Volatile organic compounds produced by Pseudomonas pseudoalcaligenes alleviated drought stress by modulating defense system in maize (Zea mays L.)
Funding information: King Saud University, Riyadh, Saudi Arabia, Grant/Award Number: RSP-2020/180
Abstract
Research on plant growth-promoting bacteria (PGPR) revealed an effective role of bacterial volatile organic compounds (VOCs) in stress alleviation. Out of 15 PGPR strains, infection with VOCs from Pseudomonas pseudoalcaligenes' resulted in maximum germination, growth promotion, and drought tolerance in maize plants. The VOCs of P. pseudoalcaligenes caused induced systemic tolerance in maize plants during 7 days of drought stress. The VOCs exposed plants displayed resistance to drought stress by reducing electrolyte leakage and malondialdehyde content and increasing the synthesis of photosynthetic pigments, proline, and phytohormones contents. Maize plants revealed enhanced resistance by showing higher activities of antioxidant defense enzymes both in shoots and roots under drought stress. Activities of antioxidant enzymes were more pronounced in shoots than roots. Gas chromatography and mass spectrophotometric (GC–MS) analysis comparing VOCs produced by the most efficient P. pseudoalcaligenes strain and inefficient strains of Pseudomonas sp. grown in culture media revealed nine compounds that they had in common. However, dimethyl disulfide, 2,3-butanediol, and 2-pentylfuran were detected only in P. pseudoalcaligenes, indicating these compounds are potential candidates for drought stress induction. Further studies are needed to unravel the molecular mechanisms of VOCs-mediated systemic drought tolerance in plants related to each identified VOC.
1 INTRODUCTION
Maize (Zea mays L.) is one of the highest yielding staple crops in the world. Due to the expeditiously increasing population, the world is facing major food problems. However, maize being stapled crop can share the essential portion for global food security due to associated multiple purpose benefits like industrial usage, animal and human feed etc. (Ghorchiani et al., 2018). Biotic and abiotic stresses are responsible for significant yield losses of maize (Li et al., 2017).
Among the abiotic stresses, drought stress has a huge impact on maize growth by altering its morphological responses and physiological functions (Zhang et al., 2020). Drought stress affects maize during vegetative growth stages as it prolongs the growth stage and conversely, the reproductive growth stage is reduced (Wang et al., 2019). Oxidative stress is part of the plant defense response under water-scarce conditions. Plants produce reactive oxygen species (ROS) in response to stress which causes injury to macromolecules and DNA (Ghosh et al., 2018). Plants have an internal protective enzyme-catalyzed cleanup system to minimize the effects of oxidative stress.
Various strategies have been used to reduce the devastating effects of drought-like water management, such as genetic engineering, and the development of drought-tolerant varieties through conventional breeding (Birhanu et al., 2019). Technical, economic, and ecological limitations associated with these strategies, however, have brought an interest in searching for alternate cheap, natural, and ecofriendly approaches such as plant growth-promoting rhizobacteria (PGPR) (Goswami & Deka, 2020).
PGPR are the soil bacteria that colonize the roots of the plants and aid the plant growth. Their role in plant growth nutrient management and biocontrol activity is very well established (Rincón-Molina et al., 2020; Yasmin et al., 2020; Zeffa et al., 2020). PGPR alter plant defense response under drought by modifying morphological, biochemical, and genetic responses of maize plants (García et al., 2017; Yasmin et al., 2017). PGPR triggers the ROS scavenging machinery to scavenge more cells damaging ROS, ultimately inducing systemic resistance in plants against drought stress (Ghosh et al., 2018; Tayyab et al., 2020).
Previous studies suggested that even without inoculation or physical contact PGPR can trigger growth by releasing fumes (Hashem et al., 2019; Rojas-Solís et al., 2018). These fumes are composed of volatile organic compounds (VOCs) which are products of secondary metabolism in bacteria (Cappellari et al., 2017). They stimulate numerous signal transduction pathways in plants to start a cascade of complex metabolic reactions which not only alleviate the stress signs but also enhance plant growth (Schulz-Bohm et al., 2018). Tahir et al. (2017) reported that 846 possible VOCs produced by 350 bacterial strains and revealed that Bacillus subtilis GBO3 triggers the growth promotion and abiotic stress tolerance in Arabidopsis. It produced albuterol, 3-Methyl-3-buten-1-01, 2-butyl-1-octanol, and dimethyl silanediol. Moreover, Pseudomonas stutzeri E5 and Stenotrophomonas maltophilia CR71 endophytes were reported to produce antifungal VOCs such as S-methylthiobutyrate, isobutyl isothiocyanate, 2-methylthioethanol and DMDS (Cappellari et al., 2017). However, the knowledge about the mechanism of VOC-mediated induce systemic resistance (ISR) in plants against abiotic stresses specifically in drought stress is limited. Therefore, we screened the VOC's producing PGPR and observed their growth-promoting and drought alleviating potential. To the best of our knowledge, this is the first report about the role of bacterial VOC's in drought tolerance of maize by regulating the photosynthetic, antioxidant, and phytohormonal responses. The main objective of this study was to explore the physiological responses of maize plants to bacterial VOCs (BVOC's) to alleviate drought stress.
2 MATERIAL AND METHODS
2.1 Bacterial strains
The 15 PGPR strains used in this study were obtained from the Applied Microbiology and Biotechnology lab (Department of Biosciences, Comsats University Islamabad). Their characteristics and application against different stresses on different crops are given in Table 1. The revival of these strains was done by growing them on Luria-Bertani (LB) medium at 37°C for 24 h (Quiroz-villareal et al., 2012).
| Sr no# | Strain code | Accession number | Strain name | Stress | Crop | Characteristics | Reference |
|---|---|---|---|---|---|---|---|
| 1 | SRM-3 | MG733991 | Bacillus subtilis | Salt stress | Sugarcane | Halotolerant (20% NaCl), ACC deaminase activity, IAA production | Yasmin et al. (2020) |
| 2 | SRM-9 | MG733989 | Bacillus megaterium | Salt stress | Sugarcane | Halotolerant (20% NaCl), ACC deaminase activity, IAA production, siderophore production | Yasmin et al. (2020) |
| 3 | SRM-16 | MG736962 | Pseudomonas pseudoalcaligenes | Salt stress | Sugarcane | Halotolerant (20% NaCl), ACC deaminase activity, IAA production, siderophore production, exopolysaccharide production | Yasmin et al. (2020) |
| 4 | SRM-20 | MG733990 | Bacillus sp. | Salt stress | Sugarcane | Halotolerant (20% NaCl), ACC deaminase activity, IAA production | Yasmin et al. (2020) |
| 5 | MU2 | MG562498 | B. megaterium | Drought stress | Wheat | Siderophore production, phosphorous solubilization, potassium solubilization, exopolysaccharide production | Harun-Or-Rashid et al. (2017) |
| 6 | MU8 | MG012485 | Bacillus licheniformis | Drought stress | Wheat | Siderophore production, phosphorous solubilization, potassium solubilization, exopolysaccharide production | Harun-Or-Rashid et al. (2017) |
| 7 | KFP1 | HQ007938.1 | Pseudomonas aeruginosa | Pyricularia oryzae/Blast pathogen | Rice | Phosphorus solubilization, siderophore production, zinc solubilization | Rais et al. (2016) |
| 8 | KFP2 | HQ007939.1 | P. aeruginosa | P. oryzae/Blast pathogen | Rice | Phosphorus solubilization, siderophore production, zinc solubilization | Rais et al. (2016) |
| 9 | KFP3 | HQ007940.1 | P. aeruginosa | P. oryzae/Blast pathogen | Rice | Phosphorus solubilization, siderophore production, zinc solubilization | Rais et al. (2016) |
| 10 | CW1 | Pseudomonas putida | MT604992 | Fungal stress | Soybean | Antagonist, hydrolytic enzymes production, zinc solubilization | Yasmin et al. (2020) |
| 11 | HY13KI | KJ191560.1 | Proteus sp. | Drought stress | Maize | Siderophore production, phosphorous solubilization, potassium solubilization, zinc solubilization | Yasmin et al. (2017) |
| 12 | HY13KR | KM016981.1 | Pseudomonas sp. | Drought stress | Maize | Siderophore production, phosphorous solubilization, potassium solubilization, zinc solubilization | Yasmin et al. (2017) |
| 13 | HYJW | KT 003271.1 | Bacillus pumilus | Drought stress | Maize | Siderophore production, exopolysaccharide production | Yasmin et al. (2017) |
| 14 | HY8N | KJ191560.1 | Pseudomonas sp. | Drought stress | Maize | Siderophore production, phosphorous solubilization, potassium solubilization | Yasmin et al. (2017) |
| 15 | HY9K | DQ289055.1 | Bacillus cereus | Drought stress | Maize | Siderophore production, phosphate solubilization, potassium solubilization, zinc solubilization | Yasmin et al. (2017) |
2.2 In vitro screening of best VOCs producing PGPR under drought stress
An in vitro experiment was conducted to observe the effects of VOCs produced by all PGPR strains to promote germination and seedling growth of maize. The maize seeds (Soan 3) were collected from the National Agriculture research council, Islamabad. Seeds were treated with 70% ethanol (V/V) for 1 min followed by 10% chlorox (V/V) for 2 min with subsequent washing with sterile water for sterilization (Tayyab et al., 2020). The sterilized seeds were planted on sterilized Petri plates with soaked filter papers. The experiment was divided into two sets, that is irrigated and drought-stressed. A 5 mL solution of PEG 2000 (20%) was applied to the drought stress group while sterile deionized water was used to moisten the irrigated set. The PGPR strains were grown on MS media and incubated at 30°C for 48 h. The Petri plates with PGPR strains and planted seeds were placed in a closed container, that is airtight plastic box with their lids sealed with parafilm. These boxes were placed in the dark for seed germination and subsequently transferred to the growth room for 10 days with light and dark periods of 13/11 h. After 5 days of drought, stress seedling was sampled for further analysis. The effect of BVOCs on plant growth was analyzed by measuring the germination index, promptness index, seedling vigor index, germination percentage, shoot, and root lengths and their fresh and dry weights.
2.3 Pot experiment
A pot experiment was conducted in the greenhouse of COMSATS University, Islamabad provided with rain shelters (33.7294°N, 73.0931°E, average temperature = 15°C, humidity = 64%) to assess the abilities of VOC's released by PGPR to improve growth and drought tolerance of maize (Zea mays L.) plants. For this, a completely randomized design (CRD) was used with three replicates per treatment and five plants per pot in the growing season of maize. The experiment included four treatments: T1 = control, T2 = drought, T3 = VOC's exposed seedlings T4 = VOC's exposed seedlings under drought stress.
The germinated control and BVOCs exposed seedlings (as described in the above section) of equal size were selected and planted in pots (15 × 13 cm) with drainage holes filled with 1.5 kg sterilized soil and clay (3:1). The control set of treatments were irrigated with deionized water until water seeps down from the bottom of pots to attain the field capacity (FC) of 65%–70%. Then watering was done regularly to maintain the FC and moisture level of the irrigated set.
After 3 weeks, the drought stress was subjected by a restrictive water supply to attain 25% of FC at the three-leaf stage of maize seedlings for 7 days. The FC of drought set was maintained by weighing the pots in the early morning using water with an equal volume of lost moisture. After 7 days of drought stress, plants were harvested, and roots and shoots were saved separately. Their phenotypic traits (like length, fresh and dry weights, and leaf area) were recorded. The rest of the samples were saved in −20°C for biochemical and physiological analysis.
2.4 Relative water content
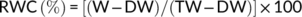
2.5 Photosynthetic pigments
The extraction and quantification of photosynthetic pigments (chlorophyll a, chlorophyll b, and carotenoids) from the leaves were done by following the procedure of Shoaf and Lium (1976). The 0.05 g of the chopped leaf sample was put in 10 mL of dimethyl sulphoxide (DMSO) and kept for shaking in a water bath for 4 h at 65°C. After that, the absorbance of the supernatant was recorded for chlorophyll a, chlorophyll b, and carotenoids at 663, 645, and 480 nm, respectively, on the spectrophotometer.
2.6 Total soluble protein
The method of (Lowry et al., 1951) was used for the assessment of the total protein content of maize plants. The 0.1 g of fresh leaves were ground in 1 mL of phosphate buffer (pH = 7) and was centrifuged at 4200g for 10 min. After that, in the 0.1 mL of supernatant, an equal amount of alkaline CuSO4 reagent was added, followed by mixing for 10 min. Then, 0.1 mL of Follin regent was added in the solution and kept for incubation for 30 min at 28 ± 2°C. The optical density of each sample was noted at 650 nm and phosphate buffer at pH = 7.5 was used for blank reading. The standard curve of bovine serum albumin (BSA) was used as a reference to calculate the amount of protein in samples.
2.7 Proline
The quantification of proline was done by the technique of Bates et al. (1973). The 0.1 g of foliage was ground in 80% ethanol and were incubated in a water bath at 80°C for 1 h. The supernatant was collected after centrifugation at 13,500g for 10 min followed by the addition of 0.5 mL of distilled water and 1 mL of phenol (5%). After the incubation of 1 h, 2.5 mL of sulfuric acid was added and the absorbance of the solution was measured at 490 nm on a spectrophotometer.
2.8 Malondialdehyde and electrolyte leakage
In the lipid peroxidation test, the amount of lipid peroxides was determined by estimating the malondialdehyde (MDA) produced by thiobarbituric acid (TBA) by the procedure described by (Buege & Aust, 1978). Leaf and root samples (0.5 g) of maize plants were crushed and homogenized by 100 mM phosphate buffer (pH = 8) to obtain the final volume of 8 mL. Samples were centrifuged at 11700 g at 4°C for 15 min. Reaction solution (RS) comprised of 5% trichloroacetic acid (TCA) and TBA. The 1.5 mL of enzyme extract and 2.5 mL of RS were warmed up in a water bath for 15 min at 95°C. After cooling the supernatant was collected by centrifuging at 4800 rpm for 10 min. The optical density was recorded at 532 nm on the spectrophotometer. The RS of 5% TCA and 20 mL of TBA was used as a blank standard.
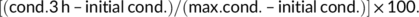
2.9 Total soluble sugars
To enumerate the total soluble sugars (TSS) in maize leaves the method of DuBois et al. (2002) was followed. The leaf extract was prepared by grinding 0.5 g of fresh leaves with deionized water. After centrifugation, phenol (5%) and H2SO4 (98%) were added in the supernatant followed by incubation at room temperature for 60 min. Then optical density was recorded with a spectrophotometer at 485 nm.
2.10 Free amino acids
The free amino acid content of maize leaves was estimated by using the method of Hamilton and Van Slyke (1943). The reaction mixture was composed of leaf extract, 10% pyridine, and 2% ninhydrin solution. It was boiled in a water bath for half an hour and optical density was recorded at 570 nm using a spectrophotometer.
2.11 ROS-scavenging enzyme analysis
2.11.1 Enzyme extracts preparation
For sample preparation and conduction of biochemical assays, 0.5 g of each sample (shoots and roots) was separately ground in 5 mL (100 mM) phosphate buffer with adjusted pH 7.8. Then, the ground sample was centrifuge at 11700g for 20 min. The supernatant was saved and stored at 4°C away from the light for the analysis of biochemical assays to avoid enzyme degradation.
2.11.2 Superoxide dismutase activity
The protocol of Beyer and Fridovich (1987) was followed for the estimation of superoxide dismutase activity (SOD) activity. The rate of inhibition in the photoreduction of nitro blue tetrazolium (NBT) is a measure of SOD enzyme activity. The reaction mixture (RM) was formed by the combination of 50 mM sodium phosphate buffer (pH 7), 0.1 mM EDTA, 50 mM sodium carbonate, 12 mM L-methionine, 50 μM NBT, 10 μM riboflavin and 100 μL crude extract. The standard solutions were used to prepare dark and light controls containing H2O (0.25 mL) and RM (2.5 mL). All the treatments were kept in light under the conditions of 100 μmol photons m−2 s−1 for 20 min while the dark samples were kept in 100% dark conditions. The samples were recorded on a spectrophotometer at 560 nm.
2.11.3 Catalase activity
Catalase activity (CAT) was done by the technique of (Mendoza et al., 2018) with few alterations. The 0.1 mL of 300 mM H2O2, 2.8 mL of 50 mM phosphate buffer, 0 and 0.1 mL of extracted enzyme solution was combined as a reaction mixture. The absorbance was measured at 240 nm at 0 min and 3 min.
2.11.4 Ascorbate peroxidase activity
The technique of (Starlin & Gopalakrishnan, 2013) will be followed to estimate the activity of ascorbate peroxidase activity (APX). The reagents used were 0.1 mL enzyme extract, 2.7 mL 50 mM of phosphate buffer, 0.1 mL 7.5 mM ascorbate peroxide and 0.1 mL of 300 mM H2O2. For zero reading, the standard reaction solution (RS) prepared contained distilled water instead of enzyme solution. The optical density was taken at the time range of 0–60 s at 290 nm.
2.11.5 Polyphenol oxidase activity
The technique of Kahn (1975) was adapted to measure the activity of the polyphenol oxidase activity (PPO) enzyme. The composition of RS was enzyme extract (200 μL), 0.1 M sodium phosphate buffer (pH = 6.5) (1.5 mL), and 0.01 M catechol (200 μL). The composition of the blank mixture was identical to RS except distilled water was used as an alternative to the enzyme extract. The absorbance was recorded at 496 nm, at the time intervals of 30 s for 3 min by spectrophotometer. The activity of PPO was computed as a variation in absorbance at 485 nm min-1 mg−1 protein.
2.11.6 Phenylalanine ammonia lyase activity
The method of (Suzuki et al., 2003) was adapted to analyze the phenylalanine ammonia lyase activity (PAL) activity of the maize leaves and roots. The RS consisted of 30 μL of enzyme extract, 300 μL distilled, and 670 μL of 3 mM L-phenylalanine. The composition of the blank mixture was the same except distilled water was used in place of enzyme solution. The absorbance was taken at the intervals of the 30s for 3 min at 270 nm.
2.11.7 Peroxidase activity
The peroxidase activity (POD) activity was estimated by the technique of (Vetter et al., 1958). The composition of RS was 1% H2O2, enzyme extract, and 0.05 M pyrogallol at the ratio of 1:1:3. The composition of the blank mixture was the same except the enzyme solution was substituted with 0.5 mL of 0.1 M phosphate buffer (pH = 7). Incubation of RS at 28 ± 2°C for 10 min was done. Then, the optical density of RS was recorded at 240 nm after the intervals of 30 s, 1 min, and 3 min and.
2.11.8 Phytohormones analysis
The phytohormones (IAA, GA, and ABA) contents in the leaves of maize plants were done by extracting them in 80% methanol by using the technique described by Kettner and Dörffling (1995). The extracted solvent was saved and replaced with an equal volume of fresh methanol successively, for 3 days. Then, after centrifugation at 10,600g a thin-film rotary evaporator (RFE) was used to reduce the 50% volume of the supernatant at 35°C. The four-times half volume of ethyl acetate was used for portioning of the extract (pH 2.3–3.0). After that, extracted ethyl acetate was dehydrated with RFE and dissolved in 100% pure methanol (1 mL). High-performance liquid chromatography (HPLC) (Agilent 1100, Germany) was used for the analysis of the sample. A UV- detector, C-18 column and mobile phase comprised of methanol, acetic acid, and water (30:1:70) was used. The wavelength for IAA was 280 for GA and ABA was 254 (Li et al., 2015; Sarwar et al., 1992). Quantification of these hormones was done using peak area and retention time with reference to the standards of respective hormones.
2.11.9 GC–MS profile of VOCs produced by rhizobacteria
To collect the VOCs produced by Pseudomonas pseudoalcaligenes, freshly grown cells were inoculated in MS agar and incubated at 28°C for 5 days. After that, divinylbenzene/PDMS (DCP, 50/30 mm) solid-phase microextraction (SPME) FIBER (Supelco, Bellefonta) was inserted in the headspace of the vial containing the bacteria and incubated for 30 min at 50°C. The removal of SPME fibers was done at 22°C for 5 min and GC–MS (Agilent® 6890 Gas Chromatography coupled with Time-of-flight Mass Spectrometry and equipped with silica capillary column) was run for 25 min with helium gas as a carrier (flow rate 1 mL min−1). The column's initial temperature was 35°C for 3 min which was increased to 180 and 240°C at the final stages for 10 and 5 min, respectively. All the compounds released by P. pseudoalcaligenes were identified by comparing them with NIST/EPA/NIH MS library for compounds identification/profiling developed since 2004 (Rath et al., 2018).
2.11.10 Data analysis
Data were statistically evaluated by the technique of one-way analysis of variance (ANOVA) by software Statistix (version 8.1). A comparison between the means of three replicates was done using the least significant difference (LSD) technique at P ≤ 0.05 (Steel and Torrie, 1962).
3 RESULTS
3.1 Effect of VOCs produced by PGPR strains on germination and growth of maize (Zea mays L.) in an in vitro experiment
VOCs of all the tested PGPR strains exhibited a marked increase in the germination of exposed maize seeds except Pseudomonas sp. (HY13KR) in vitro. Drought caused a decline in germination percentage, germination index, promptness index, and seedling vigor index by 7.5%, 36.6%, 40%, and 22.31% as compared to the irrigated control. VOCs exposed plants showed a significant rise in germination percentage and germination index in irrigated control ranged from 11.11% to 108.8% and 63.6% to 245.4% and in stressed group from 8.2% to 104.3% and 71.4% to 321.4%, respectively. However, under drought stress, VOCs of P. pseudoalcaligenes increased germination percentage and germination index by 104%–321% compared to the non-exposed control under a stressed condition (Table 2).
| Germination percentage | Germination index | Promptness index | Seedling vigor index | |||||
|---|---|---|---|---|---|---|---|---|
| Control | PEG (20%) | Control | PEG (20%) | Control | PEG (20%) | Control | PEG (20%) | |
| Strain name | 45 m | 41.6 n | 2.2 n | 1.4 p | 4.5 r | 2.7 t | 251 p | 195 r |
| Bacillus subtilis | 56 i | 48 k | 4.1 i | 2.9 l | 7.2 m | 4.3 r | 334 l | 287 o |
| Bacillus megaterium | 68 e | 60 g | 5.3 e | 4.1 i | 9.7 f | 6.8 n | 422 h | 383 j |
| Pseudomonas pseudoalcalignes | 94 a | 85 b | 7.6 a | 5.9 d | 12.33 a | 8.75 h | 661 a | 483 f |
| B. megaterium | 69 e | 61 g | 5.4 e | 4.2 i | 9.8 e | 6.9 n | 499 e | 388 j |
| Bacillus licheniforms | 55 i | 48 k | 4 i | 2.8 l | 7.1 m | 4.1 r | 332 m | 286 o |
| Pseudomonas aeruginosa | 51 j | 46 l | 3.7 j | 2.5 m | 6.4 o | 3.7 s | 330 m | 283 o |
| P. aeruginosa | 50 j | 45 l | 3.7 j | 2.5 m | 6.3 o | 3.6 s | 297 n | 229 q |
| P. aeruginosa | 50 j | 45 l | 3.6 j | 2.4 m | 6.3 o | 3.6 s | 295 n | 221 q |
| Pseudomonas putida | 59 h | 55 i | 4.4 h | 3.2 k | 8.5 i | 5.5 q | 396 j | 320 n |
| Proteus sp. | 64 f | 58 h | 4.9 f | 3.7 j | 9.2 g | 6.1 p | 412 i | 351 k |
| Pseudomonas sp. | 38 o | 36 o | 1.9 o | 1.2 q | 1.9 u | 1.5 v | 178 s | 122 t |
| Bacillus pumilus | 72 d | 63 f | 5.8 d | 4.7 g | 10.7 d | 7.3 l | 535 d | 409 i |
| Pseudomonas sp. | 60 g | 56 i | 4.5 h | 3.3 k | 8.6 i | 5.6 q | 398 j | 322 n |
| Bacillus cereus | 78 c | 67 e | 6.8 b | 5.3 e | 11.1 b | 8.1 j | 61 b | 445 g |
Exposure of plants to VOCs improved promptness index and seedling vigor index in irrigated by 40%–174% and 17%–163.3% and in stressed group by 33.3%–224% and 13.3%–147.7%, respectively, ranges from in comparison to non-exposed plants. However, a maximum increase in promptness index and seedling vigor index by 224% and 147% of plants were exhibited by VOCs of P. pseudoalcaligenes under drought stress than the non-inoculated control (Table 2).
3.2 Plant biomass
VOCs of all the tested PGPR strains revealed an obvious increase in the biomass of exposed maize except Pseudomonas sp. (HY13KR). Exposure of drought stress exhibited a decrease in shoot and root length by 15% and 20%, respectively, as compared to irrigated control. Shoot and root length of VOCs exposed plants increased by 33.3%–148.7% and 20%–131%, respectively, and in stressed group by 24.2%–118.2% and 30.5%–133.3% compared to the non-exposed control. Maximum increase in shoot and root length was shown by VOCs of P. pseudoalcaligenes with an increase by 118.2% and 133.3% relative to the non-exposed plants exposed to drought stress (Table 3).
| Shoot length (cm) | Root length (cm) | Fresh weight (g) | Dry weight (g) | |||||
|---|---|---|---|---|---|---|---|---|
| Control | PEG (20%) | Control | PEG (20%) | Control | PEG (20%) | Control | PEG (20%) | |
| Strain name | 3.9 s | 3.3 t | 4.5 s | 3.6 t | 0.38 r | 0.29 s | 0.27 l | 0.12 n |
| Bacillus subtilis | 5.5 n | 4.8 q | 6.4 k | 5.2 p | 0.71 j | 0.51 o | 0.39 g | 0.21 n |
| Bacillus megaterium | 7.6 d | 5.8 m | 8.8 e | 6 m | 0.98 d | 0.7 j | 0.5 d | 0.3 kj |
| Pseudomonas pseudoalcalignes | 9.7 a | 7.2 e | 10.4 a | 8.4 f | 1.3 a | 0.94 e | 0.7 d | 0.41 a |
| B. megaterium | 7.6 d | 5.9 i | 9.2 d | 6.2 i | 0.98 d | 0.71 j | 0.54 c | 0.31 j |
| Bacillus licheniforms | 5.5 n | 4.8 q | 6.3 k | 5.1 p | 0.7 j | 0.49 p | 0.39 g | 0.21 n |
| Pseudomonas aeruginosa | 5.3 o | 4.2 r | 5.9 n | 4.9 q | 0.66 k | 0.42 q | 0.34 i | 0.19 o |
| P. aeruginosa | 5.3 o | 4.1 r | 5.5 o | 4.7 r | 0.65 k | 0.41 q | 0.43 i | 0.18 o |
| P. aeruginosa | 5.2 o | 4.2 r | 5.4 o | 4.7 r | 0.65 k | 0.41 q | 0.31 j | 0.16 p |
| Pseudomonas putida | 6.9 f | 5.1 p | 7.2 i | 5.4 o | 0.76 i | 0.5 n | 0.42 f | 0.23 m |
| Proteus sp. | 7.1 e | 5.5 n | 8.2 g | 5.4 n | 0.82 g | 0.63 l | 0.45 e | 0.28 l |
| Pseudomonas sp. | 3.5 u | 2.5 v | 3.2 u | 2.8 v | 0.32 t | 0.25 u | 0.24 m | 0.1 q |
| Bacillus pumilus | 8.5 c | 6.2 h | 9.6 c | 6.7 j | 1.13 c | 0.79 h | 0.55 c | 0.31 j |
| Pseudomonas sp. | 6.9 f | 5.1 p | 7.8 h | 5.4 o | 0.76 i | 0.59 m | 0.45 e | 0.26 l |
| Bacillus cereus | 9.1 b | 6.7 g | 10.1 b | 7.1 i | 1.22 b | 0.87 f | 0.66 a | 0.37 h |
Drought stress decreased fresh and dry weight by 23% and 55% in non-treated plants compared to the irrigated control. Application of VOCs improved fresh and dry weight of plants by 71%–242.1%, and 14.8%–159.2% in irrigated while in stressed group caused an increase by 41.4%–224.1% and 33.3%–241.6%. VOCs of P. pseudoalcaligenes showed a maximum increase in fresh and dry weight by 224% and 241% of plants, respectively, as compared to the non-inoculated control under a stressed condition (Table 3).
VOC's produced by P. pseudoalcaligenes alleviate drought stress in maize (Zea mays L.) plants. The VOC's produced by rhizobacterial strain P. pseudoalcaligenes helped in the reduction of stress symptoms and growth promotion in association with variations in phenotypic and physiological features in the maize plants. Drought stress showed a visibly negative impact on maize seedlings like pale or gray leaves with wilted and rolled edges. However, VOC's exposed seedlings showed a pronounced reduction in the symptoms of drought stress on plants.
3.3 Root and shoot length
Drought stress reduced the length of roots and shoots of maize seedlings significantly by 16.6% and 16.1% as compared to the irrigated control. Exposure of irrigated maize seedlings to VOC's of P. pseudoalcaligenes increased the lengths of roots and shoots by 38.5% and 45.2%, respectively, as compared to the unexposed maize seedlings. Whereas, bacterial VOCs showed a marked rise in length of roots and shoots by 32.16% and 50%, respectively, in drought stress than drought control not exposed to VOCs (Figure 1).
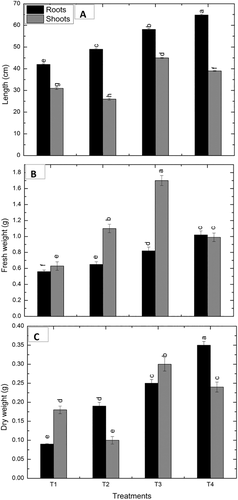
The fresh and dry weight of roots and shoots showed a marked decrease by 16, 111, and 42, 44% under induced drought. Fresh weight of roots and shoots was increased by 46.4% and 54.5%, respectively, in VOCs exposed irrigated maize seedlings as compared to the non-treated control. Drought affected VOCs exposed seedlings showed an obvious rise in fresh weight of roots and shoots by 56.9% and 57.1%, respectively (Figure 1).
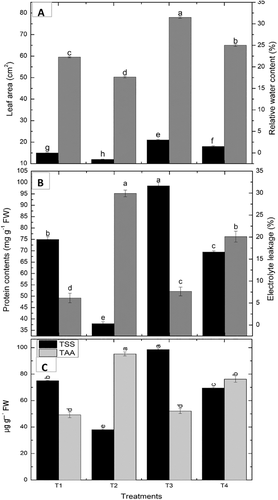
In non-stressed conditions, a remarkable increase in dry weight of roots, and shoots by 177.7% and 66.7%, respectively was observed by VOCs exposed maize seedlings, compared to the non-treated control. However, the dry weight of roots and shoots was increased by 84.2% and 140%, respectively, in drought-stressed, VOCs exposed maize seedlings relative to the non-exposed control. Similarly, the leaf area of VOCs exposed maize plants was higher than the non-exposed plants under irrigated and drought stress as compared to their respective controls (Figure 2).
3.4 Relative water content
Plants subjected to drought stress showed an obvious decrease in water content of leaves by 15.5% which was visible in the form of wilting. Seedlings treated with VOC's showed a marked upsurge in RWC by 31% than the irrigated plants. VOC's exposed seedlings showed improved RWC by 29.2% then the non-exposed control under drought stress (Figure 2).
3.5 Protein
An obvious decline in the protein contents by 49.3% was observed in plants subjected to drought stress. VOC's treated plants showed a marked increase in the protein content by 31.3% relative to the irrigated control. However, in comparison with the non-exposed control plants, a marked upsurge in the level of proteins by 82% was manifested by BVOC's exposed plants as compared to non-exposed drought-affected plants (Figure 2).
3.6 Total soluble sugars
Drought stress negatively impacted the level of TSS causing a reduction of 49.3% compared the irrigated plants. A marked upsurge in the TSS was revealed by VOC's treated plants by 31.3% in well-watered conditions as compared to the non-treated control. However, VOC's maize plants exposed to drought stress showed a significant decrease in TSS content by 82.9% then the non-exposed seedlings (Figure 2).
3.7 Total amino acids
A clear decline in the level of TAA by 93.5% was detected in plants subjected to drought stress. VOC's treated plants showed an increase in the TAA content by 6% then the irrigated control. Whereas, in comparison with the non-exposed control plants a marked increase in the level of TAA by 20% was manifested by BVOC's exposed plants under drought stress (Figure 2).
3.8 Electrolyte leakage (EL) and MDA
It was observed that EL increased in drought exposed maize plants by 93% then the irrigated control. Exposure of maize seedlings to VOCs revealed improved drought tolerance which is manifested by a marked reduction in the EL by 20% under drought stress than the non-exposed control plants (Figure 2).
The activity of MDA in the roots and shoots of maize seedlings was increased by 343 and 136%, respectively, under drought stress. In the case of irrigated conditions, VOCs exposed maize seedlings; P. pseudoalcaligenes reduced the activity of MDA significantly in shoots by 34.5% and 62% in roots, as compared to the non-exposed seedlings. However, under drought stress, in VOCs exposed seedlings, MDA activity was enhanced by 47.16% in roots and 24.8% in shoots, as compared to the non-exposed seedlings (Figure 3).
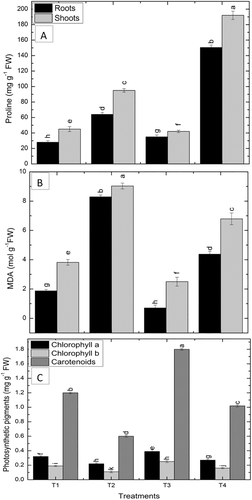
3.9 Proline
The quantity of roots and shoot proline significantly increased in the drought-affected seedlings by 73, 101% then the irrigated control. An obvious decrease was observed by the VOC's by 25% in roots and 6.6% in shoots of proline synthesis as compared to the non-treated control plants under irrigated conditions. Maize seedlings exposed to VOC's exhibited an increase by 135% in roots and 102% in shoots in proline contents than the control plants under drought stress than the drought control (Figure 3).
3.10 Photosynthetic pigments
Drought decreased chlorophyll a, b, and carotenoids contents by 31%, 65%, and 50%, respectively, as compared to irrigated control. VOCs exposed maize plants revealed a noteworthy rise in the synthesis of photosynthetic pigments, that is chlorophyll a, b, and carotenoids by 22%, 31%, and 50% as compared to the irrigated non-exposed control plants. Under drought stress, an obvious surge in chlorophyll a, b, and carotenoids content was manifested by 23%, 45%, and 70%, respectively, by VOCs exposed plants then the drought-affected plants (Figure 3).
3.11 VOCs produced by PGPR modulated the antioxidant defense system of maize (Zea mays L.) plants under drought stress
3.11.1 Superoxide dismutase
In non-stressed conditions, VOCs exposed maize seedlings, the significant increase in the SOD activity was observed by 48.2% in roots and 37.6% in shoots, as compared to VOCs unexposed seedlings. SOD activity was enhanced in drought-stressed conditions. Best results were shown by VOCs exposed seedlings which showed a further obvious increase by 25.7% in roots and 19.7% in shoots, as compared to non-exposed seedlings (Figure 4).
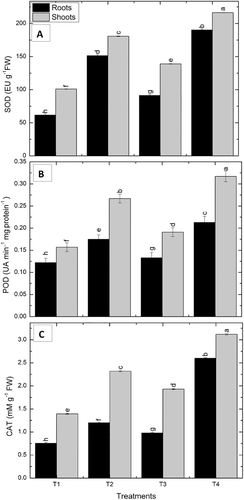
3.11.2 Peroxidase
Enhanced activity of POD was observed in drought-stressed maize roots and shoots by 43.4% and 70%, respectively, then the irrigated control. An increased POD activity by 9% in roots and 21.6% in shoots of non-stressed, VOCs exposed maize seedlings was observed, as compared to the control seedlings. Drought stress further enhanced the POD activity by 21.7% in roots and 18.8% in shoots in VOCs exposed seedlings as compared to non-exposed seedlings (Figure 4).
3.11.3 Catalase
The drought-affected plants exhibited a marked increase in CAT activity in roots and shoots by 59% and 66.5%, respectively. In VOCs exposed maize seedlings, the increase in the CAT activity was observed by 29.7% in roots and 38.6% in shoots, as compared to VOCs unexposed seedlings under irrigated conditions. However, CAT activity was enhanced in drought-stressed, P. pseudoalcaligenes VOCs exposed seedlings by 116% in roots and 34.5% in shoots, as compared to unexposed, drought-stressed seedlings (Figure 4).
3.11.4 Ascorbate peroxidase
The augmented activity of the APX enzyme was manifested in VOCs exposed maize seedlings under non-stresses and drought-stressed conditions. In the case of non-stressed VOC'S exposed maize seedlings, a higher increase in APX activity was observed in roots and shoots by 12.5% and 31.8%, respectively, as compared to the control non-exposed seedlings. VOCs exposed seedlings induced higher APX activity in roots and shoots by 26.1% and 77.9% as compared to unexposed drought-stressed seedlings under drought stress (Figure 5).
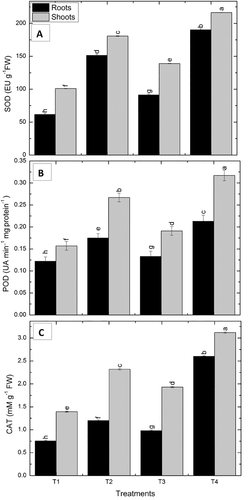
3.11.5 Polyphenol oxidase
The activity of PPO in the drought-affected roots and shoots of maize seedlings was enhanced significantly by 100% and 62.5%, respectively, as compared to non-exposed irrigated control. In the case of unstressed, VOCs exposed maize seedlings; P. pseudoalcaligenes increased the activity of the enzyme by 52% in roots and 75% in shoots, as compared to the unexposed control seedlings. In drought-stressed, VOCs exposed seedlings; the PPO activity was significantly enhanced by 36% in roots and 32% in shoots, as compared to unexposed drought-stressed seedlings (Figure 5).
3.11.6 Phenylalanine ammonia-lyase
Increased activity of PAL enzyme was observed in drought-affected maize roots and shoots by 9.3% and 234% relative to the irrigated control. In the case of non-stressed, VOCs exposed maize seedlings, a 45.4% increase in PAL activity was observed in roots and 61% in shoots, by P. pseudoalcaligenes as compared to control seedlings. Under drought-stressed conditions, VOCs exposed seedlings; the PAL activity was enhanced by 52.5% in roots and significantly increased in shoots, that is 28.8%, which was induced by P. pseudoalcaligenes as compared to unexposed drought-stressed seedlings (Figure 5).
3.12 Phytohormones
Drought stress exposed seedlings exhibited a pronounced decrease in the IAA and GA content by 66.7% and 50% while the level of ABA and CK revealed an obvious upsurge by 64.7% and 42.3%. In well-watered conditions, IAA, ABA, and CK exhibited a clear rise by 255.5%, 48.6%, and 90.8% in the VOCs exposed maize plants in comparison with their respective non-exposed controls. An obvious increase in IAA, ABA, and CK by 666%, 122%, and 67.3% was detected in drought-affected VOCs exposed seedlings then their non-exposed seedlings. In well-watered conditions, GA exhibited a clear rise by 4.8-fold while drought exposed plants showed sevenfold increases in VOCs exposed plants as compared to their respective non-exposed controls (Figure 6).
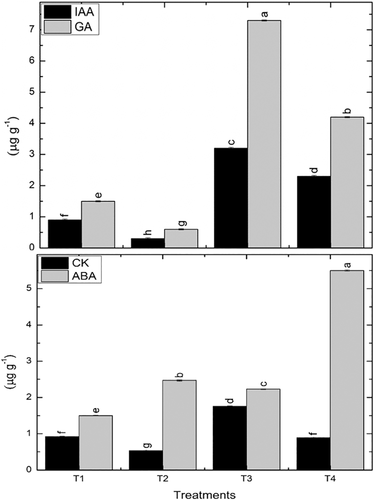
3.13 Identification of volatile organic compound in P. pseudoalcaligenes
Gas chromatography and mass spectrophotometric (GC–MS) analysis of VOCs produced by most efficient strain P. pseudoalcaligenes and non-effective strain Pseudomonas sp. grown in culture media revealed the presence of nine compounds with different retention times (Table 4). All compounds were detected in the fumes of both strains except dimethyl disulfide, 2,3-butanediol, and 2-pentylfuran that were detected only in P. pseudoalcaligenes.
| RT (min) | Name of compounds | Chemical formula | MW | Peak area (%) | |
|---|---|---|---|---|---|
| P. pseudoalcaligenes | Pseudomonas sp. | ||||
| 4.068 | Dimethyl disulfide | C2H6S2 | 94.199 | 8.10 | ND |
| 5.2 | 2,3-butanediol | C4H10O2 | 90.1210 | 12.4 | ND |
| 5.5 | Toluene | C7H8 | 92.1384 | 6.11 | 2.33 |
| 5.8 | 2-Pentylfuran | C9H14O | 138.2069 | 6.5 | ND |
| 6.89 | m-Xylene | C8H10 | 106.1650 | 1.67 | 1.1 |
| 9.60198 | Ethyl hexanoate | C8H16O2 | 144.21 | 1.4 | 1.0 |
| 18.9257 | Ethyl octanoate | C10H20O2 | 172.26 | 2.71 | 1.1 |
| 18.9257 | Phenylethyl acetate | C10H12O2 | 164.2 | 1.03 | 0.7 |
| 30.1194 | Phenylethyl alcohol | C8H10O | 122.17 | 21.57 | 12.7 |
- Abbreviations: MW, molecular weight; ND, not detected; RT, retention time.
4 DISCUSSION
The ability of VOCs produced by P. pseudoalcaligenes was analyzed in a pot experiment to ameliorate the adverse effects of water stress. We found a significant reduction in growth parameters and plant biomass as compared to the VOCs exposed seedlings. Hence, we proposed that VOCs produced by P. pseudoalcaligenes induce a systemic response in maize plants against drought stress conditions. The positive impact of PGPR inoculation on drought stress mitigation on plants has already been reported in many studies (Amna et al., 2020; Azmat et al., 2020; Ghosh et al., 2018; Ilyas et al., 2020; Yasmin et al., 2017).
Cho et al. (2008) has demonstrated the role of bacterial VOCs (BVOCs) on drought stress mitigation in Arabidopsis thaliana. However, the role of BVOCs on alteration in ROS scavenging machinery to impart resistance against drought stress has not yet been studied. In the present investigation, BVOCs exposed maize seedlings were observed to acquire resistance against moisture stress by increasing the biomass, photosynthetic activities, and enhanced activities of antioxidants enzymes like SOD, POD, CAT, APX, PPO, and PAL and phytohormones IAA, GA, CK, and ABA. Here, drought stress inhibits the growth of maize plants in terms of biomass and photosynthetic efficiency as compared to unexposed control. Lack of water availability reduces plant growth by slowing down the metabolic activities and cellular processes, reduction in turgidity, and by causing cell death (Li et al., 2019).
We showed a noticeable reduction in the adverse effects of drought stress on maize seedlings exposed to VOCs of P. pseudoalcaligenes by enhanced growth (length, fresh and dry weight of roots and shoots and leaf area) under drought as compared to unexposed control. These results were in accordance with the results of Bhattacharyya and Kha (2012) who found a marked increase in fresh weight, root and shoot length, the number of lateral leaves, and leaf surface area of Arabidopsis plant exposed to VOCs of P. vulgaris. Several other studies reported similar results for different species of Pseudomonas strain VOCs of P. fluorescens SS101 increased biomass in tobacco plants (Park et al., 2015), P. simiae AU in Glycine max L. (Vaishnav et al., 2016) and P. fluorescens SBW25 (Cheng et al., 2016).
RWC is an indication of the health status of plants. Maize seedlings exhibited a pronounced reduction in RWC on exposure to drought stress for 7 days. However, exposure to VOCs manifested a distinct increase in RWC of the drought-affected plant. This result ties well with the previous study of Cho et al. (2008) who found marked reduction in water loss by doubling the stomatal closure by treatment with VOC (2R, 3R -butanediol) of P. chlororaphis 06 in Arabidopsis plants subjected to drought stress.
Our results cast a new light on the cellular and physiological response of maize seedlings to VOCs both in well water and withheld water conditions. We demonstrated a significant rise in protein, total amino acids (TAA) and total soluble sugar (TSS) content of VOCs exposed seedlings in both irrigated and drought conditions. Kim et al. (2015) and Zhang et al. (2007) reported differential expression of genes associated with the metabolism and cellular processes in BVOCs exposed Arabidopsis seedlings. They have demonstrated upregulation in the enzymes involved in the synthesis of asparagine, and starch in Arabidopsis seedlings exposed to VOCs of GBO3 (Zhang et al., 2007) and chlorophyll a/b binding proteins, cellulose synthase, sucrose transporters, and P-proteins in tobacco seedlings exposed to VOCs of Bacillus subtilis strain (Zhang et al., 2008). However, the current study is first to report the BVOCs exposed plant's responses to drought stress by increasing the protein, TSS, and TAA content.
Water shortage generates stress in the cell membrane which induces permeability resulting in higher electrolyte leakage (EL) while higher malondialdehyde (MDA) induce the peroxidation of the lipid content of membrane. Generally, both EL and MDA indicate the cellular damage caused by the stress in plants (AbdElgawad et al., 2016; Azmat et al., 2020; Cao et al., 2017; Ilyas et al., 2020; Shi et al., 2017). Exposure of maize seedlings to drought stress exhibited elevated levels of EL, proline, and MDA content. These results are directly in line with previous findings (Li et al., 2017; Xu et al., 2016; Yasmin et al., 2017) which observed higher accumulation of proline, MDA and electrolyte leakage in plants under drought stress. Together, the present findings confirmed that drought alleviation in maize seedlings exposed to BVOCs is directly correlated with the reduction of EL and MDA content as well as higher accumulation of proline. These results are in accordance with the findings of Vaishnav et al. (2016) who found BVOCs enhanced salt tolerance in Glycine max L. by increased accumulation of proline which reduced the osmotic stress and decreases the EL and MDA under salinity stress.
In cells, ROS are reduced or oxidized form of molecular oxygen (Azmat et al., 2020; Khan et al., 2020). The function of ROS in the cells is to regulate signal transductions processes as well as act as a toxic by-product of aerobic metabolism (Khan et al., 2020). In plants, drought stress induced the production of ROS which is scavenged by cellular ROS scavenging machinery (You & Chan, 2015). In the case of drought stress, the amount of ROS rises in plant cells at toxic levels, which are scavenged by antioxidant enzymes (Y. Li et al., 2019).
Drought stress generates ROS in cells that harm the important macromolecules like protein and DNA (Ghosh et al., 2018). Plants have well organized ROS scavenging system to protect the plant by detoxification of ROS (Ghosh et al., 2018; Tayyab et al., 2020; Xu et al., 2016; Yasmin et al., 2017). We studied the effects of VOCs produced by PGPR on the activity of ROS scavenging enzymes in drought-stressed plants.
Among ROS scavenging enzymes SOD works in the front line to degrade the peroxide radicles into H2O2 and O2 while CAT degrades the H2O2 into O2 and H2O (Ghosh et al., 2018; Yasmin et al., 2017). Our results demonstrated two things, first elevated levels of antioxidant enzymes SOD, POD, CAT, APX, PPO, and PAL in drought-affected maize seedlings represent that plant defense system triggers to disintegrate the toxic ROS. Secondly, VOCs exposed seedlings further augmented the ROS scavenging machinery and exhibited higher activity of SOD, POD, CAT, APX, PPO, and PAL and reduced the drought stress than non-exposed control. The role of PGPR in the alleviation of drought stress by augmenting the ROS scavenging enzyme system is well documented in the literature (Azmat et al., 2020; Ilyas et al., 2020; Khan et al., 2020; SkZ et al., 2018; Yasmin et al., 2017). However, for the first-time current study showed BVOCs-mediated alleviation of drought stress by modulating ROS scavenging machinery.
Drought tolerance is a complex mechanism in which several metabolic processes interact with each either synergistically or antagonistically to cope up with the stress (Gupta et al., 2020). Of all phytohormones, ABA played a key role in water stress management in plants (Negin & Moshelion, 2016). However, recent studies showed that it is the complex interaction of phytohormones (IAA, ABA, GA, CK) that play a major role to alleviate the adverse effects of drought (Gupta et al., 2020; Liu & Zhang, 2015). Our results have shown that seedlings subjected to drought stress showed an obvious decline in IAA and GA content while a marked increase in levels of ABA and CK than the irrigated control plants. Numerous studies reported the same trend of these phytohormones under drought stress (Gupta et al., 2020; Yasmin et al., 2017; Yoshida et al., 2006; Zhao et al., 2016). We reported maize seedlings exposed to BVOCs showed a prominent increase in growth hormones (IAA and GA) under irrigated and drought conditions than their respective non exposed plants. Cho et al., 2008 demonstrated that Arabidopsis mutants malfunctioning with the phytohormone signaling pathway exposed to VOC (2R, 3R -butanediol) of P. chlororaphis 06 was unable to tolerate drought stress as compared to wild type plants that showed prominent resistance to drought when exposed to VOCs. This clearly showed the mechanism of BVOCs involved with the communication of phytohormones to alleviate the drought stress in plants. Zhang et al. (2008) reported that VOCs of Bacillus subtilis GBO3 upregulated IAA synthesis and responsive genes under salt stress.
ABA plays a prominent role in drought stress tolerance. Contrary to the findings of Cho et al. (2008) and Zhang et al. (2010) who reported that VOCs-mediated drought tolerance is not related to ABA synthesis we found obvious participation with higher accumulation of ABA in drought tolerance of maize seedlings exposed to VOCs. CK can positively and negatively regulate drought tolerance (Gupta et al., 2020). Here we found a positive role of CK accumulation in drought tolerance of maize seedlings which was further augmented by exposure to BVOCs. Reguera et al. (2013) found drought tolerance in rice plants was associated with elevated production of CK. Moreover, Arnaud et al. (2017) reported the exogenous application of CK-mediated drought tolerance in Arabidopsis by strong interaction with ROS scavenging machinery which resulted in higher stomatal closure. This drought tolerance mechanism was independent of ABA. However, it is important to note, that the present evidence relies on the physiological and biochemical quantification of stress indicators, antioxidants, and a detailed study at the molecular level is needed to understand the complex mechanism involved in the signaling of VOCs in growth promotion and drought tolerance. Moreover, application of detected potent VOC of P. pseudoalcaligenes will be studied in the next experiments to analyze its direct effect on plant responses to drought stress.
The effectivity of BVOCs for plant growth promotion and biotic and abiotic stress tolerance depends upon the type of compounds. Tahir et al. (2017) reported 846 potential VOCs secreted by 350 such bacterial strains. Numerous studies reported different active BVOCs directly involved in signal transduction pathways for stress tolerance and growth promotion. For example Ryu et al. (2003) reported mutant deficient in 2,3-butanediol and acetoin production was unable to promote Arabidopsis plant growth. Similarly, Rath et al. (2018) reported that B. subtilis and B. amyloliquefaciens produce 3-hydroxy-2-butanone (acetoin) and 2,3-butanediol that help in plant growth promotion. In the current study, the presence of dimethyl disulfide, 2,3-butanediol, and 2-pentylfuran only in P. pseudoalcaligenes strains indicated that these compounds are directly involved in the systemic induction of drought tolerance in maize plants when plants were exposed to the blend of BVOCs. These results are directly in line with Cho et al. (2008) who reported that 2,3 butanediol was responsible for the alleviation of drought tolerance in Arabidopsis plants. Meldau et al. (2013) reported that dimethyl disulfide produced by rhizobacteria promotes the growth of Nicotiana attenuata by supplementing with the sulfur based nutrients. Moreover, 2-pentylfuran produced by Bacillus megaterium was reported by Zou et al. (2010) which showed a remarkable increase in Arabidopsis plant biomass.
In the current study, the mechanism of action of bacterial VOCs to alleviate drought stress was found to be associated with the systemic modulation of cellular and metabolic processes through signal transduction. Bacterial volatiles induce drought tolerance by lowering the level of ROS in maize leaves and roots by upregulating the activities of antioxidant enzymes and production of phytohormones.
5 CONCLUSIONS
In conclusion, VOCs produced by P. pseudoalcaligenes promotes the growth of the plant and confer the resistance to drought stress in maize. These VOCs elicit the induced systemic resistance against the drought stress by a complex mechanism including modulation of osmolytes, photosynthetic pigments, phytohormones, and antioxidant enzymes activity in plants. Our results provide a basis for further analysis of identified VOCs on plants for growth promotion and drought tolerance and to understand the molecular mechanism of VOCs-mediated systemic tolerance in plants against drought stress.
ACKNOWLEDGMENTS
Mohammed Nasser Alyemeni extends his appreciation to the Researchers Supporting Project Number (RSP-2020/180), King Saud University, Riyadh, Saudi Arabia.
AUTHOR CONTRIBUTIONS
Humaira Yasmin: Supervision, data curation, formal analysis, conceptualization, investigation, writing original draft, review and editing. Urooj Rashid: Investigation, formal analysis, methodology, data curation. Muhammad Nadeem Hassan: Resources, project administration, review and editing, methodology. Asia Nosheen: Project administration, review and editing, methodology, formal analysis. Rabia Naz: Project administration, review and editing, methodology, formal analysis. Noshin Ilyas, Project administration, formal analysis, data curation, review and editing. Muhammad Sajjad: Data curation, formal analysis, Review and editing. Ammar Azmat: formal analysis, methodology, data curation. Mohammed Nasser Alyemeni: formal analysis, review and editing.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request




