Transcription factors as key molecular target to strengthen the drought stress tolerance in plants
Abstract
Amid apprehension of global climate change, crop plants are inevitably confronted with a myriad of abiotic stress factors during their growth that inflicts a serious threat to their development and overall productivity. These abiotic stresses comprise extreme temperature, pH, high saline soil, and drought stress. Among different abiotic stresses, drought is considered the most calamitous stressor with its serious impact on the crops' yield stability. The development of climate-resilient crops that withstands reduced water availability is a major focus of the scientific fraternity to ensure the food security of the sharply increasing population. Numerous studies aim to recognize the key regulators of molecular and biochemical processes associated with drought stress tolerance response. A few potential candidates are now considered as promising targets for crop improvement. Transcription factors act as a key regulatory switch controlling the gene expression of diverse biological processes and, eventually, the metabolic processes. Understanding the role and regulation of the transcription factors will facilitate the crop improvement strategies intending to develop and deliver agronomically-superior crops. Therefore, in this review, we have emphasized the molecular avenues of the transcription factors that can be exploited to engineer drought tolerance potential in crop plants. We have discussed the molecular role of several transcription factors, such as basic leucine zipper (bZIP), dehydration responsive element binding (DREB), DNA binding with one finger (DOF), heat shock factor (HSF), MYB, NAC, TEOSINTE BRANCHED1/CYCLOIDEA/PCF (TCP), and WRKY. We have also highlighted candidate transcription factors that can be used for the development of drought-tolerant crops.
Abbreviations
-
- ABA
-
- abscisic acid
-
- APX
-
- ascorbate peroxidase
-
- bHLH
-
- basic Helix Loop Helix
-
- bZIP
-
- basic leucine zipper
-
- CAT
-
- catalase
-
- CDPK
-
- calcium dependent protein kinase
-
- DHAR
-
- dehydroascorbate reductase
-
- DOF
-
- DNA binding with one finger
-
- DREB
-
- dehydration responsive element binding
-
- GolS
-
- galactinol synthase
-
- HSF
-
- heat shock factor
-
- HSP
-
- heat shock protein
-
- LEA
-
- late embryogenesis abundant protein
-
- MAPK
-
- mitogen activated protein kinase
-
- MDHAR
-
- monodehydroascorbate reductase
-
- MSR
-
- methionine sulfoxide reductase
-
- PIMT
-
- protein L-isoaspartyl methyl transferase
-
- ROS
-
- reactive oxygen species
-
- SOD
-
- superoxide dismutase
-
- TF
-
- transcription factor
1 INTRODUCTION
The molecular and biochemical homeostasis is essential for taking multiple, yet precise, decisions for systematized cellular growth and development in any organism. These processes' functioning is deregulated when exposed to an unfavorable environmental condition that results in the transition of the appropriate growth phase progression into the survival mode (Zhu, 2016). The unfavorable environmental factors that impose a damaging impact on crop productivity can be termed as stress. Based on the agents that impose stress on the plants, these can be broadly classified as biotic and abiotic stress. Stress mediated through the living organism is termed as biotic stress, which includes the fungal, viral, and bacterial infection. Stress imposed by non-living factors, such as drought, salinity, pH, nutrient, and extreme temperature, can be termed as abiotic stress (Suzuki et al., 2014). Among all these stressors, drought stress is one of the major factors contributing to the agrarian distress that not only severely curtails the global economy but also disturbs the livelihoods of a large population by compromising the agronomic growth and productivity of the crop (AghaKouchak et al., 2015). At the field level, drought stress experienced by a crop can be represented as lower precipitation for an extended period that may vary between months to years, depending on the crop genotype. Furthermore, the anthropogenic activities play significant role in the dramatic reduction of soil water level, resulting in a substantial reduction of area suitable for sustainable crop growth. Also, drought stress is often accompanied by high temperature stress, which is a major stressor affecting the reproductive stage with eventual impact on seed set or grain development. Although some natural accessions or specific genotype possessing an intrinsic potential to endure such extreme environmental conditions are available, their acceptability is rather limited due to the lack of one or more agronomically-important traits. Over the years, the conventional plant breeding approach has added several members to the germplasm repertoire for tolerant genotypes. The amalgamation of the breeding with molecular tools and advanced genomics/proteomics approach, that is, the molecular breeding for the trait enhancement strategy, is preferred over any other method. Understanding the molecular mechanisms underlying drought stress is a prerequisite for effective utilization of genetic engineering tools for crop improvement.
Stress perception, signaling, and response by cells are dynamic yet highly regulated processes that comprise diverse molecular players with specific roles. However, stress exposure impairs cellular homeostasis, eventually culminating to cell death. To tackle the suffering, plants tactfully respond by manipulating the cellular system at molecular, physiological, and biochemical levels. These responses are largely dependent on the type of tissue, duration of the stress, and the plant developmental stage at the moment of the stress (Pierik & Testerink, 2014). Some of the key adaptive strategies employed by the plants comprise of enhanced accumulation of osmo-protectants, anti-stress proteins, protein repairing enzymes, reactive oxygen species (ROS) detoxifying enzymes, so on (Kaur et al., 2015; Negi et al., 2017; Salvi et al., 2016; Saxena et al., 2013, 2020). Phytohormones (including abscisic acid, jasmonic acid, cytokinin, so on) also play imperative roles in the drought stress signaling by regulating the aforesaid cellular process in one or the other way. The inter- and intra-crosstalk of these hormones with other signaling or transcriptional module are associated with the plants' drought stress response. All these mechanisms of drought stress adaptation are essentially regulated at the transcription level leading to accumulation of stress-responsive cellular factors. Such molecular plasticity of gene expression is majorly governed by the activation of a specific set of genes encoding transcription factors (Ramanjulu & Bartels, 2002). Transcription factors (TFs) are DNA binding proteins that regulate gene expression by interacting with a pre-initiation complex of transcription and binding in a sequence-specific manner to the cis-regulatory elements present in the promoter regions of the respective genes (Table 1). This interaction results in the activation or inhibition of RNA polymerase, thereby reorienting the gene expression (Nakashima et al., 2007, 2014). This regulatory nature of the TFs makes them key targets for modulating the downstream gene regulatory networks and hence, the development of climate-resilient crops. This review deals with an in-depth analysis of key transcription factors (namely, basic leucine zipper (bZIP), dehydration responsive element binding (DREB), DNA binding with one finger (DOF), heat shock factor (HSF), myeloblastosis (MYB), No Apical Meristem or NAM/Arabidopsis Transcription Activation Factor or ATAF/CUp-shaped Cotyledon or CUC (NAC), TEOSINTE BRANCHED1/CYCLOIDEA/Proliferating Cell Factors or PCF (TCP), and Tryptophan or W, Arginine or R, Lysine or K and Tyrosine or Y containing motif (WRKY) involved in drought stress tolerance in plants. Their structures, DNA binding sites, and mechanisms of action for drought tolerance have been comprehensively focused upon. Moreover, recent efforts involving the utilization of these transcription factors for designing drought-tolerant transgenic plants have been discussed.
| Sl. No | TF family | Distribution | DNA binding sequences (or, cis-element sequences) | Reference(s) |
|---|---|---|---|---|
| 1. | bZIP | Eukaryotes | TACGTA, GACGTC, CACGTG, TGAAAA, GTGAGTCAT |
Glover and Harrison (1995) |
| 2. | DREB | Plants | TACCGACAT, AGCCGCC or GCC box |
Yamaguchi-Shinozaki and Shinozaki (1994), Sakuma et al. (2002) |
| 3. | DOF | Plants | (AT)/AAAG |
Plesch et al. (2001) |
| 4. | HSF | Eukaryotes | AGAANNTTCT |
|
| 5. | MYB | Eukaryotes | CNGTT(A/G) |
Dubos et al. (2010), Ng et al. (2018) |
| 6. | NAC | Plants | CGT(G/A), CACG |
Tran et al. (2004) |
| 7. | TCP | Plants | GGNCCCAC, G(T/C)GGNCCC |
Kosugi and Ohashi (2002) |
| 8. | WRKY | Eukaryotes | TTGAC(C/T) or W-Box, TGCGCTT, TAAAGATTACTAATAGGAA |
Sun et al. (2003), Cai et al. (2008), Bakshi and Oelmüller (2014) |
2 MOLECULAR MECHANISMS OF THE DROUGHT STRESS RESPONSE
Drought stress is accompanied by cellular dehydration that mediates cell damage through secondary stress, such as osmotic and oxidative stress (Dias et al., 2014). Stress perception provoked a signaling cascade resulting in the generation of ROS that imparts a detrimental effect on the macromolecules and negatively affects the cellular functionality (Campalans et al., 1999; Sinha et al., 2015). However, several pieces of research have highlighted the positive role of ROS (up to some extent) to execute the appropriate signaling cascade and metabolic events necessary during drought stress (Manna et al., 2019; Miller et al., 2010). But, prolonged stress exposure results in elevated ROS accumulation, which causes destabilization of the macromolecule and results in compromised cellular activity. Plants have acquired diverse strategies to endure such devastating milieus, such as accumulation of osmolytes, ROS detoxification, and generation of anti-stress proteins. During a stress response, cells lower the water potential (ψ) by osmotic adjustment and increase the solute concentration (Turner, 2018). These solutes do not interfere with the cellular metabolic processes even at higher cytosol concentrations, thus termed as “compatible solutes". These compatible solutes are biomolecules remaining neutral at physiological pH; they majorly belong to amino acids, sugars, quaternary amines, polyhydric alcohol, so on. (Hussain Wani et al., 2013; Yancey, 2005).
ROS detoxification is sustained by an antioxidative defense system that comprises the enzymatic and non-enzymatic components. Along with the SOD (superoxide dismutase) and CAT (catalase), there are four enzymes of the ascorbate-glutathione cycle, which include APX (ascorbate peroxidase), MDHAR (monodehydroascorbate reductase), DHAR (dehydroascorbate reductase), and GR (glutathione reductase) that coordinately regulate the cellular redox status. The non-enzymatic component includes low molecular mass compounds such as ascorbic acid, glutathione, carotenoid, tocopherol, and polyphenols (Negi et al., 2017).
There is a profound enrichment of the anti-stress proteins level during drought stress, which is an important determinant for managing adverse arid circumstances. The intricate network of protein turnover is significantly modulated during drought stress. For instance, there is the induction of HEAT SHOCK PROTEIN (HSP), LATE EMBRYOGENESIS ABUNDANT PROTEIN (LEA), GALACTINOL SYNTHASE (GolS) (Campalans et al., 1999; Kaur et al., 2015; Rao et al., 2018; Salvi et al., 2020). Besides, some protein repairing enzymes, such as protein L-ISOASPARTYL METHYL TRANSFERASE (PIMT) and METHIONINE SULFOXIDE REDUCTASE (MSR), also dynamically respond to counteract the proper functioning of proteins. These protein-repairing enzymes own the distinct capability to exert a profound influence on the enzyme activity by restoring and stabilizing it (Ghosh et al., 2019; Mata-Pérez et al., 2015; Petla et al., 2016). A large body of evidence has represented the regulatory role of phytohormone and their crosstalk during the multitude of stressful events. The raised crosstalk of these phytohormones establishes a systemic response at molecular level, which is a crucial adaptive strategy. Notably, ABA is a key stress hormone that facilitates the major regulation of drought stress adaptation by influencing the induction of stress responsive genes and pathways. ABA was originally recognized as an abscission-promoting hormone but was later found to have a variety of biological functions (Umezawa et al., 2010). Among several important roles played by ABA, mediation of stomatal regulation is crucial in drought stress response. ABA level alteration is a key factor in launching an effectuate response in guard cell ion transport that eventually induces the stomatal closure to reduce water loss (Umezawa et al., 2010). ABA acts as a link between stress perception and cellular transcriptional reprogramming for stress adaptation. This reprogramming is largely governed by transcription factors that play a decisive role in stress responses.
The TFs perceive the environmental stress stimuli through sensors located in the cell wall and plasma membrane of the plant cells. The extracellular signal is then transduced into intracellular ones through various secondary messengers present in cells, such as ROS, cyclic nucleotides (cAMP, cGMP), sugars, calcium ions, inositol phosphate, and nitric oxide (Bhargava & Sawant, 2013). These secondary messengers-mediated signal transduction leads to activation of protein kinases like CALCIUM DEPENDENT PROTEIN KINASES (CDPKs) and MITOGEN ACTIVATED PROTEIN KINASES (MAPKs) and various phosphatases which either activate or repress the activity of the targeted TFs by phosphorylation or de-phosphorylation (Danquah et al., 2014; Huang et al., 2012). Eventually, the stress susceptibility or tolerance in plants is mainly dependent on the coordinated activity of phytohormone and TF that controls the spatio-temporal regulation of the stress-responsive genes.
3 TRANSCRIPTION FACTORS AND THEIR REGULATORY ROLE IN DROUGHT STRESS TOLERANCE
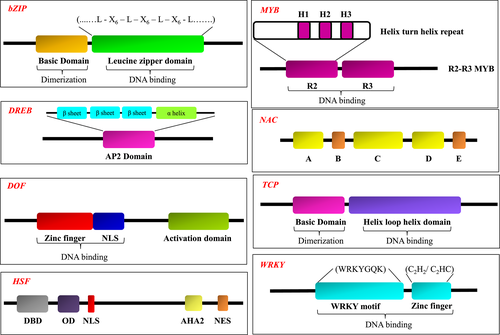
Transcription factors are proteins associated with the transcription of DNA into RNA. They constitute a wide array of proteins (excluding RNA polymerase), that are involved with initiation, regulation, and transcription of genes. TFs can be characterized by the presence of DNA-binding domains (Figure 1) that give them the ability to bind to specific sequences of DNA called promoter or enhancer sequences. Other domains include dimerization domain, activation domain, nuclear localization signal domain, nuclear export domain (Figure 1). The TFs can initiate transcription either by binding to a DNA promoter sequence near the transcription start site or binding to regulatory enhancer sequences present thousands of base pairs upstream or downstream of the gene being transcribed.
Various methods are employed to identify and isolate TFs from plants. The whole-genome sequencing projects of recent times have generated a huge number of sequences and various databases specific to TF sequences are maintained. A few such important databases are REGIA collection (Paz-Ares and Consortium, 2002), PKU Yale collection (Gong et al., 2004), TF only library (Mitsuda et al., 2010). These libraries also maintain the clones of the TFs in GATEWAY-compatible cloning vectors to help researchers validate the TFs and screen them for their role in plant development or stress via their overexpression in various plant systems (Wehner et al., 2011). Various bioinformatics tools are also employed to screen the TF genes from genome-scale protein or nucleotide sequence collections. PlantTFcat is one such high-performance web-based TF analysis tool which is utilized to identify and categorize plant specific TFs. This prediction tool uses comprehensive prediction logics involving conserved domains of various TFs (Dai et al., 2013).
The regulation of gene expression at specific time, tissue, duration, and abundance is a corner stone for the sustainable development of cells. The entire process of gene expression largely entails the reprogramming of DNA topology and energy consumption. It would be a costly affair for the cell to constitutively express the stress-responsive genes as it demands a constant flux of energy for transcription and the unwinding of DNA. The TF acts as a molecular switchboard to facilitate the regulated gene expression. TFs either induce or repress the expression of the downstream genes whenever necessary. There are several reports on the regulation of these regulators having a beneficial impact on the plant physiology against stressful conditions. For example, in Arabidopsis, the promoters region of WRKY TF members possess W box (TTTGAC/T) elements, to which WRKT TFs (WRKY33) bind to its own promoter during pathogen attack and regulate its expression (Birkenbihl et al., 2012; Phukan et al., 2016; Qiu et al., 2008; Rushton et al., 2010). Other WRKY TFs, like WRKY60, WRKY40, and WRKY18 were found to cross-regulate each other to mediate ABA signaling during drought stress (Yan et al., 2013). Similarly, the expression of some MYB TF member genes is also regulated by auto- or cross-regulation. For instance, the expression of MYB10 of apple is auto-regulated and further regulates anthocyanin accumulation, an important secondary metabolite associated with stress endurance (Brendolise et al., 2017; Espley et al., 2009). The expression of some DREB TFs is also regulated by cross-regulation. Chinnusamy et al. (2003) reported that a TF from bHLH (basic Helix Loop Helix) family, ICE1 (INDUCER OF CBF [C-repeat Binding Factor] EXPRESSION 1), binds to the promoter of DREB1C and activates its expression during cold stress (Chinnusamy et al., 2003). Apart from the transcriptional regulation, TFs are also regulated by epigenetic and post-transcriptional regulations that play an important role in modulating the expression of TFs during abiotic stresses. For instance, the expression of ZmNAC111, a positive regulator of drought stress tolerance, is repressed by the promoter methylation of the TF by H3K9 methylase (Mao et al., 2015). In Arabidopsis, the abiotic stress-mediated accumulation of ABA triggers the accumulation of miRNA159 that is further involved in the degradation of MYB33 and MYB101 and implicates in stress susceptibility (Reyes & Chua, 2007). Besides, miRNA164 has been found to degrade a NAC (OMTN) transcript in rice, thereby conferring drought tolerance (Fang et al., 2014). Protein–protein interactions and translocation of proteins to various subcellular compartments also modulate the activities of TFs. In the presence of ABA, WRKY40 (a negative regulator of ABA signaling) interacts with ABA receptor (ABAR) localized on the outer chloroplast membrane leading to the translocation of WRKY40 from the nucleus to chloroplasts, thereby eliminating the chanced of WRKY40-mediated inhibition of the stress-responsive gene expression in the nucleus (Shang et al., 2010). Furthermore, in Arabidopsis, abiotic stress signals facilitate the translocation of bZIP17 and bZIP28 from the endoplasmic reticulum to the Golgi, where they are processed and stimulated to activate the downstream stress-responsive genes in the nucleus (Liu & Howell, 2010; Tajima et al., 2008). In Medicago falacata, an S-palmitoylated NAC TF translocates to the nucleus after dissociating from the plasma membrane to initiate the transcription of various stress-responsive genes during drought stress (Duan et al., 2017). Post-translational modifications, such as MAPKinase-mediated phosphorylation of bZIP, also play a role in activating TFs for downstream gene expression (Banerjee & Roychoudhury, 2017).
When the abiotic stresses withdraw, it is important for the plants to stop the activity of the TFs to prevent futile consumption of energy; TFs are then degraded via the ubiquitin-proteasome system (UPS), which is the most common method of protein degradation in all eukaryotes. For example, the high expression of VuDEREBA2A in cowpea upon desiccation is stopped upon stress withdrawal by ubiquitin-mediated degradation of the TF (Sadhukhan et al., 2014).
In the following section, we will describe the important transcription factors found in plants, with their gene regulatory network, having a role in drought stress tolerance.
3.1 Basic leucine zipper
The basic leucine zipper (bZIP) TF family is a very large class of TF widely distributed in eukaryotes. The characteristic feature of this TF is the presence of a highly conserved bZIP domain, which is 60–80 amino acids long and contains two functional regions: a conserved basic region at N-terminus and a variable leucine zipper region towards the C-terminus (Glover & Harrison, 1995). Genome-wide studies in many plant species have identified numerous bZIP family genes, including 75 in Arabidopsis (Jakoby et al., 2002), 89 in rice (Nijhawan et al., 2008), 125 in maize (Zou et al., 2012), 92 in sorghum (Wang et al., 2011), 89 in barley (Pourabed et al., 2015), 96 in Brachypodium distachyon (Liu & Chu, 2015), 69 in tomato (Li, Fu, et al., 2015a), 160 in soybean (Zhang et al., 2018), 55 in grapevine and 247 in Brassica napus (Zhou et al., 2017).
Extensive studies have revealed the diversified role of the bZIP TF family genes. They play crucial roles in plant developmental processes, like initiation of floral organs, embryogenesis, and seed maturation (Abe et al., 2005; Alonso et al., 2009; Jakoby et al., 2002). However, in plants, they are well known for responding to abiotic stresses, like drought (Xiang et al., 2008), high salinity (Hartmann et al., 2015), and extreme temperature (Liao, Zou, Wang, et al., 2008b) conditions. For instance, transgenic rice plants overexpressing OsbZIP66 conferred drought tolerance (Yoon et al., 2017). Drought, high salinity, and extreme temperature stresses culminate into osmotic stress in the plants. The plant adaptation response to osmotic stress is a complex phenomenon and the precise molecular mechanism behind the water-deficit stress response remains unclear. Nonetheless, the plant hormone abscisic acid (ABA) plays an indispensable role during drought stress by participating in the signal transduction pathway that induces expression of the drought stress-responsive genes (Lee & Luan, 2012). The bZIP genes mainly belonging to group A have been shown to enhance plants' tolerance to abiotic stresses in an ABA-dependent manner. Recent works revealed the roles of group A of bZIP TFs, like AREB2, ABF4, ABF3 ABI5, ABF1, ABF2/AREB1, ABF3, and ABF4 of Arabidopsis thaliana, in positively regulating ABA-dependent gene expression (Choi et al., 2000; Saxena et al., 2013). Similarly, in rice, the bZIP TFs like OsbZIP23, OsbZIP46, TRAB1, OsbZIP2, OsbZIP42, OsbZIP46, OsABF1, and OsABI5 also play an important role in ABA signal transduction and osmotic stress responses (Joo, Lee, & Song, 2019; Yoon et al., 2017; Zou et al., 2008). Furthermore, transgenic Arabidopsis plants overexpressing wheat TabZIP60 or TaZIP14-B were found to impart drought, salt, and freezing stress tolerance in an ABA-dependent manner (Zhang et al., 2015, 2017). Transgenic Arabidopsis and cotton overexpressing cotton GhABF2 have enhanced tolerance to drought and salt stress, which was achieved due to the induction of ABA-, drought- and salt-responsive genes (Liang et al., 2016). Overexpression of sweet potato IbABF4 containing a typical bZIP domain led to improved tolerance to multiple abiotic stresses through the ABA signaling pathway (Ding et al., 2019). Interestingly, the bZIP TFs have also been found to negatively regulate the drought stress response by modulating ABA signaling. For instance, the expression of tomato SlbZIP38 gene was downregulated under salt stress, drought stress, and ABA application and hence, the transgenic tomato plants became drought and salt stress susceptible when SlbZIP38 was overexpressed (Pan et al., 2017). Similarly, the subgroup D bZIP transcription factor CaATBZ1 in pepper negatively modulated ABA signaling and drought stress response and therefore, the CaATBZ1-silenced pepper plants became drought tolerant (Joo, Lim, & Lee, 2019).
3.2 Dehydration responsive element binding (DREB)
The dehydration responsive element (DRE) is a part of the promoter sequence in many stress-responsive genes. The proteins that bind to this DRE were termed as DREB (Yamaguchi-Shinozaki & Shinozaki, 1994). Many DREBs have been identified in various plant species and take part in abiotic stress tolerance in both ABA-independent and ABA-dependent ways. DREB genes comprise of highly conserved AP2/ERF (of 50 to 60 amino acids length) DNA-binding domain (Lata & Prasad, 2011). Though DREB is usually found to recognize the DRE, some of its members recognize the AGCCGCC cis-acting element commonly known as the GCC box (Sakuma et al., 2002). The DREB family TFs have been recognized in various plant species, like Arabidopsis (Sharoni et al., 2010), rice (Nakano et al., 2006), mangrove (Peng et al., 2013), soybean (Marcolino-Gomes et al., 2013) and potato (Bouaziz et al., 2013).
Drought, salt and cold stresses ultimately culminate into a state of dehydration in plant cells resulting in the up-regulation of many TFs, including DREB proteins. Arabidopsis and rice have 57 and 52 DREB TFs, respectively, and have been found to respond to a wide range of abiotic stresses that finally lead to dehydration stress in the plants (Nakano et al., 2006). For instance, Arabidopsis DREB2A has a dual role in combating both water and heat-shock stress responses (Sakuma et al., 2006). When OsDREB1A from rice was overexpressed in Arabidopsis, various stress-inducible genes were upregulated, resulting in the plant's improved tolerance to drought, salt, and freezing stresses. Different DREB proteins are induced by different abiotic stress treatments. For example, AtDREB1 was expressed within ten minutes of cold treatment and provided cold stress tolerance (Liu et al., 1998). But, AtCBF4, a gene encoding DREB TF, was rapidly induced during ABA treatment and drought stress, while cold stress did not induce the gene (Haake et al., 2002). OsDREB1A and OsDREB1B were induced by low temperature treatment but not by ABA treatment. OsDREB1C was constitutively-expressed throughout the stress exposure, whereas OsDREB1D expression was not induced by any stress treatment (Agarwal et al., 2007). OsDREBL accumulated quickly after exposure to low temperature, but it did not respond to ABA, dehydration and NaCl treatments (Chen et al., 2003). WCBF2 gene from wheat was up-regulated by both cold and drought treatments, but ABA treatment had no such effect (Kume et al., 2005). In groundnut, PNDREB1 gene was induced by low temperature and dehydration treatments (Zhang et al., 2009). When DREB TFs are overexpressed in transgenic plants, certain stress-responsive genes are also induced, resulting in overall fitness advantages of the transgenic plants during various environmental stress conditions. For example, Arabidopsis plants over-expressing AtDREB1A revealed that the expression of 12 stress-responsive genes was much higher than in the wild-type plants (Liu et al., 1998). Similarly, transgenic Arabidopsis plants over-expressing the rice OsDREB1A witnessed the increased expression of six stress-related genes (Kasuga et al., 1999). Therefore, DREB TFs are the principal regulators of abiotic stress tolerance in plants. Hence, plants' abiotic stress tolerance level can be significantly enhanced by overexpressing DREB TFs.
3.3 DNA binding with one finger (DOF)
DNA binding with one finger (DOF) transcription factor is a plant-specific transcription factor family widely known to regulate numerous biological processes including vascular tissue formation, abiotic stress, cell cycle, photoperiod regulation, floral organ abscission, redox homeostasis and secondary metabolite production (Diaz et al., 2002; Mena et al., 2002; Skirycz et al., 2008; Yanagisawa, 2002). DOF protein comprises a highly conserved N-terminal DNA binding domain. This DOF domain is a Cys2Cys2 (C2C2) type zinc finger motif of 50–53 amino acid and remained conserved throughout the process of evolution (Yanagisawa, 2002). Besides the highly conserved DOF domain, the transcriptional regulatory C-terminal of this protein is composed of a highly variable amino acid sequence. DOF-TFs specifically bind to a core sequence of (AT)/AAAG at the cis-regulatory element present in their respective target gene (Plesch et al., 2001). Like some other zinc finger, DOF-TF also exhibits a bifunctional role by having both DNA binding and protein binding capability. Several DOF proteins are known to interact with DOF-TF or other biologically important proteins. The intrinsic property of DOF-TF to homo- and heterodimerize and its interaction with other transcription factors (like bZIP, Myb-TF, and TCP-TF) reflect its complex regulatory network (Gupta et al., 2015; Shigyo et al., 2007).
In Arabidopsis, the overexpression and down-regulation (antisense) of AtDOF4.2 depicted a role in the phenylpropanoid pathway by limiting the flavonoid synthesis (Zou et al., 2012). The expression level of several DOF genes is unambiguously upregulated after abiotic stress, including drought stress (Wang et al., 2017). An Arabidopsis DOF-TF (CDF3) is highly upregulated during drought stress and its overexpression is associated with the modulation of some other transcription factors such as CBFs, DREB2A and ZAT12 (Corrales et al., 2017). The T-DNA insertion mutant cdf3 is hypersensitive to the drought stress. The metabolite profiling of overexpression lines also revealed the accumulation of protective molecules like proline, glutamine, sucrose etc. (Corrales et al., 2017). Further, the protein–protein interaction of DOF-TF with MYB, bZIP, or TCP is considered to be important for drought stress response. The comprehensive analysis of the molecular mechanisms underlying the interaction of DOF with drought-responsive TF will be insightful to identify the key player under drought stress. Further, it could provide an immense opportunity to investigate their downstream molecular target and their physiological and molecular response during drought stress.
3.4 Heat shock factor (HSF)
Heat Shock Factors (HSFs) are important transcription factors that are mostly induced by heat stress along with other abiotic stresses like drought, salt and cold. Though HSFs have been identified in a wide range of species, they are widely distributed in plant species. The first plant HSF was identified from tomato and later in many other plant species at genome wide scale. For example, 21 and 24 HSF genes have been identified in Arabidopsis and tomato, respectively (Scharf et al., 2012). HSF is a modular structure; it includes a DNA binding domain (DBD), which is the most conserved domain characterized by a central helix-turn-helix motif. Another important domain is an oligomerization domain (OD) that connects the DBD through a flexible linker of 15–80 amino acid residues. In addition, a nuclear localization signal (NLS), nuclear export signal (NES), activator motifs, repressor domain and functional and signature sequence are also included in the structure of HSF. Based on the flexible linker length and type of amino acid residues between DBD and OD, HSFs have been classified into three categories namely, HSFA, HSFB and HSFC (Kotak et al., 2004). HSFs function by binding to the heat stress promoter element containing a palindromic binding motif (5′-AGAANNTTCT-3′) present upstream to the TATA box of eukaryotic HSF-inducible genes (Scharf et al., 2012). As explicated by their name, they are involved in heat stress, but recent studies have illustrated their potential role in drought stress. Besides, in a field condition, drought stress is usually accompanied with heat stress. Thus, it is reasonable to ponder that plants have evolved with an intriguing transcriptional regulation mechanism to simultaneously mediate the drought- and thermo-tolerance. Also, HSFs can simultaneously regulate abiotic and phytohormone signaling pathways and, therefore, play a crucial role in the regulation of stress-responsive genes.
Plant HSFs are either positive or negative transcriptional regulators for drought stress-responsive genes depending on several factors, including their post-translation modification, interacting proteins or binding target. ABA signaling also crosstalks with HSF-mediated gene expression during abiotic stresses. The expression of Arabidopsis thaliana AtHSFA6a was enhanced in response to external ABA application, salt, drought and salt treatment. The ABA mediated expression of AtHSF6b also increased plant resistance to salt and drought stresses. The transgenic lines overexpressing HsfA2 had a higher accumulation of several dehydration-responsive genes like GALACTINOL SYNTHASE (AtGolS) and RAFFINOSE SYNTHASE (RafS) of the raffinose family oligosaccharide (RFO) pathway. The enhanced mRNA level of GolS and RafS led to the over-accumulation of galactinol and raffinose, which were implicated in the scavenging of hydroxyl radicle generated via oxidative damage (Nishizawa-Yokoi et al., 2008; Panikulangara et al., 2004).
Further, the expression of HSFA3 in response to drought and heat stress are dependent on the expression of DREB2A. Also, AtHSFA3 and AtHSFA1b take part in different signaling pathways to enhance the plant's tolerance to drought stress (Scharf et al., 2012). Overexpression of chickpea CarHSFB2 improved drought tolerance by enhancing the expression of some stress-responsive genes at the seedling stage in Arabidopsis (Ma et al., 2016). Li, Zhang, et al. (2015) demonstrated that overexpression of the drought- and heat-inducible maize ZmHSF06 in Arabidopsis improved drought and heat stress tolerance. Moreover, Triticum aestivum TaHSFs induced by ABA plays a crucial role during drought (Huang et al., 2016). The expression of Chenopodium quinoa CqHSF5 and CqHSF12 were upregulated during drought stress treatment. Likewise, CqHSF8 and CqHSF17 also play an important role in drought resistance. Some genes out of 25 SbHSF genes identified in sorghum were expressed differentially during drought, cold and salt stresses (Tashi et al., 2018). The Oryza sativa OsHSFB2b negatively regulated HSF genes during salt and drought tolerance (Yoshida et al., 2011). Thus, HSFs are one of the most important TFs influencing the expression of various stress-responsive genes, thereby enhancing the adaptation to multiple environmental stress conditions. However, the functions of some HSFs in many abiotic stresses continue to be mostly unknown (Wu et al., 2018). So, to improve stress tolerance and productivity of crops, more research on HSFs should be conducted.
3.5 MYB superfamily
The MYB superfamily of proteins is a part of a large gene family of TFs distributed in almost all eukaryotes and perform varied functions (Dubos et al., 2010). The MYB TF was first discovered and named after the avian myeloblastosis virus (Paz-Ares et al., 1987). The first plant-specific MYB TF (ZmMYBC1 gene) was isolated from maize and it primarily regulates the anthocyanin biosynthesis (Salih et al., 2016). The complete MYB protein has three regions: the DNA binding domain (DBD), the transcriptional activation domain and the negative regulatory domain (Ogata et al., 1996; Thompson & Ramsay, 1995). The Arabidopsis and rice genomes have over 198 and 183 MYB genes, respectively (Yanhui et al., 2006).
Expression studies have validated the upregulation of several MYB genes under drought stress conditions (Abe et al., 2003). For instance, MYB4 TF from rice imparted drought tolerance to Arabidopsis overexpressing the transgene and these transgenic plants accumulated several osmolytes. In addition, the genes of the proline biosynthesis pathway were also upregulated (Mattana et al., 2005). About 43 MYB genes discovered in soybean responded to desiccation (Liao, Zou, Wang, et al., 2008a). Drought stress leads to ABA accumulation in plant cells, which in turn upregulate several TFs and other proteins. MYB TFs take part in ABA-dependent response to drought stress (Lata & Prasad, 2011). For example, MYB2 TF interacted with the promoter region of a dehydration responsive rd22 gene in Arabidopsis (Abe et al., 1997) and induced several ABA-responsive genes in Arabidopsis (Abe et al., 2003). Another MYB TF of Arabidopsis, AtMYB44, down-regulated the expression of PP2C genes that are the negative regulators of ABA signaling (Jung et al., 2008). AtMYB60 plays a crucial role in regulating stomatal movement and desiccation survival (Cominelli et al., 2005) and AtMYB96 takes part in ABA and auxin signaling pathway for imparting drought tolerance to plants (Seo et al., 2009). The overexpression of OsMYB48-1 in rice triggered the upregulation of many ABA-related genes, indicating MYB's role in conferring drought and salt tolerance in an ABA-dependent manner (Xiong et al., 2014). In finger millet (Eleusine coracana), the transcript level of EcMyb1 was induced during drought stress response in drought-tolerant cultivars (Jadhav et al., 2018; Salvi et al., 2012). There are very few instances where MYB conferred drought tolerance in an ABA-independent manner. For example, overexpression of chrysanthemum CmMYB2 in Arabidopsis improved plant's survival to desiccation and high salinity but the plants became more sensitive to ABA application suggesting it functions in an ABA-independent manner (Shan et al., 2012). In conclusion, MYB TFs largely function as positive regulators of drought stress tolerance mainly in an ABA-dependent manner.
3.6 NAC transcription factor family
The NAC-TFs are named after the characterization of three functional genes containing NAC domain: NAM, ATAF, and CUC. NAC-TF family is one of the largest plant-specific TF families involved in the multitude of biological processes. Although a small proportion of these proteins is explored, NAC-TFs reprogram diverse pathways associated with plant growth and development and respond to (a)biotic stresses. These NAC proteins comprise a 150–160 amino acid-long N-terminal DNA binding domain (BD) that aids in DNA-protein interaction and nuclear localization. The transcriptional regulatory region resides in the C-terminus, which is post-translationally regulated under specific conditions (Kim et al., 2012; Lee et al., 2012). The NAC TFs generally bind to cis-regulatory elements: CGT (G/A) or CACG (Tran et al., 2004).
In transgenic rice, overexpression of stress-responsive NAC confers salinity, low temperature, and dehydration stress tolerance. Similarly, the overexpression of root-specific NAC-TF (OsNAC5, OsNAC6, OsNAC9, and OsNAC10) in rice plants altered root architecture and thereby imparted drought tolerance (Hu et al., 2006; Nakashima et al., 2007; Redillas et al., 2012; Tran et al., 2004). A transcriptomic study in rice revealed that OsNAC1 is upregulated during drought stress and ABA exposure. The overexpression of OsNAC1 entailed protection against drought stress and resulted in more fertile spikelets under drought stress. Under drought stress, the OsNAC1-OE plants maintained high water retention capability by mediating the stomatal closure without affecting the photosynthetic rate. Interestingly, the comparative transcriptomic analysis of the wild-type and SNAC1-overexpressing plants indicated the upregulation of about 40 genes associated with drought stress tolerance mechanisms in transgenic plants (Hu et al., 2006). These candidate genes facilitated osmolyte accumulation, signal transduction, and redox homeostasis. Similarly, OsNAC6 overexpression significantly improved the dehydration stress response in rice (Nakashima et al., 2007). Furthermore, expression analysis of GFP driven by OsNAC1 promoter revealed that the OsNAC1 promoter is active in the stomatal guard cells after drought stress exposure (Hu et al., 2006). In Arabidopsis, the NAC-TF AtJUB1 (JUNGBRUNNEN 1; ANAC042) is associated with the regulation of ROS signaling and mediates the drought stress response (Wu et al., 2012). The AtJUB1 directly triggers the expression of DREB2A, an AP2 type TF associated with drought stress response. AtJUB1 also acts as a negative regulator of GA or BR biosynthesis genes, thereby resulting in a decline in the cellular level of these phytohormones. The reduced level of these phytohormones resulted in the stabilization of DELLA proteins. Similarly, a homolog of AtJUB1 in tomato (SlJUB1, Solyc05G021090) directly binds to the promoter region of genes encoding SlDREB and DELLLA to regulate ROS homeostasis and stress-associated gene modulation with the evident role of SlJUB1 in drought stress tolerance (Thirumalaikumar et al., 2018). Being a transcriptional regulator, NAC-TFs are located in the nucleus. However, there are some NAC-TFs localizing at the membrane region of the endoplasmic reticulum or plasma membrane due to the presence of a transmembrane domain; they are thus called NTLs (NAC-TF associated with transmembrane motif-1- Like). Interestingly, during stress progression, these NTLs are channelized to the nucleus through different post-translational modifications, including phosphorylation, membrane proteolysis, alternative splicing, so on. (Liang et al., 2015). Duan et al. (2017) reported that in Medicago falcata, MfNACsa was found in the membrane under normal condition, while it translocated to the nucleus via de-S-palmitoylation during drought stress. This MfNACsa preserves the reduced cellular milieu during drought stress exposure by regulating the transcriptional module of GLYOXALASE1. In conclusion, these NAC-TFs integrate with diverse pathways and could be candidates to improve drought stress tolerance in different crop plants.
3.7 TCP transcription factor family
TCP family is plant-specific and regulates a plethora of plant metabolic processes. Phylogenetic analysis of TCP-TF from diverse species revealed the presence of non-canonical basic bHLH-containing DNA-binding domain known as TCP domain. Based on the conserved TCP domain, this TF family is mainly categorized into two classes named TCP-P (Class I) and TCP-C (Class II) (Cubas et al., 1999; Kosugi & Ohashi, 1997). TCP-TF exhibits protein–protein interaction that imparts functional diversity. TCP proteins are largely known to affect the phytohormonal regulation, including the jasmonic acid, auxin, cytokinin, and strigolactone pathways, directly or indirectly influencing cellular functions (Lucero et al., 2015; Nicolas & Cubas, 2016; Resentini et al., 2015). Several studies have documented their role in leaf shape and pattern regulation (Kieffer et al., 2011), flower and shoot development (Doebley et al., 1995), circadian cycle (Giraud et al., 2010), mitochondrial biogenesis (Welchen & Gonzalez, 2006). The TCP TFs generally bind to cis-regulatory elements: GGNCCCAC, G (T/C)GGNCCC (Kosugi & Ohashi, 2002).
Apart from all the roles mentioned above, few studies indicated the role of specific TCP-TF in drought stress response (Ding et al., 2019; Lei et al., 2017; Mukhopadhyay & Tyagi, 2015; Zhou et al., 2013). For instance, the overexpression of OsTCP19 imparted drought stress tolerance in Arabidopsis. The interaction of OsTCP19 and OsABI4 played a significant role during the ABA-induced drought stress response (Mukhopadhyay & Tyagi, 2015). Similarly, TCP14, a positive regulator of seed germination, acts antagonist to DOF6 TF, and regulates a specific set of ABA-related genes to ensure seed germination (Rueda-Romero et al., 2011). In Agrostis stolonifera, the Osa-miR319-mediated downregulation of TCP resulted in enhanced drought stress tolerance. The Osa-miR319-mediated stress tolerance is supported by higher leaf wax content and improved water retention capacity (Zhou et al., 2013). In a recent study, Ding et al. (2019) performed a genome-wide analysis of the TCP-family gene in Zea mays and identified a positive role of ZmTCP42 in drought stress tolerance. However, a more comprehensive analysis is needed to understand the role and regulation of the TCP-TF family in drought stress.
3.8 WRKY transcription factor family
Plants have a big family of WRKY transcriptional regulators. Their activity is not only vital for plant growth and development but also for their stress adaptation during the myriad of environmental stresses encountered during their life cycle. The first WRKY gene was identified in sweet potato (Ishiguro & Nakamura, 1994). The whole genome sequencing of various plant species has led to the identification of a variety of other WRKY genes (He et al., 2016; Wei et al., 2017). The WRKY protein domain of 60 amino acids contains highly conserved sequences: WRKYGQK along with the zinc-finger-like motifs: Cys(2)-His(2) or Cys(2)-HisCys. WRKY TFs bind to W-box [or, TTGAC(C/T)] cis-element present in the promoter of the desired genes (Bakshi & Oelmüller, 2014). There are other binding sites of WRKY TFs in addition to W-box. For example, OSWRKY13 from Oryza sativa binds to pathogen-responsive element (PRE4; TGCGCTT) and HvWRKY46 from Hordeum vulgare binds to SUgar-Responsive Element (SURE; TAAAGATTACTAATAGGAA) (Cai et al., 2008; Sun et al., 2003). WRKY TFs can either activate or repress the expression of a gene in their various homo and heterodimer combinations (Bakshi & Oelmüller, 2014). WRKY TFs are classified into three groups (namely, I, II, and III) based on protein structural characteristics (Li et al., 2010).
WRKY TFs are strongly induced by various abiotic stress conditions like drought, heat stress, excessive saline soil, water stress, so on. (Schluttenhofer & Yuan, 2015). The WRKY TFs are associated with plant hormone signaling, MAP kinase signal transduction pathway and self-regulation of WRKY (Jiang et al., 2017). WRKY TF genes are activators of ABA signaling and the phytohormone further controls downstream stress responses by integrating various stress signals. WRKY TF positively regulates ABA-mediated closure of stomatal aperture during drought stress. However, several WRKY TFs are negative regulators of abiotic stresses. In Boea hygromectrica, BhWRKY1 TF regulates the activation of BhGO1S1 (GALACTINOL SYNTHASE 1), an ABA-induced gene, to increase the dehydration tolerance of the plants (Wang et al., 2009). Several studies have highlighted the role of GolS in drought stress endurance via limiting the excess accumulation of detrimental ROS (Salvi et al., 2018). When cotton GhWRKY41 and Solanum pimpinellifolium SpWRKY1 TFs were overexpressed in tobacco, they regulated the stomatal conduction and ROS levels in plants, thereby conferring drought stress tolerance to the transgenic plants (Li, Luan, & Liu, 2015c). Contrarily, overexpression of cotton GhWRKY25 in Arabidopsis could not impart drought stress tolerance (Liu et al., 2016). Similarly, overexpression of GhWRKY68 in N. benthamiana decreased tolerance to drought and salt (Jia et al., 2015). In Arabidopsis, the expression of the sunflower HaWRKY76 induced drought stress adaptation (Raineri et al., 2015). Similarly, the overexpression of OsWRKY45 and OsWRKY72 in rice was associated with improved tolerance to salt and drought stresses (Qiu & Yu, 2009; Song et al., 2010). Interestingly, when the expression of a drought stress-inducible WRKY gene (OsWRKY11) was driven by a promoter of heat shock protein encoding gene (OsHSP101), the transgenic lines exhibited both drought- and thermo- tolerance phenotype. The tolerance mechanism in the transgenic lines was associated with the improved desiccation tolerance and reduced water loss in the transgenics during stress exposure (Wu et al., 2009). Also, overexpression of GmWRKY13/54 in Arabidopsis thaliana boosted the plant's tolerance to both salt and drought stresses (Zhou et al., 2008). Furthermore, Fei et al. (2019) identified 38 WRKY TFs in Zanthoxylum bungeanum, out of which ZbWRKY33 (homologous sequence of AtWRKY33) was the most upregulated TF during drought conditions. BnaWRKY210 TF of Brassica napus was highly upregulated during drought stress (He et al., 2016). In the presence of exogenous ABA application as well as drought stress, the expression of CsWRKY2 in tea plants was enhanced (Wang et al., 2016). The TaWRKY44 TF positively regulated drought stress response either by eliminating ROS or by activating the expression of stress-linked genes (Wang et al., 2015). Gossypium hirsutum WRKY TFs GhWRKY25 and GhWRKY27 positively influenced the salt stress-mediated dehydration tolerance and Rhizoctonia solani infection, respectively; however, both of them negatively regulated the drought tolerance response (Liu et al., 2016; Yan et al., 2015). In Vitis vinifera, VvWRKY11 was responsible for regulating drought stress tolerance when overexpressed in Arabidopsis (Liu et al., 2011). WRKYs can regulate multiple abiotic stresses simultaneously and, therefore, play a central role in response to abiotic stresses of plants. So, the regulation by WRKY in relation to both biotic and abiotic stresses should be explored in detail. Because of this tight regulation, WRKYs have become a promising target for crop improvement.
All the above molecular players directly or indirectly interact with each other differently to precisely activate the downstream effector, the TFs. The TFs act synergistically or antagonistically to ensure suitable actions that will help plants to circumvent the adverse state (Figure 2).
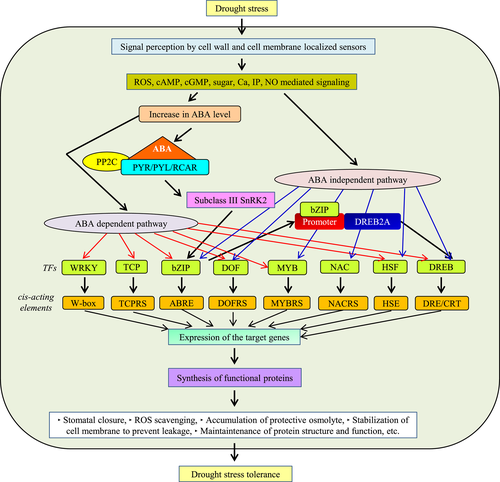
4 CHALLENGES AND PROSPECTIVE STRATEGIES TO ENGINEER TRANSCRIPTION FACTOR-MEDIATED DROUGHT STRESS TOLERANCE IN PLANTS
Drought tolerance is a complex trait that involves several genes, a cascade of intricate responses, and cross-talks between genes and signaling molecules (Shinozaki et al., 2003). Besides having technological advances, achieving the desired manipulation simultaneously in many genes is difficult. However, identifying a regulator of the genes involved in drought tolerance and subsequently targeting that regulator to get the desired changes in the trait looks more accomplishable. In this regard, understanding TF-mediated regulation is significant. The TFs with pleiotropic effects are outstanding candidates for stress tolerance (Kasuga et al., 1999). Being pleiotropic, transcription factors are master regulators that regulate several downstream stress-responsive genes networks along with the regulatory genes involved in the network, thereby developing stress tolerance. The stress tolerance mechanism against different stress factors usually involves a similar set of genes and molecular pathways. Therefore, improvement in the transcription factor activity may also develop a tolerance against other stress conditions. For instance, overexpression of the bZIP TF improved tolerance against salinity, drought, and heat stress in rice (Das et al., 2019). The TFs involved in the drought tolerance gene network are also known to interact among themselves. Such interactions make them more interesting candidates, as modulation in one transcription factor's activity may promote another transcription factor and network activity, thereby providing several amplitudes of improved tolerance (Singh & Laxmi, 2015).
Some of the wild type relatives of cultivated crop plants are highly tolerant to abiotic stresses. Allele mining and haplotype evaluation offer opportunities for searching superior alleles for stress-responsive TF genes from wild relatives and breeding populations. Using a similar strategy, a superior allele of the transcription factor OsDREB1F, having a significant role in drought tolerance, was identified through allele mining performed in Indian wild rice germplasm (Singh et al., 2015). Similarly, different alleles for NAC TFs were identified in maize and rice germplasm, and their overexpression was found to enhance the osmotic tolerance in Arabidopsis and rice, respectively (Hu et al., 2006; Lu et al., 2012).
The pleiotropic effects of transcription factors are as problematic as they are advantageous. The TF's pleiotropic nature could be detrimental, causing impaired growth and sterility, leading to reduced yield due to undesirable changes in downstream gene expression (Dubouzet et al., 2003; Vannini et al., 2007). However, this bottleneck can be overcome by using a stress-inducible or tissue-specific promoter (Engels et al., 2013). Alleles for TFs providing stress tolerance while having no negative impact on crop physiology and yield can be identified and utilized to develop drought-tolerant crops (Hu et al., 2006). Several crop plants with modulated TF expression have shown improved stress tolerance in field conditions, thereby proving the efficacy of the approach. Now the challenge for the plant science community is to explore the technological advances along with the biological understanding of TFs to make crop plants for resilient.
Transcription factors are the key factors that orchestrate the gene expression and direct an adequate response upon stress perception. This inflicts a complicated yet highly regulated alteration at the transcriptional and phytohormonal levels, which eventually facilitates the plant system to minimize the damage associated with stress progression. Table 2 enlists the candidate transcription factors encoding genes that have been manipulated in plant systems to strengthen the drought tolerance response in plants.
| TF family | Gene targeted | Gene origin | Overexpressed (O)/silenced (S) in | Regulatory mechanism | Reference(s) |
|---|---|---|---|---|---|
| bZIP | GhABF2 | Gossypium hirsutum |
Gossypium HirsutumO & Arabidopsis thalianaO |
GhABF2 upregulates genes related to ABA biosynthesis, drought response, proline biosynthesis, thiamine biosynthesis and ROS scavenging enzymes (superoxide dismutase and catalase) |
Liang et al. (2016) |
| IbABF4 | Ipomoea batatas | Ipomoea batatasO & Arabidopsis thalianaO |
IbABF4 increases the levels of endogenous ABA and ABA/stress-responsive genes (AtRD29A, AtRD29B, AtCOR47, IbRD29B, IbCOR47, IbRAB18, IbRD22) |
Wang, Qiu, et al. (2019), Wang, Xu, et al. 2019 | |
| IbbZIP1 | Ipomoea batatas | Arabidopsis thalianaO | ABA (NCED, ABA2), proline (P5CS) and ROS scavenging genes (encoding SOD, GPX, CAT, APX and DHAR) were upregulated in the transgenic lines |
Kang et al. (2019) | |
| ScAREB1 | Solanum lycopersicum |
Nicotiana tabacumO | There was upregulation of stress-responsive genes such as RD29B, LEA genes (ERD10B and TAS14), the TFs (PHI-2) and a trehalose-6-phosphate phosphatase gene when ScAREB1 was overexpressed |
Yánez et al. (2009) | |
| OsbZIP62 | Oryza sativa | Oryza sativaO | Upregulation of genes related to: carbohydrate metabolism, response to abiotic stimuli, cell wall organization or biogenesis, response to hormones, and cellular ion homeostasis |
Yang et al. (2019) | |
| OsbZIP71 | Oryza sativa | Oryza sativaO | Abiotic stress-related Genes like OsVHA-B, OsNHX1, COR413-TM1 and OsMyb4 were upregulated and OsbZIP71 was found to directly bind to the promoters of OsNHX1 and COR413-TM1 |
Liu et al. (2014) | |
| AtTGA4 | Arabidopsis thaliana | Arabidopsis thalianaO | Higher activity of nitrate transporter genes (NRT2.1 and NRT2.2) and nitrate assimilation activity conferred drought tolerance |
Zhong et al. (2015) | |
| SlbZIP38 | Solanum lycopersicum | Solanum lycopersicumO | The overexpression of the TF decreased drought tolerance in tomato by decreasing chlorophyll and free proline content and increasing the malodialdehyde level in leaves. Thus, downregulation of the TF is expected to improve drought tolerance |
Pan et al. (2017) | |
| OsbZIP52 | Oryza sativa | Oryza sativaO | The overexpression lines of the TF had decreased drought tolerance and downregulation of some stress responsive genes (like, OsLEA3, OsTPP1, Rab25, etc.). Thus, downregulation of the TF is expected to improve drought tolerance |
Liu et al. (2012) | |
| MYB | OsMYB6 | Oryza sativa | Oryza sativaO | Confers drought tolerance by elevation of proline content, higher CAT and SOD activities, lower relative electrolyte leakage and MDA content |
Tang et al. (2019) |
| TaMYBsm3 | Triticum aestivum | Arabidopsis thalianaO | Upregulation of drought responsive genes like P5CS1, DREB2A, and RD29A. Also increase in proline content and lowering of MDA level |
Li et al. (2019) | |
| OsMYB3R-2 | Oryza sativa | Arabidopsis thalianaO | Upregulation of stress-responsive genes, including DREB2A, COR15a and RCI2A |
Dai et al. (2007) | |
| StMYB1R-1 | Solanum tuberosum | Solanum tuberosumO | Enhanced expression of drought regulated genes such as AtHB-7, RD28, ALDH22a1 and ERD1-like protein |
Shin et al. (2011) | |
| PtrMYB94 | Populus trichocarpa | Arabidopsis thalianaO | Upregulation of ABA- and drought-responsive genes, like ABA1 and DREB2B | Fang et al. (2020) | |
| HvMYB1 | Hordeum vulgare | Hordeum vulgareO | Enhanced activity of ROS scavenging APX and GPX. Enhanced relative water content and reduced water loss rate and stomatal conductance |
Alexander et al. (2019) | |
| TaMpc1-D4 | Triticum aestivum | Triticum aestivumS | Improvement in relative water content, proline content, enhanced activity of antioxidant enzymes and upregulation of stress responsive genes leading to improved drought tolerance |
Li et al. (2020) | |
| DREB | AtDREB1A | Arabidopsis thaliana | Glycine maxO | Drought tolerance under field conditions was achieved because of lower water use due to reduced rate of transpiration | de Paiva Rolla et al. (2014) |
| DREB1/CBF | Arabidopsis thaliana | Oryza sativaO | Proteomics analysis revealed up-accumulation of proteins belonging to carbohydrate and energy metabolism and a novel protein, R40C1accumulated in root to confer drought tolerance |
Paul et al. (2015) | |
| OsDREB1F | Oryza sativa | Oryza sativaO and A. thalianaO | Activation of expression of COR genes which contain DRE/CRT element in their promoters and enhanced expression of rd29B and RAB18 genes |
Wang et al. (2008) | |
| OsDREB2A | Oryza sativa | Oryza sativaO | Elevation of soluble sugar and proline contents | Cui et al. (2011) | |
| VrDREB2A | Vigna radiata | Arabidopsis thalianaO | Elevated expression of AtCOR15A, AtCOR15B, AtKIN1, AtRD17, AtRD29A and AtRD29B which contain DRE elements in their promoters |
Chen et al. (2016) | |
TaDREB2 and TaDREB3 |
Triticum aestivum | Triticum aestivumO & Hordeum vulgareO |
Increased expression of 10 other DREB genes and a large number of stress responsive LEA/COR/DHN genes which provide protection to plants from cellular damage and desiccation under drought stress |
Morran et al. (2011) | |
| NAC | SNAC1 | Oryza sativa | Oryza sativaO | Up and downregulation of various stomatal movement genes which facilitated reduced loss of water through transpiration. A rice R2R3-MYB gene (UGS5) was upregulated and it interacted SNAC1 with to regulate stomatal movement |
Hu et al. (2006) |
| OsNAC5 | Oryza sativa | Oryza sativaO | Increased accumulation of proline and soluble sugars. Reduced accumulation of MDA, Na+ ion (due to enhanced expression of Na+/H+ antiporter genes) and H2O2 |
Song et al. (2011) | |
| OsNAC6 | Oryza sativa | Oryza sativaO | Upregulation of multiple genes related to abiotic stress tolerance (e.g. thioredoxin, peroxidase, lipoxygenase) |
Nakashima et al. (2007) | |
| TaNAC2 | Triticum aestivum | Arabidopsis thalianaO | Expression of drought stress responsive AtRD29A, AtRD29B, AtCOR47, AtRD20, AtGSTF6 and AtP5CS1 genes were enhanced |
Mao et al. (2012) | |
| TaNAC69 | Triticum aestivum | Triticum aestivumO | Upregulation of glyoxalase I family gene genes | Xue et al. (2011) | |
| GmNAC20 | Glycine max | Glycine maxO | Activation of the DREB/CBF-COR pathway genes and probable controlling of lateral root development by alteration of auxin signaling-related genes |
Hao et al. (2011) | |
| BoNAC019 | Brassica oleracea | Arabidopsis thalianaO | Overexpression lines of this TF had decreased drought tolerance, lower proline and ABA content and downregulation of various stress responsive and antioxidant protein coding genes. Thus downregulation of the TF is expected to improve drought tolerance. |
Wang et al. (2018) | |
| TCP | OsTCP19 | Oryza sativa | Arabidopsis thalianaO | Regulation of triacylglycerol biosynthesis genes and hyper-accumulation of lipid droplets in seedlings and mature plants conferred better drought stress tolerance. Upregulation of stress responsive genes: IAA3, ABI3 and ABI4 related to auxin and ABA biosynthesis respectively |
Mukhopadhyay and Tyagi (2015) |
| DOF | CDF3 | Arabidopsis thaliana | Arabidopsis thalianaO | CDF3 regulates expression of osmoprotectant and anti-oxidant genes, stress tolerance TFs (like, CBFs, DREB2A, ZAT12). Enhanced accumulation of osmoprotectants like γ-aminobutyric acid, proline, glutamine and sucrose |
Corrales et al. (2017) |
| TDDF1 | Lycopersicon esculentum | Lycopersicon esculentumO | Reduced rate of transpiration and stomatal conductance. Drought tolerant genes DREB2 and MAPK2 were significantly upregulated. Drought stressed transgenic plants over-accumulated ABA, JA and SA |
Ewas et al. (2017) | |
| PheDof12-1 | Phyllostachys edulis | Arabidopsis thalianaO | It was found to interact with drought induced protein PH01000199G0750 for possible drought stress adaptation |
Liu et al. (2019) | |
| AhDOF | Amaranthus hypochondriacus | Arabidopsis thalianaO | Increased accumulation of SOD, proline, sugars and enhanced expression of genes related to drought stress tolerance (e.g. CDPK1, Ca2+ ATPase, MYB 117 etc.) | Massange-Sanchez et al. (2016) | |
| WRKY | GhWRKY33 | Gossypium hirsutum |
Arabidopsis thalianaO | Overexpression of this gene reduces expression of drought responsive RD29A, DREB2A, RAB18 and SOS2 genes thereby reducing drought tolerance. Therefore, downregulation of ther TF is expected to improve drought tolerance |
|
| GhWRKY17 | Gossypium hirsutum |
Nicotiana benthamianaO | Decrease in the endogenous level of ABA along with lower expression of ABA-inducible genes, like AREB, DREB, NCED, ERD and LEA. Also reduction in proline content along with lower expression of ROS-scavenging genes (APX, CAT and SOD) resulting in drought susceptibility |
Yan et al. (2014) | |
| TaWRKY2 | Triticum aestivum | Triticum aestivumO | Increased accumulation of proline, soluble sugar, and chlorophyll and reduced water loss. Enhanced expression of drought responsive DREB1, DREB3, GST6, ERF5a, TaWRKY19 and TIP2 genes |
Gao et al., 2018 | |
| TaWRKY44 | Triticum aestivum | Nicotiana tabacumO | Increased accumulation of soluble sugar, proline and higher activity of SOD, catalase and peroxidase |
Wang et al. (2015) | |
| AtWRKY30 | Arabidopsis thaliana | Triticum aestivumO | Increased chlorophyll accumulation, relative water content, proline and soluble proteins content antioxidant enzyme's (CAT, SOD, peroxidase, APX) activity. Upregulation of stress responsive genes stress-responsive genes (ERF5a, DREB1, DREB3, WRKY19, TIP2 and AQP7) |
El-Esawi et al. (2019) | |
| MbWRKY5 | Malus baccata | Nicotiana tabacumO | Higher chlorophyll and proline accumulation, greater activity of GSH, peroxidase, SOD, CAT, decreased MDA and H2O2 accumulation and increased expression of stress responsive genes (NtLEA5, NtERD10D and NtP5CS) |
Han et al. (2018) | |
| HSF | HsfA3 | Arabidopsis thaliana | Arabidopsis thalianaO | HsfA3 directly Enhances the expression of galactinol synthase (GolS; GolS1 and GolS2) genes, the key enzyme in the biosynthesis of raffinose family oligosaccharides (RFOs), which function as antioxidants in plant cells |
Song et al. (2016) |
| HSFA1b | Arabidopsis thaliana | Arabidopsis thalianaO | HSFA1b directly regulates various HSE1b-element containing genes, which in turn control many drought stress responsive genes such as MULTIPROTEIN BRIDGING FACTOR1c (MBF1c) |
Bechtold et al. (2013) | |
| HSFA6b | Arabidopsis thaliana | Arabidopsis thalianaO | HSFA6b directly binds to the promoter of DEHYDRATION-RESPONSIVE ELEMENT-BINDING PROTEIN2A to enhance its expression and is a positive regulator of ABA-mediated drought stress tolerance |
Huang et al. (2016) | |
| OsHSFA3 | Oryza sativa | Arabidopsis thalianaO | Drought tolerance is conferred by reduction of water loss and ROS levels and increased biosynthesis of ABA and prolamine |
Zhu et al. (2020) | |
| OsHSP50.2 | Oryza sativa | Oryza sativaO | Lower electrolytic leakage and MDA content including higher SOD activity and increased accumulation of proline |
Xiang et al. (2018) |
- Note: The description and abbreviations of various genes and biomolecules should be referred from respective references.
5 GENOME EDITING EFFORTS FOR THE MANIPULATION OF TRANSCRIPTION FACTORS IN PLANTS
Genome editing approaches are used to generate novel desired alleles for stress-responsive genes (Osakabe et al., 2016). Similarly, novel alleles of stress-responsive TFs outperforming the existing ones can be generated by the genome-editing techniques. Engineering of cis-regulatory elements and TFs is a promising approach towards developing stress-tolerant plants (Sheshadri et al., 2016). The cis-regulatory elements located in the promoter and other regions govern the stress-responsive expression of genes, and mutation in these elements can significantly impact the expression of these genes (Brown et al., 2007; Wittkopp & Kalay, 2012). Prime editing, a powerful genome-editing technique, is capable of performing all possible base conversions. Therefore, prime editing looks highly promising to achieve the desired manipulations in the cis-regulatory elements of stress-related TF genes (Anzalone et al., 2019; Hassan et al., 2020). Prime editing may also be used to fine-tune the expression while avoiding deleterious effects. Promoter bashing is another approach utilized to improve the cis-regulatory element activity (Vats et al., 2019). It can be used in the enhancement of the promoter activity of TF genes or bringing the other drought-tolerant genes under the regulatory control of the specific promoter (Figure 3).
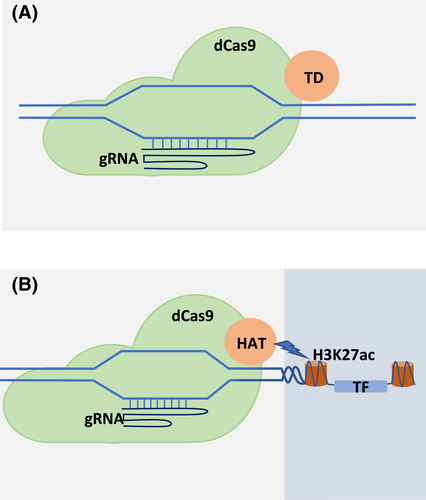
Genome editing can also be used to improve the expression of TF genes. The modified Cas9 (dCas9) fused with histone acetylation transferase domain was used for improving the promoter activity of the AREB1 transcription factor gene in Arabidopsis. The genome-edited lines showed an enhanced transcription of the AREB1 gene and developed drought tolerance through the positive regulation of drought-tolerant genes (Roca Paixão et al., 2019). Some of the TFs are also known to negatively regulate stress tolerance (Cai et al., 2017; Pan et al., 2017; Sanjari et al., 2019). These TFs acting as negative regulators can be easily knocked out using the CRISPR-Cas9 approach, which will eventually lead to the development of stress-tolerant cultivars.
6 CONCLUSIONS AND FUTURE PERSPECTIVE
Drought stress is one of the major constraints that threaten the livelihood of global population through its potential damage to crop yield. The understanding of physiological, biochemical and molecular response to drought stress is a prerequisite for decrypting the complex network and the candidate gene involved in it. Therefore, the detailed knowledge of these candidate genes and their underlying mechanism could provide key insight into the molecular regulation of drought stress response. This review is a comprehensive report on the scientific research concerning the gene regulatory networks associated with the water deficit stress and their contribution to unveil the molecular regulation of drought stress network. Since drought stress is a quantitative trait, engineering this trait with single gene integration is practically unfeasible. TF regulates multiple genes at a time by simultaneously binding to the promoter region of its different target genes. Therefore, the scientific fraternity has considered TF as an interesting target that can be utilized to engineer drought stress tolerance. Several studies have documented the promising role of the transcription factors in drought stress response, hence providing a better understanding of differential gene expression and their cumulative effect on a series of events occurring during stress progression. Therefore, it is reasonable to think that the manipulation of a candidate TF and its regulatory component would also contribute to engineer a crop with multiple stress adaptability. On the other end, there is a need to reorient our vision towards the intrinsic drought tolerance of the extremophiles (i.e., desert plants) and the available tolerant germplasm of crop plants that can be exploited to fish out the candidate target. The advent of the next-generation sequencing has paved new avenues to decipher the complex pathway associated with the drought stress response. The “big data science” spurred the progress in identifying novel candidate targets. Moreover, OMICs and molecular breeding approaches can be juxtaposed to delineate the instrumental role of transcriptional syndicate associated with drought stress. Thus, there are immense prospects to understand the indispensable role of TFs and their potential use as a modern genomic tool for the development of multiple stress-tolerant crops and eventually minimize the gap between actual and attainable yield.
ACKNOWLEDGMENT
Prafull Salvi gratefully acknowledges the Department of Science and technology (DST), Government of India for the DST-INSPIRE Fellowship Award (DST/INSPIRE/04/2018/003425). Sincere thanks to Ms Riti Sinha for her diligent proofreading of this article. We thank DBT- e-Library Consortium (DeLCON) at National Agri-Food Biotechnology Institute for providing required facilities.
AUTHOR CONTRIBUTIONS
Prafull Salvi conceptualized the title of the manuscript and supervised the authors. Mrinalini Manna, Tanika Thakur, Oceania Chirom, and Prafull Salvi wrote the original manuscript and made the figures. Mrinalini Manna, Rushil Mandlik, Rupesh Deshmukh, and Prafull Salvi revised the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




