Physio-morphological, biochemical, and anatomical traits of drought-tolerant and susceptible sorghum cultivars under pre- and post-anthesis drought
Edited by: M. Ahanger
Abstract
Understanding the physiological mechanisms that control drought tolerance in crop plants is vital for effective breeding. In this study, we characterized drought stress responses in four sorghum cultivars exhibiting differential levels of drought tolerance at pre- and post-anthesis. Greenhouse-grown plants were subjected to two types of drought treatment, water stress (WS) and desiccant-induced water stress (DA), timed to occur at pre- and post-anthesis. Multiple physiological measurements were then made revealing varying responses among the experimental cultivars. The pre- and post-flowering drought-tolerant cultivar P898012 showed a significantly higher net photosynthetic rate, higher transpiration rate, and greater stomatal conductance compared to the drought-susceptible cultivars at both pre- and post-anthesis. A significantly greater stomatal size was also detected in P898012, while the highest stomatal density was found in the drought-susceptible cultivar P721Q. Meanwhile, the two post-flowering drought-tolerant cultivars P898082 and B35 had a higher starch content and exhibited greater osmotic potential under post-anthesis water stress. Compared to WS and well-watered control plants, a greater increase in root biomass was observed in P898012 under DA at pre-anthesis. This finding suggests that plants invested more assimilates into the roots under severe DA at pre-anthesis. Overall, our results show good conformity between drought tolerance in sorghum and key physiological mechanisms of stomatal conductance, root growth patterns, and starch accumulation, all of which act as coping mechanisms during critical drought-sensitive growth stages.
Abbreviations
-
- WS
-
- water stress
-
- DA
-
- desiccant induced
-
- WW
-
- well watered
-
- RWC
-
- relative water content
-
- SPAD
-
- soil–plant analytical development
1 INTRODUCTION
Drought is one of the key abiotic stresses limiting crop productivity worldwide (Cha-um et al., 2012), affecting plant productivity by inducing stomatal closure, and thereby reducing photosynthesis (Németh et al., 2002) and growth (Bibi et al., 2012). With growing effects of climate change, the development of drought-tolerant and climate-resilient crops is essential for survival under unfavorable environmental conditions and maintenance of high yield. Plants respond to water stress via a series of cellular events involving physiological, morphological and biochemical processes. Under water stress, photosynthesis decreases, osmotic potential is reduced, and consequently, the availability of photosynthetic assimilates and energy becomes limited. During pre-anthesis assimilation, the necessary resources for grain filling temporarily accumulate within the stem as non-structural carbohydrates such as starch, sucrose, monosaccharides, isoprenoids, and fructans (Schnyder, 1993; Jensen and Wilkerson, 2017). It has been suggested that genotypes that can synthesize and store large amounts of starch could potentially exhibit improved grain yield by mobilizing these reserves to the grains when photosynthesis is inhibited (Conocono, 2002; Gutjahr et al., 2013). Osmotic potential, a significant physiological determinant of water stress (Lacerda et al., 2003), has also been highlighted as an important factor indicating drought tolerance in plants (Esperon-Rodriguez et al., 2018). Understanding stomatal behavior in drought-tolerant and -susceptible cultivars is therefore important. Although some stomatal characteristics are genetic and heritable (Orlovic et al., 1998), others are thought to be environmentally induced under certain growth conditions (Al Afas et al., 2006; Russo et al., 2014). Moreover, the density and size of stomata on the abaxial and adaxial leaf greatly affect the rate of gas exchange between inner and outer and layers (Kardiman and Ræbild, 2018).
Sorghum, which exhibits high levels of drought tolerance, is widely grown in North and South America, Africa and the Indian subcontinent, where water availability is often limited (Beyel and Bruggemann, 2005). Sorghum is therefore considered a good model species for research into the genetic and physiological mechanisms underlying drought tolerance in higher plants (Rao et al., 2016). However, no species is immune to water stress, and drought can have adverse effects on early vegetative, pre-, and post-anthesis sorghum growth (Tuinstra et al., 1997). Anthesis and grain filling are thought to be the most sensitive growth stages, with drought during these periods leading to the greatest reductions in yield (Krupa et al., 2017). Additionally, water stress during the reproductive and post-anthesis stages were found to reduce sorghum grain yield by approximately 55 (Assefa et al., 2010) and 43% (Menezes et al., 2014), respectively.
In this study, sorghum cultivars with varying degrees of drought tolerance at different development stages were therefore selected to determine whether tolerance is associated with key physiological, morphological, anatomical and biochemical mechanisms.
2 MATERIALS AND METHODS
2.1 Growth conditions and plant materials
A greenhouse study was conducted from September 2016 to February 2017 at Purdue University, West Lafayette, USA. The greenhouse temperature was maintained at 24°C during the day and 20°C at night, with relative humidity of approximately 60–70% (depending on the season) and a photosynthetic photon flux density of 350–400 μmol m−2 s−1 during a 16-h photoperiod.
Four cultivars with variable genetic backgrounds were selected: P898012, P721Q, TX7078, and B35. P898012 is tolerant to drought at both the pre- and post-flowering stages, while P721Q is susceptible to drought at both stages. TX7078 is tolerant to pre-flowering drought and susceptible to post-flowering drought, and B35 is susceptible to pre-flowering and tolerant to post-flowering drought (Tuinstra et al., 1996; Tesso et al., 2006; Leslie, 2008; Ongom et al., 2016).
The soil media comprised equal amounts of master mix (Sun Gro Horticulture), sand, and topsoil. Slow-releasing fertilizer was homogenously applied at the seedling stage using 100% resin-coated fertilizer cores. Major nutrients included nitrogen, phosphorous, and potash (15-9-12) and secondary nutrients included magnesium and sulfur, as well as other micronutrients such as boron, copper, iron, manganese, molybdenum, and zinc. Seedlings were transferred to large pots (19-l) 2 weeks after germination in trays.
2.2 Drought stress treatments
In addition to well-watered (WW) plants, two drought treatments were examined at pre- and post-anthesis growth stages: water stress (WS) and desiccant-induced (DA) treatment. Under WW treatment, plants were well watered, while under WS, water was withheld at pre-anthesis (beginning of booting) for 7–8 days and at post-anthesis (10 days after 50% blooming) for 10–12 days. Under DA treatment, 1% potassium chlorate (KClO3) was used to induce drought (Figure 1).

2.3 Relative water content (RWC)
Two fresh leaves were removed from each plant then weighed before being transferred to a scintillation vial filled with 5 ml of deionized water. The leaves were left to imbibe water for 24 h then surface dried with paper towels prior to measuring the leaf turgor weight. The tissues were then dried to a constant weight at 70°C to obtain the leaf dry weight. The RWC was then calculated as (fresh weight – dry weight) / (turgid weight – dry weight) × 100 (Chen et al., 2004).
2.4 Osmotic potential measurements
Osmotic potential was measured in three fully expanded leaves per plant. Three discs per leaf were used to represent each treatment. Measurements were conducted as described by Bumgarner et al. (2015). Briefly, multiple sections of leaves were removed, placed in microfuge tubes containing a plastic mesh insert then immediately placed in liquid nitrogen. The tubes were then closed and kept on ice until returning to the laboratory, where they were stored at −4°C and centrifuged at 25.200 g (15.000 rpm) for 5 min to extract the cell sap. The sap was then immediately placed in an osmometer (Wescor 5200, Wescor Inc.). The solute concentration was converted to yS using the van't Hoff equation: yS = –RTC, where R is the gas constant, T is the absolute temperature, and C is the molar solute concentration (Marigo and Peltier, 1996). Osmolality was then converted to osmotic potential using the equation ψπ = −CsRT, where ψπ is the osmotic potential, Cs is the osmolality, R is the gas constant, and T is the temperature.
2.5 Stomatal anatomy analyzes
Stomatal observations were carried out using microscopic slides pasted with a layer of super glue then pressed on either the adaxial or abaxial side of a fully expanded leaf at pre-anthesis. Approximately 30 s later, the slides were removed from the leaves along with the epidermal tissue. Three locations per leaf and three leaves per plant were collected in total, with three replicates per treatment. Stomata were examined using Olympus BX53 microscope (Olympus Corp.) in a leaf area of 0.48 mm2 at a resolution of 1280 × 960. Image analysis was performed using ImageJ software (https://imagej.nih.gov/ij/). Stomata size (area) ([stomata width × stomata length × π] / 4), stomata density (number of stomata per area), and stomata index (ratio of stomata to total epidermal cells, including stomata and pavement cells) were then calculated (Cortan et al., 2017).
2.6 Photosynthesis, stomatal conductance, transpiration and chlorophyll measurements
Net photosynthetic activity (Pn), transpiration (E), and stomatal conductance (gs) in the top three fully expanded leaves per plant were measured using a portable photosynthesis system (LI-6400; LI-COR). Readings were performed from 09:00 AM to 12:00 PM with an external light source set at 800 μmol m−2 s−1 and an ambient CO2 concentration of 400 μmol m−2 s−1. For the chlorophyll concentration (chl) measurements, a soil–plant analytical development (SPAD) chlorophyll meter (SPAD-502, Minolta Corp.) was used. The SPAD index was determined using the average of nine readings on the upper, middle and bottom of the top three fully expanded leaves per plant.
2.7 Stem starch content
Starch sampling was performed using a mixture of top, middle, and bottom stem samples obtained from excised leaves and immediately transferred to liquid nitrogen. The tissues were then ground to a fine powder using ceramic beads in a 2 ml tube to which 1 ml of 80% ethanol was added before incubating at 80°C for 30 min. Tubes were then centrifuged at 16.128 g for 5 min and the supernatant was discarded. Another 1 ml of 80% ethanol was then added to the tube with the pellet and the above steps were repeated. The remaining pellet was then air dried under a hood for 40 min before dissolving in 1 ml H2O and incubating at 90–95°C. The tubes were then cooled at room temperature and centrifuged for 1 min, and 50 μl of the supernatant was used for the starch measurements. A reagent blank (0.1 ml of Solution 1 and 0.05 ml of H2O) and sample solutions (0.1 ml of Solution 1 and 0.05 ml of sample) were employed. The tube contents were stirred and incubated at 55–60°C for 15 min then Solution 2 (0.5 ml) and H2O were added. The mixtures were then transferred to a cuvette and the absorbance (A1) at 340 nm was read after 3 min. Solution 3 was then added (0.01 ml) to the tube and the contents were stirred before obtaining the A2 absorbance reading 10–15 min later. The starch concentration was calculated using the Roche Results Starch Template (Lukaszewska and Gorin, 1988). The quantity of starch was determined using the UV method by measuring the amount of glucose released from the starch (Boehringer Mannheim, Cat. No. 10207748035).
2.8 Data analysis
Statistical analyzes were carried out via analysis of variance (anova) to compare parameters between control and drought treatments using MSTAT-C (Freed et al., 1991). The treatments were arranged in a completely randomized plot design with three replicates. Differences between means were also assessed to determine significance levels using the lsd test.
3 RESULTS
3.1 Morphological and biochemical parameters
Significant variations in each parameter were observed. Plant height at pre- and post-anthesis ranged from 49 to 107.7 cm and 74 to 105.3 cm, respectively (Tables 1 and 2) and was generally greater in plants in the control group than both drought treatments at pre-anthesis. However, panicle heights in plants grown under control and drought conditions were similar at both growth stages.
| Cultivars | Treatments | Plant height (cm) | Panicle height (cm) | Root biomass (g) | Panicle biomass (g) | Starch content (mg g−1) |
|---|---|---|---|---|---|---|
| P898012 | WW | 107.7a* | 14.7e** | 34.7bc** | 18.8b** | 92cd** |
| WS | 94.7b | 15.3e | 22.6cde | 11cd | 212a | |
| DA | 93.3bc | 15.8de | 51.3a | 11.3cd | 23efg | |
| Mean | 98.6A** | 15.3C** | 35.3B** | 13.8B** | 109A** | |
| B35 | WW | 79.6def | 25.4a | 43.4ab | 15.1bc | 94c |
| WS | 72.0efg | 23.7ab | 41.9ab | 8.1d | 53def | |
| DA | 82.6cde | 22.0bc | 44.3ab | 10.4d | 31efg | |
| Mean | 78.1B | 23.7A | 44.3A | 11.2C | 59B | |
| P721Q | WW | 83.3cd | 23.0ab | 26.8cd | 15.0bc | 153b |
| WS | 71.0fg | 24.3ab | 12.1ef | 15.3bc | 56cde | |
| DA | 62.7gh | 15.3e | 18.4def | 10.6d | 15fg | |
| Mean | 72.3B | 20.9B | 19.1C | 13.6B | 75B | |
| TX7078 | WW | 72.7d-g | 19.0cd | 34.5bc | 27.1a | 29efg |
| WS | 53.0hi | 16.0de | 7.1f | 14.8c | 61cde | |
| DA | 49.0i | 13.0e | 8.3f | 9.8d | 11g | |
| Mean | 58.2C | 16.0C | 16.6C | 17.2A | 34C | |
| CV (%) | 8.54 | 7.36 | 20.8 | 13.0 | 24.8 | |
| LSD (C× T) | 11.05 | 3.19 | 13.67 | 4.15 | 39.1 | |
| LSD (C) | 8.65 | 1.84 | 7.89 | 2.40 | 22.6 | |
- Note: Means that do not share a letter are significantly different.
- Abbreviations: CV, variation coefficient; DA, desiccant application; LSD, least significant difference; WS, water stress; WW, well-watering as control.
- ** and * represent P < 0.01 and for P < 0.05, respectively.
| Cultivars | Treatments | Plant height (cm) | Panicle height (cm) | Root biomass (g) | Panicle biomass (g) | Starch content (mg g−1) |
|---|---|---|---|---|---|---|
| P898012 | WW | 100 | 15 | 63.7c** | 28.3b** | 78b** |
| WS | 100 | 15 | 44.1d | 24.5bc | 64b | |
| DA | 105.3 | 15.7 | 18.3e | 14.3d | 10e | |
| Mean | 101.8A** | 15.2C** | 42B** | 22.4B** | 51A** | |
| B35 | WW | 91.3 | 28.2 | 96.3a | 24.4bc | 114a |
| WS | 88.7 | 27 | 73bc | 23.9bc | 67b | |
| DA | 85.7 | 27.5 | 78.4b | 20.6c | 35 cd | |
| Mean | 88.6B | 27.6A | 82.6A | 23.0B | 72B | |
| P721Q | WW | 74 | 25 | 37.8d | 36.3a | 57bc |
| WS | 74.7 | 24.2 | 22e | 28.5b | 16de | |
| DA | 74.7 | 23.7 | 20.8e | 21.9c | 8e | |
| Mean | 74.4C | 24.3B | 26.9C | 28.9A | 27C | |
| CV (%) | 3.6 | 8.2 | 10.4 | 10.22 | 18.9 | |
| LSD (C × T) | NS | NS | 12.37 | 5.95 | 22.2 | |
| LSD (C) | 4.39 | 2.48 | 7.14 | 3.43 | 12.8 | |
- Note: Means that do not share a letter are significantly different.
- Abbreviations: CV, variation coefficient; DA, desiccant application; LSD, least significant difference; WS, water stress; WW, well-watering as control.
- ** represents P < 0.01.
Similar results were obtained in root biomass in both the WW and DA groups at pre-anthesis. Meanwhile, at post-anthesis, the root biomass was ranked as follows: WW > WS > DA. The panicle biomass ranged from 8.1 to 27.1 g under pre-anthesis treatment and from 14.3 to 36.3 g under post-anthesis treatment (Tables 1 and 2) and was greater in control plants at both growth stages compared to drought treatment. Results were more significantly affected by severe drought treatment (DA).
Significant differences in the starch content were also observed between and within treatments and cultivars. The starch content varied from 11 and 212 mg g−1 under pre-anthesis treatment and 8 and 114 mg g−1 under post-anthesis treatment (Tables 1 and 2). Similar results were obtained under WW and WS at pre-anthesis, while DA treatment resulted in the lowest starch content. Meanwhile, under post-anthesis treatment, values were ranked as follows: WW > WS > DA. The highest starch contents were found in the drought-tolerant cultivars, P898012 and B35.
3.2 Physiological traits
Analyzes of Pn, gs, E, and chl concentrations revealed significant differences among cultivars at both growth stages. At pre-anthesis, the WS and WW groups respectively displayed Pn rates of 2.8 to 8 and 16 to 19 μmol m−2 s−1, gs values of 0.011 to 0.031 and 0.078 to 0.145 mol m−2 s−1, E-values of 0.24 to 1 and 2.56 to 4.43 mmol m−2 s−1, and chl contents of 36.0 to 51.5 and 51.5 to 60.9 μmol m−2 (Figure 2). Values at post-anthesis in the WS and WW groups were respectively 3 to 9.9 and 15 to 18.9 μmol m−2 s−1, 0.007 to 0.044 and 0.076 to 0.123 mol m−2 s−1, 0.24 to 1.65 and 1.81 to 3.46 mmol m−2 s−1, and 30.2 to 54.1 and 51.5 to 62.8 μmol m−2 (Figure 2). According to these results, the average Pn of the cultivars remained unchanged between pre- and post-anthesis at 17.2 μmol m−2 s−1 under WW and 5.5 μmol m−2 s−1 under WS.
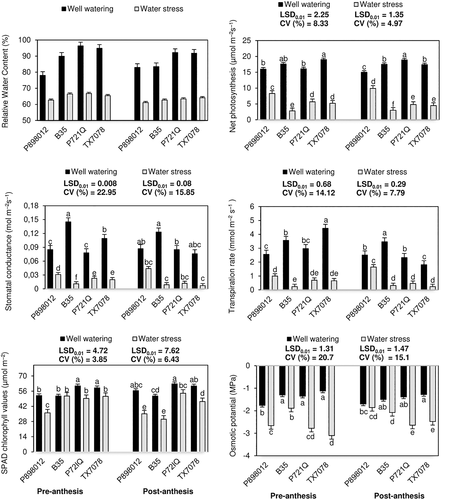
The osmotic potential of the WW and WS groups at pre-anthesis ranged from −1.12 to −1.76 and − 1.89 to −3.10 MPa, respectively. Meanwhile, at post-anthesis, values ranged from −1.28 to −1.70 and −1.86 to −2.64 MPa, respectively (Figure 2). Under control WW conditions, the lowest osmotic potential at both pre- and post-anthesis was observed in the drought-tolerant cultivar P898012 (−1.76 and −1.70 MPa, respectively), while under WS, the osmotic potential of P898012 increased to −2.66 and − 1.86 MPa at pre- and post-anthesis, respectively.
Chl in the WW and WS groups, respectively, varied from 51.5 to 60.9 and 36.0 to 51.5 μmol m−2 at pre-anthesis and from 51.5 to 62.8 and 30.2 to 54.1 μmol m−2 at post-anthesis (Figure 2). SPAD values decreased significantly in response to water stress at both growth stages, except in B35 at pre-anthesis.
The RWC of the WS and WW groups, respectively, varied from 62.6 to 66.8 and 78 to 96.4% at pre-anthesis and from 61.1 to 64.1 and 83 to 92.2% at post-anthesis (Figure 2). Lower RWC values were observed in the drought-tolerant cultivars P898012 (62.6%) and TX7078 (65.4%) under pre-anthesis water stress and the drought-tolerant cultivars P898012 (61.1%) and B35 (62.7%) under post-anthesis water stress compared to the drought-susceptible cultivars.
3.3 Stomatal anatomy
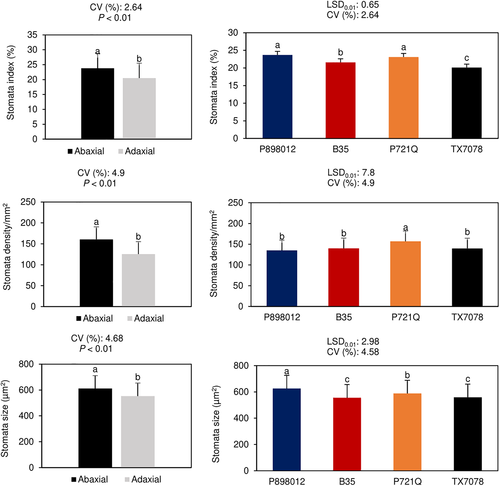
The stomatal index, size, and density differed significantly among cultivars. The stomatal index ranged from 20.1 to 23.7%, stomatal size from 556 to 626 μm2, and the stomatal density from 135.1 to 157 stomata/mm2 (Figure 3). On the abaxial and adaxial sides, stomatal indexes were 23.8 and 20.5%, stomatal sizes were 556 and 626 μm2, and stomatal densities were 135.1 and 157 stomata/mm2, respectively (Figure 3). The maximum stomatal size was observed in the drought-tolerant cultivar P898012, while the drought-susceptible cultivar P721Q had the highest stomatal density at both growth stages. The highest stomatal index, size, and density values were obtained on the abaxial side of the leaf (Figure 3.).
4 DISCUSSION
Drought treatment has been shown to lead to severe reductions in shoot traits. A previous study revealed that non-irrigated conditions lead to significant reductions in panicle weight (0.8–13.1%) and grain yield (10.5–15.5%) in grain sorghum (Talwar et al., 2009). Meanwhile, in the present study, the panicle biomass significantly differed in response to drought treatments with DA at post-anthesis leading to greater reductions than WS. Moreover, based on the reduction in panicle biomass between WW and WS, the post-anthesis drought-tolerant cultivars P898012 and B35 were less affected by WS than the drought-susceptible cultivar P721Q. Meanwhile, no significant differences among treatments were observed in terms of plant or panicle height at post-anthesis. One explanation for this is that plant and panicle height reached their maxima prior to post-anthesis drought treatment. Root biomass responded differently to pre- and post-anthesis drought treatment. At pre-anthesis, cultivars under severe drought (DA) generally invested more assimilates into the roots than those in the WS group, consistent with the conclusions of Wright et al. (1983) who found an increase in root biomass under drought when assimilates were remobilized to promote root growth. Moreover, at both pre- and post-anthesis, the root biomass was lower under WS than WW treatment. Root biomass has been shown to be associated with drought stress tolerance (Ekanayake et al., 1985), while Nour and Weibel (1978) reported that root weight could be used as an indicator of drought tolerance in grain sorghum. Passioura (1983) suggested that a smaller root system may be beneficial under drought, while Khodabin et al. (2020) revealed that drought stress in the early growth stages reduced the root biomass in canola. However, drought tolerance can also be improved by enhanced assimilate allocation to the roots and increased moisture uptake capability (Mathew et al., 2019). Compared to WW, a greater reduction in root biomass was observed in the drought-susceptible cultivar P721Q at post-anthesis WS compared to the drought-tolerant cultivars P898012 and B35. The higher root biomass in the drought-tolerant cultivars under WS may therefore be beneficial in terms of drought tolerance.
The remobilization of stem reserves is important in meeting carbon requirements during grain filling (Fortmeier and Schubert, 1995; Yang et al., 2000). In this study, the pre-anthesis drought-tolerant cultivars P898012 and TX7078 demonstrated a higher starch content under WS than WW. Meanwhile, in the drought the starch contents in the pre-anthesis drought-susceptible cultivars, P721Q, and B35 were lower under WS than WW. Yi et al. (2014) showed that drought stress in sorghum during anthesis slightly elevated starch accumulation in the panicle after which accumulation slowed down in the prophase of grain filling. Under water stress, accumulation of pre-anthesis assimilates in the stems of drought-tolerant cultivars is used to maintain the carbon transport rate during the grain filling stage (Krupa et al., 2017). Sowder et al. (1997) revealed similar results in terms of the starch content of B35 at anthesis. However, this result was not in agreement with those of Massacci et al. (1996), who reported a higher stem starch content in sweet sorghum under WS during post-anthesis than in control stems. Massacci et al. (1996) also highlight that the physiology of sweet sorghum differs from that of grain sorghum, which may explain the conflicting results obtained using grain sorghum in the present study. Here, DA treatment led to extreme reductions in starch content compared to WW and WS treatment at both growth stages. This reduction in stem starch content under pre-anthesis DA treatment may have resulted from the remobilization of starch reserves to the root system and panicle. The post-anthesis drought-tolerant cultivars B35 and P898012 also displayed higher starch contents under WS than the drought-susceptible cultivar P721Q.
Our study showed that Pn, gs, and E at both pre- and post-anthesis were significantly lower in the WS group than the WW group. Tsuji et al. (2003) reported that drought tolerance in sorghum was associated with the Pn, gs, E, RWC, small leaf area, and ability to maintain high leaf water potential. Previous studies on Pn, gs, and E in sorghum also revealed reductions in plants subjected to water stress after anthesis (Massacci et al., 1996) and for approximately 2 weeks at booting (Zhang et al., 2019). Furthermore, drought was also shown to reduce the Pn when the soil water content was at 30% field capacity over 6 days from approximately 30.5 to 3.3 μmol m−2 s−1 in B35 and 31.5 to 12.3 μmol m−2 s−1 in genotype E36-1 (Beyel and Bruggemann, 2005).
In the present study, the drought-tolerant cultivar P898012 had the highest Pn, gs, and E when subjected to pre- and post-anthesis drought stress. Compared to the WW group, the reduction in the Pn during pre- and post-anthesis water stress was 48.1 and 34% in P898012, 84.1 and 82.9% in B35, 64 and 74.6% in P721Q, and 72 and 74.1% in TX7078, respectively. The reduction in gs and E under water stress during both growth stages was very similar to that of the Pn. These results therefore suggest that P898012 possesses a different drought tolerance mechanism that enables it to maintain gas exchange throughout pre- and post-anthesis drought stress, which is consistent with previous studies. For example, it was previously suggested that drought-tolerant wheat genotypes displayed higher photosynthetic rates when subjected to post-pollination water stress (Sharifi and Mohammadkhani, 2016), while drought-tolerant species showed improved carbon fixation during drought by altering their water use efficiency and controlling gs (Lawlor, 2002). It has also been proposed that a small reduction in gs has a greater effect on E than Pn (Nobel, 1999). Furthermore, gs in drought-tolerant sorghum genotypes has been found to increase under water stress at the grain filling stage compared to drought-susceptible genotypes (Krupa et al., 2017).
Chl allows rapid assessment of chl content (Puangbut et al., 2017), which is a good indicator of the “stay-green” trait of photosynthetic tissue (Fotovat et al., 2007). It has been suggested that a high chl content is associated with a low degree of photoinhibition (Farquhar and Richards, 1984). In the present study, the lowest chl values were observed in the drought-tolerant cultivar P898012 under pre-anthesis drought stress and drought-tolerant cultivars P898012 and B35 under post-anthesis drought stress. Meanwhile, the highest chl content was observed in the drought-susceptible cultivar P721Q under WW control conditions.
In this context, the results of this study suggest that there is significant variation among drought-tolerant and -susceptible cultivars in terms of osmotic potential, particularly during post-anthesis. Lowering the cellular osmotic potential via the accumulation of solutes is considered a typical strategy used by plants against drought. A lower osmotic potential helps to reduce water loss during drought, enabling the plant to maintain cell growth and avoid damage (Tariq et al., 2018). In this study, the drought-tolerant cultivars P898012 and B35 showed high osmotic potential under post-anthesis drought, in contrast to the drought-susceptible cultivars. This was possibly related to the strategies used by drought-tolerant cultivars during post-anthesis water stress; however, future studies are needed to elucidate the underlying mechanism.
In the present study, stomatal size varied significantly among the cultivars. The greatest stomatal size was observed in P898012 (drought-tolerant at both growth stages). This result is in line with the findings from Aasamaa et al. (2001) who suggested that smaller stomata are associated with higher sensitivity to dehydration. The drought-susceptible cultivar P721Q had the highest stomatal density at both growth stages. The number and density of stomata on the leaves are highly heritable characteristics (Hofmann and Dobrenz, 1983; Schoppach et al., 2016). The results of this study therefore imply that a high stomatal density is linked to the genetic background of drought susceptibility.
5 CONCLUSIONS
Plants employ different strategies to alleviate the adverse effects of water stress, responding via morphological, physiological, anatomical, and metabolic adjustments. Measurements of the different processes involved in drought tolerance and sensitivity are therefore important in understanding the various strategies employed by plants to cope with the harmful effects of drought stress. The results presented here suggest that sorghum cultivars P898012 and P721Q, which differ in terms of both pre- and post-anthesis drought tolerance, present remarkable and contrasting behaviors in response to drought. Further molecular characterization of these contrasting cultivars may offer new insights into genetic and physiological mechanisms of drought tolerance that could enhance breeding efforts aimed at increasing drought tolerance in crop plants.
ACKNOWLEDGMENTS
The authors thank the TUBITAK-The Scientific and Technological Research Council of Turkey for the Postdoctoral Fellowship Program provided. The authors are thankful to the Zhang Lab crews to help the sampling and harvesting and Dr. Mike Gosney to share his knowledge on stomata analysis.
AUTHOR CONTRIBUTIONS
Hayati Akman, Cankui Zhang, and Gebisa Ejeta designed the experiments. Hayati Akman performed the experiments. Hayati Akman, Cankui Zhang, and Gebisa Ejeta analyzed data. Hayati Akman prepared the figures and tables and wrote the manuscript. Hayati Akman, Cankui Zhang, and Gebisa Ejeta revised the manuscript. All the authors have read and approved the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
Data openly available in a public repository that issues datasets with DOIs




