Inoculation of ACC deaminase-producing endophytic bacteria down-regulates ethylene-induced pathogenesis-related signaling in red pepper (Capsicum annuum L.) under salt stress
Abstract
Pathogenesis-related (PR) signaling plays multiple roles in plant development under abiotic and biotic stress conditions and is regulated by a plethora of plant physiological as well as external factors. Here, our study was conducted to evaluate the role of an ACC deaminase-producing endophytic bacteria in regulating ethylene-induced PR signaling in red pepper plants under salt stress. We also evaluated the efficiency of the bacteria in down-regulating the PR signaling for efficient colonization and persistence in the plant endosphere. We used a characteristic endophyte, Methylobacterium oryzae CBMB20 and its ACC deaminase knockdown mutant (acdS−). The wild-type M. oryzae CBMB20 was able to decrease ethylene emission by 23% compared to the noninoculated and acdS− M. oryzae CBMB20 inoculated plants under salt stress. The increase in ethylene emission resulted in enhanced hydrogen peroxide concentration, phenylalanine ammonia-lyase activity, β-1,3 glucanase activity, and expression profiles of WRKY, CaPR1, and CaPTI1 genes that are typical salt stress and PR signaling factors. Furthermore, the inoculation of both the bacterial strains had shown induction of PR signaling under normal conditions during the initial inoculation period. However, wild-type M. oryzae CBMB20 was able to down-regulate the ethylene-induced PR signaling under salt stress and enhance plant growth and stress tolerance. Collectively, ACC deaminase-producing endophytic bacteria down-regulate the salt stress-mediated PR signaling in plants by regulating the stress ethylene emission levels and this suggests a new paradigm in efficient colonization and persistence of ACC deaminase-producing endophytic bacteria for better plant growth and productivity.
1 INTRODUCTION
Salt stress can induce a plethora of perturbations that can prove to be detrimental to plant physiological and biochemical processes. The typical salt-induced perturbations can be attributed to ion toxicity, oxidative stress, and elevated ethylene emissions leading to the activation of stress response machinery (Chatterjee et al., 2017, 2018, 2019; Roy Choudhury et al., 2021; Samaddar et al., 2019). However, recent studies have also shown that abiotic stresses can induce pathogenesis-related (PR) signaling in plants (Pacheco et al., 2013; Roy Choudhury et al., 2022; Song et al., 2016) that are typically induced by biotic stress factors (Yim et al., 2013, 2014). PR signaling can be activated by various effector molecules and proteins in plants during pathogen attacks or herbivory (Ali et al., 2018). Ethylene emission and subsequent reactive oxygen species (ROS) accumulation are major drivers for inducing PR signaling (Dang et al., 2013; Xing et al., 2013). These physiological responses that induce PR signaling are classic responses for plants exposed to salt stress (Chatterjee et al., 2017, 2018, 2019; Roy Choudhury et al., 2021; Samaddar et al., 2019).
PR signaling is mainly responsible for inducing host defense responses and can be characterized as the primary immune response mediator (Nomura et al., 2005). There are various transcription factors, genes, and enzymes that are upregulated when the plants are exposed to biotic stress factors, such as WRKY transcription factors, PR1 and PT1 genes, β-1,3 glucanase, and phenylalanine ammonia lyase (PAL) (Pacheco et al., 2013; Roy Choudhury et al., 2022; Song et al., 2016; Yim et al., 2013, 2014). These effector molecules are important for enhancing the stress tolerance of plants and recently, tweaking PR signaling has been observed to enhance tolerance against abiotic stresses (Ali et al., 2017; Liu et al., 2013; Seo et al., 2008; Singh et al., 2013; Wu et al., 2016). In addition, inoculation with ACC deaminase producing an endophytic microbial inoculant has the potential to enhance the activities of PR proteins and upregulate gene expression profiles (Yim et al., 2013, 2014). However, there is a lack of studies that have explored the possible role of endophytic bacterial inoculation in modulating PR signaling in plants under abiotic stress conditions. On the other hand, a competent endophytic inoculant is supposed to successfully colonize and persist in the plant endosphere and prime against the host defense responses (Balmer et al., 2015; Hardoim et al., 2008). There is a need to evaluate how beneficial microbes are able to colonize and persist in the plant endosphere and bypass the hosts' innate immune response . Also, there is a lack of studies that concentrates on studying the efficiency of ACC deaminase-producing bacterial inoculant on priming against host defense responses for successful persistence in the plant endosphere. Hence, this study was conducted to evaluate the efficiency of ACC deaminase-producing endophytic bacteria in regulating ethylene-mediated PR signaling in plants under salt stress and their ability to prime against host defense responses for successful persistence in the plant endosphere. To achieve these objectives, we used a well-characterized ACC deaminase-producing Methylobacterium oryzae CBMB20 and it's ACC deaminase knockdown (acdS−) mutant as inoculants for red pepper plants (Capsicum annum cv. Soobicho). We studied plant growth parameters, ethylene emissions, ROS accumulation, PAL activity, β-1,3 glucanase activity, and expression profiles of candidate PR genes under normal and salt stress conditions. We observed that the inoculation of both the bacterial strains had induced host defense responses during the initial inoculation period and it subverted to the level of noninoculated plants under normal conditions. However, only the wild-type M. oryzae CBMB20 was able to down-regulate the ethylene emission-induced PR signaling under salt stress conditions which suggests a novel pathway in plant–bacteria interactions for efficient colonization of the plant endosphere to modulate the stress response machinery.
2 MATERIALS AND METHODS
2.1 Plant material, growth conditions, and bacterial inoculation
Red pepper seeds (Capsicum annuum cv. Soobicho; Syngenta seeds) were surface sterilized as per Samaddar et al. (2019). Briefly, the seeds were treated with 2% NaOCl followed by 70% ethanol and three washes with sterile distilled water. The seeds were incubated in dark at 30°C for 72 h on a moist sterile filter paper for germination. The germinated seeds were transplanted into plastic pots (dimensions: inner diameter = 9 cm; outer diameter = 10 cm; depth = 9 cm) containing 150 g nursery soil (composition: cocopeat 49.876%, peatmoss 25%, pearlite 12%, vermiculite 7%, zeolite 6%, pyroligneous liquor 0.004%, fertilizer 0.11%, wetting agent 0.01%; Nongwoo-Bio Co., Ltd., Yeoju-gun; Gyeonggi-do) and grown in greenhouse conditions with 32°C/28°C day/night temperature, 70% humidity, and under natural illumination.
Methylobacterium oryzae CBMB20 (WT), acdS knockdown M. oryzae CBMB20 (acdS−), gfp-tagged wild-type CBMB20 (gfp+ M. oryzae CBMB20) and gfp-tagged acdS− CBMB20 (gfp+ acdS− M. oryzae CBMB20) were grown in ammonium mineral salt (AMS) medium, supplied with 0.5% sodium succinate as the sole carbon source at 25°C. The construction of acdS knockdown M. oryzae CBMB20, gfp+ M. oryzae CBMB20 and gfp+ acdS− M. oryzae CBMB20 is provided in the supplementary material. The induction of dCas9 for acdS knockdown was carried out by adding 0.1% cumate at an OD600 ~ 0.4–0.5 and grown until OD600 ~ 0.8–1. The bacterial suspension was prepared using 0.03 M MgSO4 and the absorbance at OD600 was maintained at 0.8 (~108 CFU mL − 1). Bacterial inoculation was performed at 7 days after transplantation by inoculating 10 mL of bacterial suspension into the rhizosphere zone. Each treatment contained three replicates and a total of six plants were used for each of them.
2.2 Salt stress application
The salt stress was applied to the plants after 1 day of inoculation (8 days after transplanting). Salt stress was applied by adding 10 mL of 150 mM NaCl into the rhizosphere zone. The control plants were watered with tap water throughout the experimental period and water leaching was prohibited by watering the plants below the water-holding capacity of the soil. The plants were harvested at 2, 9, 16, and 23 days after NaCl treatment. Plant growth parameters such as shoot length, root length, and number of leaves were recorded while harvesting and the dry weight of the plants was measured after drying the plants at 70°C for 72 h.
2.3 Determination of ethylene emissions
Ethylene emissions from red pepper seedlings were determined in a completely discrete experimental setup, as mentioned in Roy Choudhury et al. (2021). Bacterization of 30 red pepper seeds, each with wild-type M. oryzae CBMB20 and acdS− M. oryzae CBMB20, were carried out separately by soaking them in bacterial inoculum for 4 h with gentle shaking in a sterile flask. Noninoculated seeds were treated with 0.03 M MgSO4. The noninoculated and bacterized seeds were placed inside a GC bottle. Distilled water (2 mL) was added to the seeds and allowed to grow for 7 days in a growth chamber (DS 54 GLP; DASOL Scientific Co., Ltd.). The seedlings were treated with 2 mL of a 150 mM NaCl solution for the salt stress set up, distilled water was used for the control setup and the seedlings were incubated for 4 h. The accumulated ethylene in the headspace was analyzed using gas chromatograph (dsCHROM 6200, Donam Instruments Inc.) equipped with a Poropak-Q column.
2.4 Determination of hydrogen peroxide concentration
The hydrogen peroxide (H2O2) concentration was measured as per the protocol mentioned in Theocharis et al. (2012). Fresh leaf and root samples (0.5 g) were ground with liquid nitrogen, homogenized with 5 mL cold acetone in an ice bath, and the homogenate was centrifuged at 6000 g for 10 min. The supernatant (1 mL) was transferred into a fresh test tube and 5% titanium sulfate (0.1 mL) and ammonia (0.2 mL) were added to it. The contents were centrifuged at 10 000 g for 10 min at 4°C and the supernatant was discarded. The pellet was resuspended in 2 mM H2SO4 (5 mL) and the absorbance was recorded at 415 nm using a UV–Vis spectrophotometer (UV-1601, Shimadzu Corporation). H2O2 content was determined using a standard curve plotted with a known concentration of H2O2.
2.5 Determination of β-1,3-glucanase activity
The β-1,3-glucanase activity was assayed by the laminarin-dinitrosalicylic acid method (Pan et al., 1991). Briefly, an equal volume (62.5 μL) of 4% laminarin and plant extract were mixed and incubated at 40°C for 10 min. Dinitrosalicylic acid (DNSA) reagent (375 μL) was added to finish the reaction and further incubated in a boiling water bath for 5 min. The resulting mixture was diluted with 4.5 mL distilled water and the absorbance of the colored solution was recorded at 500 nm using UV–Vis spectrophotometer (UV-1601, Shimadzu Corporation). The enzyme activity was finally expressed as 1 ng of reduced glucose min−1 mg−1 protein.
2.6 Determination of PAL activity
PAL activity was measured following the method of Dickerson et al. (1984). The plant extract (100 μL) was mixed with 500 μL 50 mM Tris HCl (pH 8.8), and 600 μL 1 mM l-phenylalanine. The reaction mixture was incubated at room temperature for 1 h and the reaction termination was carried out by adding 2 N HCl. The organic phase was extracted with 1.5 mL toluene and the absorbance of the toluene phase was recorded at 290 nm using UV–Vis spectrophotometer (UV-1601, Shimadzu Corporation). Finally, the enzyme activity was expressed as nmol trans-cinnamic acid released min−1 mg−1 protein.
2.7 Determination of superoxide dismutase and catalase activities
Superoxide dismutase (SOD) and catalase (CAT) were extracted by homogenizing ground leaf samples in 50 mM potassium phosphate buffer supplemented with 1% (w/v) polyvinylpyrrolidone. The SOD activity was measured by monitoring the photochemical reaction of nitro-blue tetrazolium at 560 nm, whereas CAT activity was measured by monitoring the ability of the enzyme extract to convert hydrogen peroxide at 390 nm using a UV–Vis spectrophotometer (UV-1601, Shimadzu Corporation; Roy Choudhury et al., 2021).
2.8 Determination of culturable bacterial endophytes in the red pepper endosphere
The colony-forming units (CFU) of wild-type M. oryzae CBMB20-gfp and gfp+ acdS− M. oryzae CBMB20 were determined from surface sterilized red pepper plants. Culturable bacterial populations were noted after serial dilution and plating of 1 g crushed plant root or shoot in ammonium salt media supplemented with 50 μg mL − 1 kanamycin, incubated in 28°C for 3 days.
2.9 RNA extraction and quantitative reverse-transcriptase PCR (qRT-PCR)
Total RNA was isolated from ground leaf tissues using the RNeasy Plant Mini Kit (QIAGEN) following the manufacturer's protocol and stored at −80°C. cDNA was synthesized using Superscript III first strand synthesis system (Invitrogen). The gene expression analyses were carried out by quantitative reverse transcription (qRT)-PCR using the CFX96 real-time system (Bio-Rad Laboratories) with the SYBR Green master mix (iQ SYBR Green Supermix, Bio-Rad). Ubiquitin was used as the internal control for the normalization of data. All experiments were performed in triplicates with three repeats to ensure data validity. A list of primers is available in Table S1.
2.10 Statistical analysis
The experiments were carried out in a randomized block design. The data were subjected to analysis of variance (ANOVA) and the significant differences between means were determined by least significant difference (LSD) at p < 0.05 using the SAS package, Version 9.4.
3 RESULTS AND DISCUSSION
3.1 ACC deaminase is important for improving plant growth parameters under salt stress
Salt stress reduces plant growth and ACC deaminase-producing bacteria have been documented to enhance plant growth parameters (Chatterjee et al., 2017; Roy Choudhury et al., 2021; Samaddar et al., 2019). In this study, the plant growth parameters were measured in terms of shoot length, root length, plant dry weight, and number of leaves (Table 1). The growth parameters had significantly decreased under salt stress across all the treatments irrespective of bacterial inoculation. However, the plants inoculated with wild-type M. oryzae CBMB20 had recorded significantly higher growth parameters compared to noninoculated and acdS− M. oryzae CBMB20 inoculated plants under salt stress. The number of leaves, shoot length, root length, and plant dry weight were approximately 11%, 14%, 16%, and 11% higher respectively for wild-type M. oryzae CBMB20 inoculated plants compared to the noninoculated and acdS− bacteria inoculated plants at 23 days after salt stress (Table 1). On the other hand, both the wild type and acdS− M. oryzae CBMB20 inoculated red pepper plants had shown significantly higher plant growth parameters compared to the noninoculated plants under normal conditions. The ability of the acdS− M. oryzae CBMB20 to enhance plant growth can be attributed to its multifunctional PGP characteristics such as IAA production, cytokinin production, etc. which are important for enhancing plant growth (Madhaiyan et al., 2006, 2007). This observation can be supported by a previous report where inoculation of a ACCD deletion mutant Streptomyces spp. lacking indigenous IAA production ability was not able to enhance plant growth under normal and salt stress conditions (Jaemsaeng et al., 2018). Hence, ACC deaminase might not hold much significance under nonstress conditions; however, it can be highly regarded for enhancing plant growth under environmental stress conditions.
| DAS | Inoculation | Salt conc. (mM) | Number of leaves | Shoot length (cm) | Root length (cm) | Plant dry weight (mg) |
|---|---|---|---|---|---|---|
| 2 | NI | 0 | 5.67 ± 0.23a | 8.38 ± 0.19b | 10.59 ± 0.39b | 63.67 ± 3.91a |
| WT | 0 | 6.06 ± 0.22a | 9.47 ± 0.26a | 14.27 ± 0.37a | 68.00 ± 4.47a | |
| acdS− | 0 | 6.06 ± 0.28a | 9.38 ± 0.20a | 4.03 ± 0.52a | 67.00 ± 3.72a | |
| NI | 150 | 5.11 ± 0.35a | 7.56 ± 0.20b | 10.46 ± 0.43b | 51.33 ± 5.80b | |
| WT | 150 | 5.39 ± 0.13a | 8.91 ± 0.20a | 13.86 ± 0.85a | 60.67 ± 3.16a | |
| acdS− | 150 | 5.33 ± 0.23a | 8.48 ± 0.41a | 12.98 ± 1.00a | 54.67 ± 2.94ab | |
| 9 | NI | 0 | 6.72 ± 0.36b | 9.83 ± 0.19b | 11.99 ± 0.44b | 86.00 ± 8.81b |
| WT | 0 | 7.50 ± 0.23a | 10.95 ± 0.18a | 15.02 ± 0.89a | 100.33 ± 2.15a | |
| acdS− | 0 | 7.44 ± 0.05a | 10.66 ± 0.14a | 14.84 ± 0.76a | 99.67 ± 3.44a | |
| NI | 150 | 5.56 ± 0.40b | 8.28 ± 0.67b | 10.72 ± 0.25c | 69.67 ± 11.39b | |
| WT | 150 | 6.56 ± 0.13a | 9.63 ± 0.31a | 14.20 ± 0.70a | 79.67 ± 4.88a | |
| acdS− | 150 | 5.72 ± 0.25b | 8.94 ± 0.03b | 12.99 ± 0.18b | 71.00 ± 5.87ab | |
| 16 | NI | 0 | 7.39 ± 0.42b | 10.47 ± 0.71b | 13.78 ± 1.08b | 89.74 ± 4.25b |
| WT | 0 | 8.39 ± 0.57a | 11.46 ± 0.41a | 15.69 ± 0.44a | 110.56 ± 11.57a | |
| acdS− | 0 | 8.06 ± 0.57ab | 11.36 ± 0.52a | 15.44 ± 0.72a | 105.89 ± 5.49a | |
| NI | 150 | 6.11 ± 0.30b | 9.01 ± 0.27b | 12.41 ± 0.35c | 76.16 ± 2.81b | |
| WT | 150 | 6.50 ± 0.17a | 10.2 ± 0.12a | 14.49 ± 0.37a | 85.56 ± 3.20a | |
| acdS− | 150 | 6.22 ± 0.28b | 9.22 ± 0.57b | 13.02 ± 0.12b | 77.91 ± 1.28b | |
| 23 | NI | 0 | 8.61 ± 0.10a | 12.01 ± 0.17b | 14.13 ± 0.49b | 111.32 ± 3.51b |
| WT | 0 | 8.72 ± 0.05a | 12.29 ± 0.11a | 17.23 ± 0.37a | 129.54 ± 6.73a | |
| acdS− | 0 | 8.56 ± 0.18a | 12.01 ± 0.30a | 16.75 ± 0.71a | 132.84 ± 7.46a | |
| NI | 150 | 6.50 ± 0.09b | 9.32 ± 0.36b | 12.81 ± 0.75b | 84.42 ± 1.79b | |
| WT | 150 | 7.33 ± 0.54a | 10.68 ± 0.51a | 15.12 ± 0.12a | 94.28 ± 3.92a | |
| acdS− | 150 | 6.50 ± 0.17b | 9.34 ± 0.35b | 13.10 ± 0.48b | 85.90 ± 2.43b |
- Note: All data represent the mean ± SD of three replicates and the differences between means were analyzed using LSD test (p < 0.05). Different letters indicate significant differences within the salt treatments.
- Abbreviations: acdS−, ACC deaminase knockdown mutant of M. oryzae CBMB20; DAS, days after salt stress; LSD, least significant difference; NI, Non-inoculation; WT, wild-type M. oryzae CBMB20.
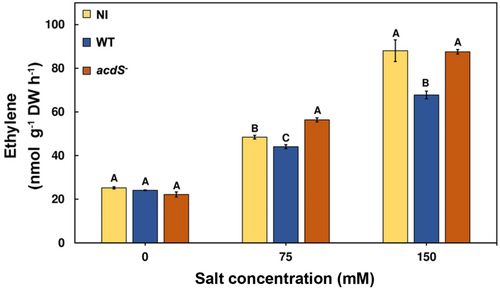
3.2 Salt stress-induced elevated levels of ethylene in red pepper seedlings
Elevated ethylene emissions are a major response of plants under environmental stress conditions (Chatterjee et al., 2017; Madhaiyan et al., 2006; Roy Choudhury et al., 2021; Samaddar et al., 2019). Similar observations were drawn in the current study, where ethylene emission was observed to be significantly higher under salt stress across treatments irrespective of bacterial inoculation. In addition, the noninoculated and acdS− M. oryzae CBMB20 inoculated red pepper seedlings had shown significantly higher ethylene emission levels compared to the wild-type bacteria inoculated plants under salt stress (Figure 1). The wild-type M. oryzae CBMB20 inoculation resulted in approximately 23% reduction in ethylene emissions compared to noninoculated and acdS− M. oryzae CBMB20 inoculated plants. The decrease in ethylene emissions from wild-type M. oryzae CBMB20 inoculated plants were due to its ACC deaminase activity which enables them to scavenge the precursor of ethylene, ACC and use it as a nitrogen source (Madhaiyan et al., 2006).
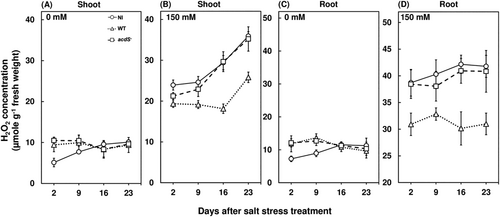
3.3 Salt-induced ethylene emissions enhanced ROS accumulation in red pepper
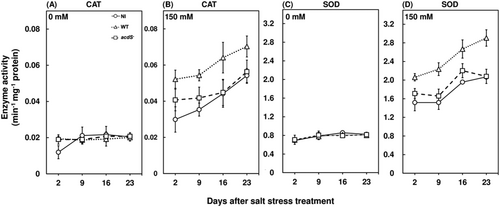
Salt stress can exert oxidative stress on plants through an accumulation of ROS (Chatterjee et al., 2017; Roy Choudhury et al., 2021; Samaddar et al., 2019), which has been reported to be regulated by ethylene emissions (Yao et al., 2017). In this study, the hydrogen peroxide concentrations of red pepper plants were significantly higher for salt-stressed plants compared to the control conditions in both shoot and root, irrespective of bacterial inoculation (Figure 2). Salt stress accumulated 2.5 fold and 3 fold higher H2O2 in the shoots and roots of wild-type M. oryzae CBMB20 inoculated plants (Figure 2B, D) compared to the plants grown under nonstress conditions. Whereas H2O2 accumulation of 3.5 fold and 4 fold higher was recorded for noninoculated and acdS− M. oryzae CBMB20 inoculated plants at 23 days after salt stress treatment compared to the plants grown under nonstress conditions (Figure 2B,D). The higher ROS accumulation under salt stress can be attributed to ethylene emission dependent membrane-bound RBOHD protein which catalyzes the reaction for synthesis of superoxides (Yao et al., 2017). However, the wild-type M. oryzae CBMB20 inoculation resulted in a 40.37% and 32.59% decrease in H2O2 accumulation for both shoots and roots compared to the noninoculated and acdS− M. oryzae CBMB20 inoculated plants at 23 days after salt stress treatment, respectively (Figure 2B, D). These results are in agreement to a previous study where M. oryzae CBMB20 inoculation was able to decrease hydrogen peroxide concentration of tomato plants under salt stress by enhancing the antixodant enzyme activities (Chanratana et al., 2019). The CAT and SOD activities were significantly increased under salt stress across all treatments compared to the plants grown in nonstress conditions (Figure 3). Furthermore, in this study, the wild-type M. oryzae CBMB20 inoculated plants had shown a 30.29% and 55.57% increase in CAT and SOD activities compared to the noninoculated and acdS− M. oryzae CBMB20 inoculated plants (Figure 3B,D). Hence, these results support the potential of ACC deaminase-producing endophytic bacteria in ameliorating the toxic effect of ROS accumulation under salt stress.
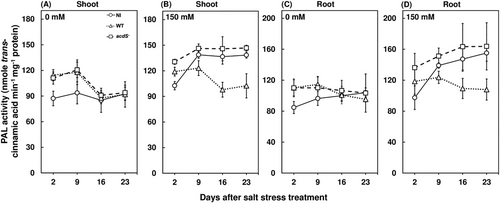
3.4 Salt stress-induced ethylene emissions and ROS accumulation enhanced PR protein activities
The increase in ROS concentrations can directly up-regulate PAL activity, which can induce chloroplast dysfunction and cell death (Xing et al., 2013). In the present study, the PAL activity was observed to be significantly higher in salt-stressed plants across treatments in both shoots and roots compared to the plants grown under nonstress conditions (Figure 4). These results are in agreement with previous studies where the up-regulation of PAL was observed under salt stress in sugar cane (Pacheco et al., 2013) and rice (Roy Choudhury et al., 2022). The increase in PAL activity under salt stress conditions can be attributed to plant's ability to catalyze the synthesis of secondary metabolites, which is important for stabilizing ROS (Gao et al., 2011). Furthermore, the PAL activity was observed to be significantly lower for the wild-type M. oryzae CBMB20 inoculated salt-stressed plants at 2, 9, 16, and 23 days after stress treatment (Figure 4B, D). The inoculation of wild-type M. oryzae CBMB20 had recorded a 28.63% and 27.27% decrease in PAL activities of shoots and roots at 23 days after salt stress treatment compared to the noninoculated and acdS− M. oryzae CBMB20 inoculated plants, respectively (Figure 4B,D). This can be attributed to the reduction in ROS accumulation due to a decrease in ethylene emissions and enhanced antioxidant enzyme activities by ACC deaminase-producing endophytic bacteria.
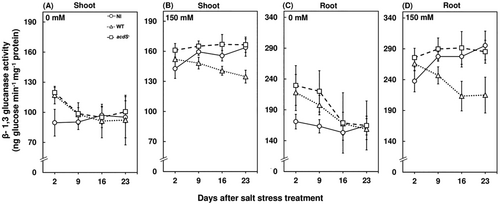
Salt stress also significantly enhanced β-1,3 glucanase activity compared to the plants grown in control conditions (Figure 5). The increase in β-1,3 glucanase activity are observed to be regulated by ethylene emissions (Felix & Meins, 1987), as well as being attributed to the expression of wounding-related genes under salt stress that are typical in enhancing β-1,3 glucanase activity (Choudhury et al., 2010; Dombrowski, 2003). Moreover, the ability of M. oryzae CBMB20 to produce ACC deaminase and reduce the salt-stress-mediated ethylene emission levels, resulted in a significant decrease in β-1,3 glucanase activity compared to noninoculated and acdS− M. oryzae CBMB20-inoculated red pepper plants in both shoots and roots under salt stress at 9, 16, and 23 days after stress treatment (Figure 5B,D). The wild-type M. oryzae CBMB20 inoculation showed a 21.8% and 36.6% decrease in β-1,3 glucanase activity in shoots and roots of red pepper plants at 23 days after stress treatment compared to the noninoculated and acdS− M. oryzae CBMB20-inoculated plants, respectively (Figure 5B, D). The decrease in PR protein activities during inoculation can be attributed to the ability of ACC deaminase-producing endophytic bacteria in reducing stress susceptibility by lowering ethylene emission levels and in turn resulting in the modulation of ethylene-induced downstream signaling.
3.5 PR genes are regulated by salt-induced ethylene emission levels
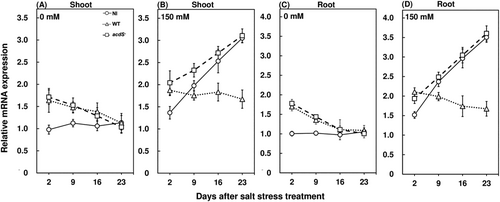
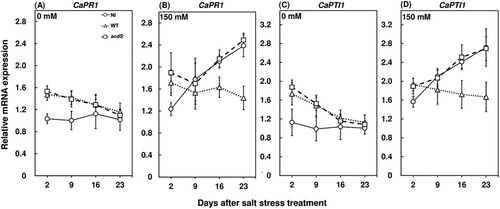
The expression profiles of PR genes were characterized to evaluate whether salt stress can induce their expression. WRKY gene expression profiles were significantly higher in the shoots and roots of salt-stressed plants compared to the plants grown under control conditions (Figure 6). These observations are in agreement with a previous study where the high-throughput sequencing of RNAs extracted from Capsicum annuum leaves showed higher expression of WRKY genes under salt stress (Song et al., 2016). Also, WRKY gene expressions and ethylene emissions have been observed to be positively correlated under biotic stress conditions (Dang et al., 2014), which might be a causation of the increase in WRKY expressions upon enhanced salt stress-induced ethylene emissions. Salt stress also significantly enhanced the expression of PR1 expression profiles in red pepper leaves compared to the plants grown in control conditions (Figure 7A, B). Another study showed similar trends in increased PR1 expression profiles in cucumber plants imposed with salt stress (Chojak-Koźniewska et al., 2017). A typical root localized ethylene response factor in red pepper, the CaPTI1 expression profile, was also significantly higher under salt stress compared to the plants grown in normal conditions (Figure 7C, D). Moreover, the wild-type M. oryzae CBMB20-inoculated plants had significantly lower WRKY expression profiles at 16 and 23 days after stress treatment in shoots and roots at 9, 16, and 23 days after stress treatment compared to the noninoculated and acdS− M. oryzae CBMB20 inoculated plants under salt stress (Figure 6B,D). Similarly, the shoot CaPR1 and root CaPTI1 expression profiles were significantly lower for wild-type M. oryzae CBMB20-inoculated plants compared to noninoculated and acdS− M. oryzae CBMB20 inoculated plants under salt stress (Figure 7B,D). Overall, the inoculation of wild-type M. oryzae CBMB20 showed an approximate range of 1.8–3 fold decrease in WRKY, CaPR1, and CaPTI1 gene expression profiles compared to the noninoculated and acdS− M. oryzae CBMB20-inoculated red pepper plants under salt stress. The down-regulation of PR genes upon inoculation of ACC-producing endophytic bacteria can be attributed to its ability in decreasing ethylene emission levels and enhancing the stress tolerance of red pepper plants. These results are validated by previous studies which established that WRKY, CaPR1, and CaPTI1 expression profiles are modulated by the ethylene emission pathway under stress conditions (Dang et al., 2013, 2014; Huang et al., 2004; Jin et al., 2016; Xu et al., 2007). Hence, the ACC deaminase activity of M. oryzae resulting in decreasing the salt stress-mediated ethylene emission levels is a characteristic trait in reducing the expression profiles of these particular PR genes in planta.
3.6 Beneficial bacteria can induce host defense responses during initial inoculation period
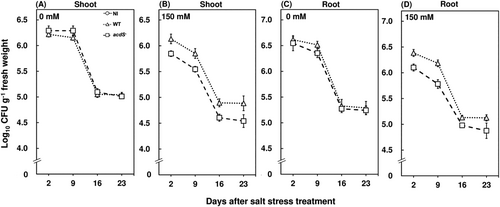
Host defense responses are generally up-regulated during pathogen infection (Yim et al., 2013, 2014). However, bacterial endophytes need to be competent to colonize and persist in the plant endosphere and it is also noteworthy that the colonization of bacterial endophytes can induce host defense responses (Compant et al., 2005; Hardoim et al., 2008). In this study, both the wild type and acdS− M. oryzae CBMB20-inoculated plants showed significantly higher hydrogen peroxide concentrations in red pepper plants grown under control conditions during the early inoculation period (Figure 2A, C). Similar observations were also incurred for PAL (Figure 4A, C) and β-1,3 glucanase activities (Figure 5A,C) where the bacteria-inoculated plants were observed to have significantly higher enzyme activities compared to the noninoculated plants grown under control conditions. These results are in agreement with a previous study where the inoculation of Burkholderia sp. enhanced the host defense responses by an accumulation of polyphenol in grapevines (Compant et al., 2005). Similar observations were recorded for the expression profiles of WRKY in shoots and roots (Figure 6A, C), shoot CaPR1 (Figure 7A) and root CaPTI1 (Figure 7C) expression profiles. The activation of these genes by red pepper plants during the early stages of inoculation can be attributed to the colonization of non-native bacterial endophytes and the eventual decrease can be a potential response of bacteria to successfully prime and sustain in the endosphere. In addition, these are not dependent on the jasmonate biosynthesis pathway; rather, they are tightly regulated by ethylene levels in plants which minimize the restriction of colonization by bacterial endophytes (Iniguez et al., 2005; Miché et al., 2006). Also, the CFU count of wild type and acdS− M. oryzae CBMB20 in the endosphere of shoot and root compartments of red pepper plants were invariant when plants were grown under control conditions (Figure 8A,C). These results are in line with another study, where the CFU count of wild type and ACC deaminase knockout mutant of Pseudomonas putida did not show any difference in the Arabidopsis thaliana endosphere (Ravanbakhsh et al., 2019). Both the wild type and the mutant bacterial strains were able to persist in the endosphere of red pepper plants which validates the competence of M. oryzae CBMB20 as an effective endophytic inoculant. On the other hand, the wild-type M. oryzae CBMB20 CFU count was significantly higher under salt-stress conditions compared to the CFU count of acdS− M. oryzae CBMB20 (Figure 8B,D). These results might infer that the ability of ACC deaminase-producing bacteria in utilizing the ethylene precursor ACC under environmental stress conditions can enhance a mutualistic relationship between the plant and the endophyte (Hardoim et al., 2008; Pieterse et al., 2014).
4 CONCLUSIONS
ACC deaminase is important for enhancing plant growth under salt-stress conditions and holds minimal significance under normal environmental conditions. On the other hand, salt stress can also induce the activation of ethylene emission-mediated PR signaling in plants and the inoculation of ACC deaminase-producing endophytic bacteria has the ability to down-regulate PR signaling by modulating stress-induced ethylene emissions. Also, plants inoculated with non-native bacterial endophytes can induce PR signaling during the early stages of inoculation but the beneficial effect as well as the competence of the inoculant can subvert the host defense responses and successfully persist in the plant endosphere. Overall, this study extrapolates the reasons behind the efficiency of M. oryzae CBMB20 in alleviating various environmental stresses and their beneficial interaction with multiple plant hosts.
AUTHOR CONTRIBUTIONS
Aritra Roy Choudhury and Tongmin Sa conceptualized the study. Aritra Roy Choudhury designed the experimental setup. Aritra Roy Choudhury, Jeongyun Choi and Wonho Choi performed the experiments. Jung-Ho Park provided the resources. Aritra Roy Choudhury and Pankaj Trivedi did the data analysis and writing of the original draft. Aritra Roy Choudhury, Pankaj Trivedi, Munusamy Madhaiyan, Jung-Ho Park, and Denver I. Walitang wrote, critically reviewed, and edited the work. Tongmin Sa was responsible for the supervision and funding acquisition.
ACKNOWLEDGMENTS
We would like to thank Prof. Tobias J. Erb and Dr. Dae-Hee Lee for providing the required plasmids for this study. This study was supported by the Basic Research Program through the National Research Foundation (NRF) funded by the Ministry of Education, Science and Technology (2021R1A2C1006608), Republic of Korea.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




