants dramatically changes; thereby, the plant architecture and the use of photo-assimilates are redistributed to those organs in active growth. These responses were well studied in crops such as sunflower (Libenson et al., 2002) and wheat (Ugarte et al., 2010). In our work, we demonstrated that 2 weeks of simulated shade significantly reduced tuber yield in potato (Figure 4). In addition, the far-red signals reflected and transmitted by surrounded leaves in shade increase the susceptibility to attack by necrotic pathogens such as fungi in leaves and tubers of potato plants (Figures 8 and 9). Future studies should be performed to maximize plant density to guarantee maximal sunlight interception and to avoid the reduction in tuber yield and/or the attack of necrotrophic pathogens in potato crops.
AUTHOR CONTRIBUTIONS
Gabriel Gómez Ocampo and Javier F. Botto conceived the experiments; Gabriel Gómez Ocampo and Jimena Cascales performed physiological and molecular experiments; Ana L. Medina-Fraga and Gabriel Gómez Ocampo performed defense experiments; Anita I. Mantese prepared the samples and took photographs for anatomical measures; Edmundo L. Ploschuk provided technical assistance in photosynthesis experiments; Carlos D. Crocco, Daniel Matsusaka, Javier F. Botto, and Diego H. Sánchez performed the transcriptomic analysis. Javier F. Botto, Gabriel Gómez Ocampo, and Jimena Cascales analyzed the data and wrote the article. All authors read and accept the article.
ACKNOWLEDGMENTS
We thank Roberto Tornese for his technical assistance in the adjustment of light chambers for the experiments. This work was supported by grants from the University of Buenos Aires (UBACYT 20020170100265BA and PIDAE2020/2022), and the Agencia Nacional de Promoción Científica y Tecnológica Argentina (PICT2017-0583 and PICT2019-2807) to Javier F. Botto.
CONFLICT OF INTEREST STATEMENT
Authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The transcriptomic data are deposited in ArrayExpress (www.ebi.ac.uk/arrayexpress/) under accession number E-MTAB-12771.




