Coping with low moisture stress: Remembering and responding
Edited by: M. Ahanger
Abstract
Low-moisture stress, also referred to as drought, is one of the major factors that negatively impact the agricultural yield. The present scenario of climate change is expected to aggravate it further. Considering the extended time required to develop resistant crops, it is important to prioritize research efforts for coping with low moisture, prevalent in arid and semi-arid regions of the world. While agricultural yield is a tradeoff between many choices, tolerance to biotic and abiotic stresses comes with yield penalties. To balance the tradeoffs and maximize productivity, the use of region-specific cultivars and/or introgression of precise genetic proportions in an elite variety may prove useful. Stress memory is an emerging approach that helps plants to record and respond to repeated stress in an effective manner. In this context, we discuss the role of “stress memory” in imparting drought tolerance in plants. Future research efforts for its effective deployment for “drought hardening” in agricultural settings, along with a discussion on the yield tradeoff involved, is implicated.
1 INTRODUCTION
The most important abiotic factor that adversely influences crop performance is low moisture stress also known as drought. Many strategies, including the identification of resistant sources, agronomic practices, and genetic engineering, have been explored to impart drought tolerance in plants for enhanced yield or nutrition. The challenges posed by climate change require a continuous reorientation of the efforts. This also necessitates a detailed understanding of the drought stress and the response to it. Furthermore, low moisture stress is often associated with high-temperature stress in various limited-resource agro-ecologies of the world, making breeding for drought tolerance more difficult (Choudhary et al., 2019). Hence, understanding the molecular nature of short-term and long-term responses to low moisture in plants is essential to design effective interventions to mitigate it.
In recent years, “stress memory” is receiving increasing attention: priming a plant to cope rapidly and more effectively with future stress events (Penn State, 2020). The nature of low moisture stress plays an important role in determining the response to it. The stress may be single-time or reiterated (repeated events). Further, it may be periodic (intermittent) or chronic (continuous). In Arabidopsis, some traits, like increased seed dormancy, pass on to the next generations by a phenomenon called transgenerational plasticity (Herman & Sultan, 2011). The development of a “memory” in a plant, based on differential treatment (with and without stress condition), is often associated with changes that signal downstream events (Herman & Sultan, 2011). This review highlights the molecular response to drought stress in plants, discussing how the low moisture conditions can be variable with regard to season, location, and their effect on crop. The “memory response” in crop, useful for a better crop management, is also reviewed.
2 THE BENEFITS OF “MEMORY”
Stress memory is an evolving concept that enables plants to remember the experiences (in terms of metabolic changes and gene expression) from previous stress for protection from any further stress (Bruce et al., 2007; Ding et al., 2012; Galis et al., 2009; Ramírez et al., 2015). Drought stress memory capacitates the plant to respond differently to similar (drought) or different (waterlogging/heat) stresses, based on signaling or adaptation developed from the first encountered drought stress. For example, the prior treatment with stress-signaling molecules, like jasmonic acid, salicylic acid, or abscisic acid, is also an example of stress memory improving drought tolerance (Bruce, 2014; Farooq et al., 2012; Goh et al., 2003; Slaughter et al., 2012). Prior exposure to stress improves water retention (physiological efficiency) in Arabidopsis and maize (Ding et al., 2012, 2014; Virlouvet & Fromm, 2015). The establishment of stress memory occurs through a well-coordinated reaction at the genome, cellular and whole plant level. While the singular events in response to drought stress have been deciphered, the response strategy to repeated stress episodes remains to be investigated. At the molecular level, it still remains to be understood whether the expression of genes under repeated stress episodes occurs through a similar response pathway (as was activated during the initial encounter of drought stress) or through different response pathways. In the case of Arabidopsis seedlings, drought calcium-signaling pathway exhibited variable patterns depending on the history, i.e. duration and intensity of stress (Knight et al., 1998). Hence, it is important to understand the molecular factors that lead to the “maintenance” of stress memory to manipulate their expression for developing drought-tolerant crops.
The stress memory can be mainly classified at physiological and transcriptional level (Ding et al., 2012, 2014; Virlouvet & Fromm, 2015). The physiological memory involves the synthesis of metabolites such as anti-oxidants and stress proteins, whereas transcriptional memory involves the changes at the chromatin or transcript level, which ultimately regulates the gene expression, particularly drought stress genes. Furthermore, as plants can encounter drought stress at seed germination, vegetative growth and/or reproductive growth (Kinoshita & Seki, 2014; Munné-Bosch & Alegre, 2013; Ramírez et al., 2015), drought stress memory can be understood at three levels: seed priming, post-embryonic stage and transgenerational. Seed priming establishes a memory in the seed, used for an effective response to future drought stress at the seedling stage (Bruce et al., 2007). For example, polyethylene glycol-treated seeds in Arabidopsis exhibited better survival once the drought stress was resumed. On the other hand, if the seedling is well established without encountering drought stress at seedling stage but encounters a drought episode at the vegetative state, it may develop post-embryonic stage memory. This memory helps in imparting drought tolerance at reproductive or post-reproductive stress. The mild-drought-exposed rice seedlings exhibited less oxidative damage during subsequent drought stress (Li et al., 2011). Similarly, drought-exposed wheat seedlings showed limited membrane and oxidative damage under subsequent drought stress (Selote et al., 2004; Selote & KhannaChopra, 2006; Selote & Khanna-Chopra, 2010). The memory for drought tolerance was found to be connected with levels of antioxidant enzymes produced by the first drought exposure and the levels were maintained thereafter. Similar responses were observed in maize seedlings under recurring drought (Virlouvet et al., 2018). Wang et al. (2014) reported that memory established by applying drought stress at six-leaf and stem elongation stages can help to counteract moisture stress at grain filling stage in wheat. Banik et al. (2016) also reported beneficial effects of drought stress memory on yield and plant vigor in potato. The memory developed in one generation can also be transferred to the next generation (to offspring) via transgenerational “memory” (Luna et al., 2012). As per Boyko and Kovalchuk (2011), transgenerational memory mainly includes the heritable changes (DNA and chromatin structure change), but may also include non-heritable components: epigenetic changes. The non-inheritable memory stress factors include signaling molecules (metabolites), proteins/hormones and transcription factors in seed (Bruce, 2014; Vriet et al., 2015). Ćuk et al. (2010) demonstrated that modified activity (upregulation) of antioxidative enzymes, like catalase and ascorbate peroxidase in Arabidopsis progenies, is a sign of drought stress transgenerational memory. Walter et al. (2011) studied drought tolerance in a grass, Arrhenatherum elatius, by applying recurring drought. The authors reported enhanced drought tolerance in plants stressed at two occasions, when compared to the unstressed and once-stressed plants. Further, the functionality of drought stress memory persisted during the entire vegetative growth period and even in the next generation (vegetative propagation). In barley, offspring of the parental lines stressed for drought at the reproductive stage showed better drought stress response by developing longer roots than the offspring of non-drought-stressed parental lines (Nosalewicz et al., 2016). However, the stability of the transgenerational memory over the generations needs to be tested in crops.
3 MOLECULAR MECHANISM OF “STRESS MEMORY” IMPRINTS
The general mechanism behind drought stress memory is the maintenance of major physiological activities via safeguarding and regulating the photosynthetic capacity and the ROS (reactive oxidative species) scavenging capacity by upregulation of Rubisco activase and ascorbate peroxidase, respectively (Wang et al., 2014). Histone modifications (mainly epigenetic marks) regulate the rate of transcription and hence acts as putative markers for drought stress memory (Conrath et al., 2015). The epigenetic changes provide flexibility to the genome for conveying persistent stress memory (Van Oosten et al., 2014). The signaling pathway proteins, such as MITOGEN-ACTIVATED PROTEIN KINASES (MAPK), transduce signals to the chromatin structure via chromatin-modifying enzymes. Hence, chromatin acts as memory “storage” for the reprogrammed expression, derived from the interactions of signal transduction pathways and transcription factors (Johnson & Dent, 2013). The criterion to confirm the presence of drought stress transcriptional memory genes is that the level of transcripts or transcription factors (imparting drought tolerance) produced at recurring stresses (after recovery) should be significantly different from that at first stress, provided enough recovery period is given (Ding et al., 2012; Liu et al., 2014) as shown in Figure 1. This represents the phenomenon of genomic imprinting. In the case of non-stress memory genes, the level of transcripts/proteins remains similar during the first and recurring stresses. The other difference is the presence of a maintained state of chromatin modifications known as memory marks, like the histone methylation H3K4me3 (Ding et al., 2012), in drought stress memory genes. The imprints (modifications) remaining after removal of the stress and maintaining their ability to regulate transcription (for drought memory genes) after the recovery period and during subsequent stress are true memory marks. For example, the histone modificationsH3K4me3in the drought stress memory genes during the first drought stress (Ding et al., 2012; Jaskiewicz et al., 2011) is a classical example of an epigenetic mark; although its level decreases during the recovery period, it still remains as effective as under the previous stress. Such memory marks decide the fate of the transcriptional regulation upon incidents of future drought stresses as revealed by diverse whole-genome transcriptome patterns in Arabidopsis (Ding et al., 2013). However, the demethylation removes H3K4me3 in some genes and thus fails to regulate the transcriptional activity during future stress and, hence, those genes cannot qualify as true drought stress memory genes (Kim et al., 2012).

The physiological and transcriptional type of memory can be better understood using advanced omics approaches. For example, recently, Forestan et al. (2020) carried out transcriptome and genome-wide histone modifications in maize subjected to mild and prolonged drought stress (prior to flowering) and revealed the presence of transcriptional memory genes (maintenance of the stress tolerance-associated transcripts during recovery period) and epigenetic memory candidate genes (stress-induced chromatin changes persisting longer than the stimulus). In addition, the study identified a new type of stress memory genes known as delayed memory genes (expressed as a delayed response to stress, instead of immediate response). The examples of transcriptional memory genes in maize are ZEP1, NCED6, and AP2/EREBP, NAC and WRKY transcription factors family, whereas MADS4 and MADS15 are delayed memory genes. Furthermore, Liu et al. (2014) coined the term “super-induced transcription memory genes” in Arabidopsis to indicate genes that have the ability to stimulate higher transcript production in subsequent drought stresses. Histone modifications (epigenetic marks) effectively regulate the rate of transcription (Conrath et al., 2015). For example, enhanced expression of H3K4me3 led to the activation of two stress memory genes, RD29B and RAB18 (Ding et al., 2012). Ding et al. (2013) found four different memory patterns involving >2000 drought stress memory genes. Further, the gene ontology analysis indicated four general approaches for drought stress memories: (1) damage repair and detoxification, (2) photosynthetic machinery maintenance, (3) homeostasis for adaptation, and (4) interactions between drought and other stresses (like waterlogging and heat) pathways. The complexity of the coordinated reaction for drought stress memory arises from the presence of a combination of genes that govern a specific physiological function and the genes that govern many different physiological functions, including the intra-genic interactions. For example, Virlouvet et al. (2018) carried out a co-expression network analysis for repeated drought stress in maize and reported the presence of 10 gene modules for specific physiological processes, like photosynthetic and photo-protective responses. In addition, one gene module exhibited a strong correlation with different processes, suggesting the presence of coordinated responses across networks. Further, a comparative study on transcriptomic responses in maize and Arabidopsis confirmed the presence of conserved acclimation and species-specific gene regulation patterns, drought stress memory and responses (Ding et al., 2014). MicroRNAs (miRNAs) are 20–22 nt long RNA responsible for the post-transcriptional gene silencing via the upregulation or downregulation of the target genes. To impart drought tolerance, drought downregulates the miR169 in Arabidopsis (Li et al., 2010), whereas upregulates miR398 and miR408 in Medicago truncatula (Trindade et al., 2010). Post-transcriptional modifications, like enhanced acetylation of H3 in drought stress-responsive genes in Arabidopsis (Kim et al., 2008) and activation of AhDREB1, an APETALA2/Ethylene Respond Factor (AP2/ERF2) in peanut (Arachis hypogaea), impart drought tolerance (Zhang et al., 2018). ARABIDOPSIS HOMOLOG OF TRITHORAX1 (ATX1) is a histone methyltransferase conveying drought stress memory by H3K4me3 deposition on target genes (Ding et al., 2012). The affinity of ATX1 to bind NINE-CIS-EPOXYCAROTENOID DIOXYGENASE 3 (involved in ABA biosynthesis) increases due to its methylation. It hence regulates the expression of the ABA-dependent and ABA-independent drought stress genes (Qin & Zeevaart, 1999). Therefore, acetylation and methylation of histones are epigenetic marks for drought stress memory, although the former is more prevalent (Hyun et al., 2017). Li et al. (2019) studied gene expression by whole-transcriptome strand-specific RNA sequencing (ssRNA-seq) for recurrent drought in rice. They revealed the involvement of long noncoding RNA, DNA methylation, and abscisic acid for imparting short-term drought memory. These changes act as memory imprints and induce drought-related memory transcripts (photosynthesis and proline biosynthesis) when encountering further drought stress.
Another mechanism for epigenetic marks (on chromatin structure)-based memory development is through the association of the transcriptional machinery with stalled RNA Polymerase II (Ding et al., 2012). This ensures that RNA Polymerase II is readily available for the transcription of genes associated with stress memory. Recently, the involvement of certain proteins, which remain in the cell for a long time, was found associated with memory response (Schneider et al., 2019). These proteins had a slow rate of turnover, thus, remained in the metabolism for a longer period of time.
4 MEMORY AND TRADEOFFS
To deploy and manage stress memory for increasing crop productivity, it is important to characterize the nature of low moisture stresses against which the tolerance needs to be built. Several studies on water-stress across different locations and different time points, including future predictions, were conducted (Kholová et al., 2013; Watson et al., 2017). Low moisture stresses are highly variable, differing in the onset, intensity, and stages of crop phenology when they occur. Projections indicate that the frequency of drought stress incidences will rise in the future (Naumann et al., 2018; Spinoni et al., 2018). This, together with the possibilities of recurrent stress episodes, makes the utilization of “stress memory” a promising strategy. However, at the same time, it is important to understand the tradeoffs that will accompany drought stress memory in plants (Skirycz & Inzé, 2010). For example, stomatal conductance and non-photochemical quenching were found to have contrasting memory patterns, indicating the existence of tradeoffs for metabolic costs against cellular protection. Therefore, to build drought tolerance, stress memory imposes adverse effects (costs) resulting from an altered metabolism (Crisp et al., 2016). As a result, plants have developed the mechanism of erasing or resetting the unfitting memories (which are associated with a metabolic cost if maintained for a long time) via a mechanism similar to cell cycle checkpoints (Cools & De Veylder, 2009). For example, decrease in DNA METHYLATION 1 (DDM1) and MORPHEUS' MOLECULE 1 (MOM1) regulates the erasing or resetting of the transgenerational memory (Iwasaki & Paszkowski, 2014). The resetting of the memory brings most of the transcripts, stress-associated proteins, and metabolites to a prestress state (Crisp et al., 2016). This helps the plant to harness the favorable conditions (stress-free environment) by exhibiting maximum growth via photo-assimilate synthesis. Persistence of the memory may lead to a metabolically-expensive liability of chromatin marks that, in the absence of future events of stress, reduce the overall performance of plants. Figure 2 depicts a conceptual framework evaluating the relative significance of utilizing stress memory for drought mitigation. Since the maintenance of memory is associated with metabolic burden, the yield levels can be different in well-watered or fully-irrigated conditions. In the hypothetical framework depicted in Figure 2, the yield at 0S (without stress and stress memory) is higher than the yield at 0S-M (when stress memory is there, but no stress). This can be compared with scenarios where multiple stresses occur, but due to the absence of stress memory (in genotypes that do not code for necessary “memory marks”), yield losses are severe. Hence, there is a need to identify the genotypes with optimized system memory-associated tradeoffs to achieve a higher yield advantage in recurring drought stresses. It is clear that this evaluation needs to be done on a case-by-case basis. Evaluation of local weather conditions, possibility of recurrent low moisture stresses in the eco-geographical region, and availability of locally-adapted germplasm from where the “memory” genetic loci can be introgressed, are expected to be the key parameters. These will determine the rationale of introgressing stress memory in elite cultivars for a specific eco-geographical region.
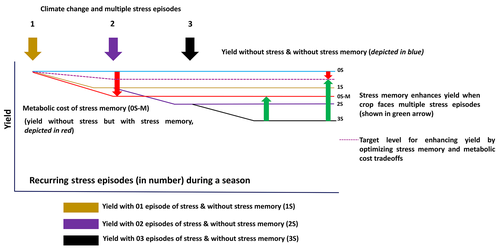
Hence, while characterizing the nature of stress memory, it may be helpful to precisely determine the tradeoffs, in particular the yield penalty, associated with each “memory mark.” Depending on the case scenario, it may be helpful to maintain “bare minimum” stress memory, rather than maintaining the full repertoire of the memory marks. Figure 3 describes the concept, by considering the potential drought tolerance and metabolic burden associated with the maintenance of memory marks. Hence, the genotypes that possess a stress memory giving higher drought tolerance but with lower metabolic costs need to be identified and adopted in drought stress-prone environment.
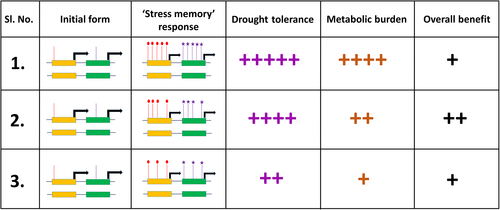
The model presented in Figure 3 can be used to categorize stress memory marks and to decide which genetic loci to deploy, depending on the assessment of the required level of tolerance. Hence, while there is need of properly evaluating the need for stress memory (in case of recurrent droughts in a season), there is a need to categorize the molecular nature of stress memory marks, including the advantage of drought tolerance conferred by each, together with the metabolic energy necessary to maintain each. A detailed understanding of the tradeoffs associated with stress memory and its maintenance will aid decision-making in its effective deployment and assessment.
5 INTEGRATING STRESS MEMORY WITH VARIETAL DEVELOPMENT
While more mechanisms are at play at cellular levels and efforts are ongoing to elucidate them, the knowledge gained so far should be integrated with breeding efforts to develop tolerant varieties. The nature of stress memory in mitigating the next incident of stress episode can be utilized in plant breeding specifically for stress memory. Figure 4 describes how phenomics and genomics can be utilized to incorporate the “stress memory” trait in breeding programs by large-scale germplasm screening. In this case, plants are subjected to stress, followed by a recovery period and a subsequent stress. Plants that are able to better respond to the subsequent stress have a genotype with better potential for deployment of “stress memory.” The transgenerational memory can be well explored for crops, especially self-pollinated crops, as seeds of varieties (with transgenerational stress memory) can be reused for cultivation by farmers. However, reuse of such seed will depend upon the generations up to which memory can last. Promising existing varieties with drought stress memory can be directly recommended in ecologies prone to drought stress. Such genotypes can also be crossed with elite varieties to obtain recombinants possessing better stress memory. In addition, the development of inbreds and chromosome substitution lines from the crossed material can help determine the relative advantage of memory marks in particular genetic segments, as described in Figure 3. Epigenetic breeding can be of great help in understanding the molecular mechanism of the stress memory. The plants with stress memory crossed with that off non-stress memory type and segregation studies (of epigenetic prints) can reveal novel genetic regions for drought stress memory (Banik et al., 2016). Further, the selection in the segregating generations should be focused on the magnitude of drought stress memory and metabolic tradeoffs. In fact, the integration of drought stress memory to the existing ideotype of crops can help to establish the refined drought tolerant ideotype in various crops.
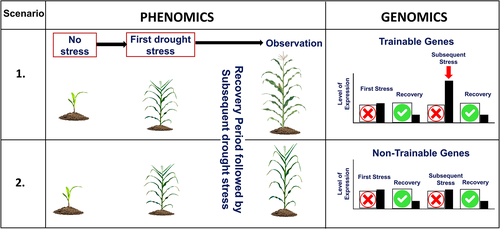
Ding et al. (2012) have classified the genes related to stress memory in two categories: trainable and non-trainable. Both the trainable and non-trainable stress memory-associated genes get transcribed after the onset of stress. Upon rewatering and return to normal conditions, both these gene classes return to the basal level of expression. However, upon a subsequent stress, the transcript levels of trainable genes rise to a higher level, while the transcript level of non-trainable genes does not increase more than the level during the first exposure. In their analysis on Arabidopsis, they showed that genes RD29B and RAB18 are trainable and can be utilized for stress memory, while non-trainable genes like RD29A and COR15A do not contribute to the development of stress memory. Next generation sequencing can help to identify the stress memory-associated trainable genes in the genome. These can be utilized as markers for introgression in locally-adaptable varieties.
A new dimension in epigenetic engineering has arrived with the advanced abilities in CRISPR/Cas9 systems (Pulecio et al., 2017). Cas9 Endonuclease Dead (dCas9) is deficient in endonuclease activity, but capable of homing in a sequence using single guide RNA (sgRNA). dCas9 can be coupled to various proteins to construct molecular editing systems for gene expression or repression and histone modifications. Using this, stress memory marks can be engineered depending upon the occurrence of drought stress events. This would enable the translation of fundamental research in “stress memory” to the applied “stress memory engineering.”
6 CONCLUSIONS
The concept of stress memory can be utilized to develop better crops to cope with low moisture stress. With the possibilities of more frequent stresses due to climate change, it is necessary to effectively deploy “stress memory” in field conditions. It is possible to integrate the “stress memory” trait in breeding programs and develop segregating material that can be screened to obtain a balance between drought tolerance and crop productivity. The integrated approaches of precise phenotyping with advanced approaches, such as methylome, transcriptome and proteome, can help to precisely understand and harness the potential of drought stress memory for crop improvement. The rapidly evolving science present an opportunity to design tailored crops that may be referred to as SMART (Stress Memory Adapted and Resilience Targeted)-Breed crops to utilize this physiological trait for stress management. The biggest hurdle in the process remains in its precise understanding. The selection of the desirable segregation is contingent upon identifying transgressive segregants with desirable memory markers. This can be done through precise phenotyping and subjecting the population to methylome, transcriptome, and proteome analyses. The use of advanced biotechnologies and low cost genomic sequencing will help in delineating the mechanism of memory and utilizing it in cultivar identification. Though a tradeoff exists in the process, it is worth exploiting to develop climate-smart cultivars.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the support received from Indian Council of Agricultural Research.
AUTHOR CONTRIBUTIONS
A.S. and M.C. conceived the idea and formulated the hypothetical framework. A.S. and M.C. prepared the draft and figures. A.S., M.C., and S.R. carried out the proof reading and revision of the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




