Functional characterization and expression profiling of glyoxalase III genes in date palm grown under abiotic stresses
Abstract
Methylglyoxal (MG), a by-product of various metabolic processes, including glycolysis, is a highly reactive cytotoxic metabolite. The level of MG in the cell is maintained at a non-toxic level via MG detoxification pathways such as the universal glyoxalase system, including glyoxalase I/II/III enzymes. Glyoxalase III (DJ-1) can breakdown MG to d-lactate in a single step without reducing glutathione (GSH). Elucidating the function of the DJ-1 gene family may provide further knowledge about its role in plants under abiotic stresses. Here, we characterize four glyoxalase III genes (PdDJ-1B1, PdDJ-1B2, PdDJ-1C, and PdDJ-1D) encoding the conserved DJ-1 domain in the genome of the date palm, a crop with high drought and salinity tolerance. The expression level of the PdDJ-1 genes increased in date palm leaves upon salinity treatment. In addition, overexpression of PdDJ-1 genes in Escherichia coli and the complementation in yeast hsp31Δ knockout mutant cells enhanced their growth rate and reduced the accumulation of reactive oxygen species (ROS) under MG and oxidative stress conditions as shown by the flow cytometry assay. Subcellular localization using confocal microscopy revealed the accumulation of PdDJ-1B1, PdDJ-1C, and PdDJ-1D in the chloroplast, whereas PdDJ-1B2 was localized to the cytosol. Remarkably, constitutive expression of the PdDJ-1C gene in Arabidopsis thaliana Columbia (Col-0) resulted in the generation of non-viable albino plants implying that PdDJ-1C plays a critical function in chloroplast development. These findings suggest that PdDJ-1 protein has an important function in MG-detoxification and maintaining the redox balance in date palm plants under abiotic stress conditions.
Abbreviations
-
- AGE
-
- advanced glycation end products
-
- AKR
-
- aldo-keto reductase
-
- anova
-
- analysis of variance
-
- GLYI
-
- glyoxalase I
-
- GLYII
-
- glyoxalase II
-
- GSH
-
- reduced glutathione
-
- H2DCFAD
-
- 2′,7′-dichlorodihydrofluorescein diacetate
-
- HMM
-
- Hidden Markov Model
-
- IPTG
-
- isopropyl β-d-1-thiogalactopyranoside
-
- LB
-
- Luria-Bertani
-
- MFI
-
- mean fluorescence intensity
-
- MG
-
- methylglyoxal
-
- pI
-
- isoelectric point
-
- RCS
-
- reactive carbonyl species
-
- ROS
-
- reactive oxygen species
-
- SD
-
- synthetic defined
-
- SVM
-
- support vector machine
-
- Ura
-
- uracil
1 INTRODUCTION
Salinization of arable land imposes a detrimental effect on crop production globally. Various factors such as overexploitation of underground water, high evaporation rate, lack of precipitation, and the use of brackish water for irrigation have further increased the salinization of arable land (Butcher et al., 2016). With the advancement of climate change and the rise of sea levels, this phenomenon of soil salinization will exponentially increase, thereby affecting crop production (Thomas and Middleton, 1993; Yaish and Kumar, 2015). Under salinity, plants are affected in two main phases: (1) a rapid initial osmotic stress leading to a decrease in the growth of new shoots and (2) slower ionic stress that causes an increase in leaf senescence (Munns and Tester, 2008; Assaha et al., 2017; Al-Harrasi et al., 2020a; Al-Harrasi et al., 2020b). Various physiological and metabolomic changes occur in the cells under saline stress conditions. Among these, the accumulation and detoxification of reactive carbonyl species (RCS) have gained attention in relation to salinity stress tolerance (Mano, 2012; Biswas and Mano, 2015).
The RCS are highly reactive α-oxoaldehydes generated in all living organisms, from prokaryotes to eukaryotes (Islam and Ghosh, 2018). Among the RCS, methylglyoxal (MG) is a major endogenous toxin produced in plants under salt stress (Singla-Pareek et al., 2003; Mano, 2012). Under normal conditions, MG is synthesized by various enzymatic and non-enzymatic metabolic reactions. MG is also a by-product of glycolysis via the spontaneous decomposition of triose phosphate intermediates under aerobic glycolysis. In addition, MG is formed during protein and fatty acid metabolism (Desai et al., 2010), as well as oxidative degradation of carbohydrates, nucleic acids, and lipids under stress conditions (Degenhardt et al., 1998).
MG is an essential signaling molecule in plant adaption to stress conditions (Hasanuzzaman and Fujita, 2011; Hoque et al., 2016). However, hyperaccumulation of MG is detrimental to plants as it is a potent cytotoxic macromolecule (Hoque et al., 2012; Jana and Yaish, 2020a). To overcome MG hyperaccumulation in the cell and maintain it at a non-toxic level, MG is detoxified via the highly conserved glyoxalase pathway (Kalapos, 1994; Ferguson et al., 1998; Yadav et al., 2005) or via the NAD[P]H-dependent aldo-keto reductase (AKR) pathway (Vander Jagt et al., 1992; Schumacher et al., 2018). The glyoxalase detoxification pathway comprises of the GSH-dependent glyoxalase I (GLYI; lactoylglutathione lyase; EC 4.4.1.5), which converts MG to S-lactoylglutathione (Thornalley, 1990), and glyoxalase II (GLYII; hydroxyacylglutathione hydrolase; EC 3.1.2.6), which converts S-lactoylglutathione to d-lactate (Norton et al., 1990; Ghosh et al., 2014). Glyoxalase III, also known as DJ-1, (GLYIII; DJ-1/PfpI/Hsp31 domain-containing proteins) represents a GSH-independent pathway that converts MG to d-lactate in a single step (Ghosh et al., 2016) and has been previously reported in humans (Lee et al., 2012), Saccharomyces cerevisiae (Bankapalli et al., 2015), Candida albicans (Hasim et al., 2014), Arabidopsis thaliana (Kwon et al., 2013), Oryza sativa (Ghosh et al., 2016), and Glycine max (Islam and Ghosh, 2018). DJ-1 has been shown to play an important role in neuronal protection against oxidative stress and ROS homeostasis, while DJ-1 mutations are associated with the onset of Parkinson's disease in humans (Biosa et al., 2017). Previous reports have shown that overexpression of AtDJ1a enhances abiotic stress tolerance in Arabidopsis plants subjected to environmental stresses, however loss-of-function of the gene resulted in accelerated death in aging plants (Xu et al., 2010). It has also been reported that the loss-of-function of AtDJ1C resulted in non-viable, albino seedlings indicating its essential role in chloroplast development (Lin et al., 2011).
Our long-term aim is to functionally characterize the glyoxalase III gene family in date palm (Phoenix dactylifera L.), which is a plant that tolerates a wide range of abiotic stresses including drought, high temperature, and salinity; to understand the nature of this tolerance and then use this knowledge to enhance it in date palm and other crops.
Here, we report that mining of the date palm genome helped us to identify four PdDJ-1 genes as DJ-1 orthologs. qPCR analysis revealed that the genes were expressed in a tissue-specific manner under the different stress conditions tested, and the epitope expression combined with confocal microscopy localization assay showed that PdDJ-1 proteins have accumulated either in the chloroplast or the cytosol. Heterologous expression of the PdDJ-1 genes enhanced growth and MG detoxification in E. coli cells under salinity stress, oxidative stress, and exogenous MG stress. Further, PdDJ-1 was also able to complement the loss-of-function of the yeast hsp31Δ knockout lines, enhancing their MG stress tolerance, antioxidant activity, and salinity stress tolerance. Overexpression of most PdDJ-1 in Arabidopsis did not yield viable plants; however, functional characterization of the PdDJ-1C resulted in albino and unusual Arabidopsis phenotypes, which may indicate that PdDJ-1C plays a role in chloroplast development in date palms.
2 MATERIALS AND METHODS
2.1 Plant growth conditions and treatments
The date palm (P. dactylifera L.) seeds (cv. Khalas) were extracted from the fruit and thoroughly washed under tap water to remove any debris adhering to the surface. The seeds were then surface sterilized using 70% ethanol for 10 min and further rinsed twice for 10 min each using sterile distilled water. The seeds were allowed to germinate by incubation in moist sterile vermiculite at 37°C for 10 days. The germinated seeds were then transferred to 2-l pots containing multipurpose compost (Bulrush Horticulture Ltd). The pots were maintained in the greenhouse under controlled environmental conditions at 30°C, 350 μmol m−2 s−1 photon flux and a photoperiod of 16/8 h-light/dark cycle. The pots were irrigated to field capacity with distilled water on a weekly basis for about 3 weeks, after which the pots were divided into four sets according to the irrigation solutions (treatments): control (distilled water), salt stress (300 mM NaCl), oxidative stress (10 mM H2O2), and methylglyoxal stress (10 mM MG). The treatments were applied for 6 weeks before the plants were harvested, and various parameters were measured.
2.2 Identification of PdDJ-1 in P. dactylifera L.
BLASTP search was carried out on the date palm (taxid:42345) genome on the NCBI database to identify putative PdDJ-1 proteins using the specific domain sequence (KVTVAGLAGKDPVQCSRDVMICPDTSLEDAKTQGPYDVVVLPGGNLGAQNLSESPMVKEILKEQESRKGLIAAICAGPTALLAHEVGFGCKVTTHPLAKDKMMNGSHYSYSESRVEKDGLILTSRGPGTSFEFALAIVEALV), retrieved from the Pfam database. Hidden Markov Model (HMM) on the Pfam database was used to analyze the retrieved protein sequences for the presence of the DJ-1/PfpI domain (PF01965.24), thus confirming the identity of the retrieved sequences.
2.3 In silico analyzes and subcellular localization prediction
The theoretical molecular weight (MW) and the calculated isoelectric point (pI) of the PdDJ-1 proteins were predicted using the pI/MW tool on the ExPASy online platform (Bjellqvist et al., 1993). The PlantCARE tool was utilized to predict the presence of cis-acting elements, 1.5 kb upstream in the promoter region of the genes (Lescot et al., 2002). The GSDS 2.0 tool was implemented to verify the presence of introns in the genomic DNA (Hu et al., 2015). The MEME tool was used to predict the occurrence of conserved motifs (Bailey et al., 2015). The SOPMA consensus prediction from multiple alignment tool was applied to predict the protein secondary structure (Geourjon and Deleage, 1995). For evaluating the evolutionary relationship between the DJ-1 proteins of P. dactylifera L., O. sativa, and A. thaliana, an unrooted phylogenetic tree was constructed by using the neighbor-joining model with pairwise deletion and a bootstrap value of 1000 replications utilizing MegaX software (Kumar et al., 2018). The subcellular localization of the deduced amino acid sequences of the DJ-1 gene family was predicted using the CELLO v.2.5 prediction tool (Yu et al., 2006).
2.4 Cloning of the PdDJ-1 coding regions and quantitative PCR (qPCR)
Total RNA was isolated from date palm root and leaf samples using the MRIP method (Xiao et al., 2012), and cDNA was synthesized using the SuperScript IV Reverse Transcriptase (Invitrogen). PdDJ-1 genes from date palm were amplified using the cDNA with the DreamTaq Master Mix (ThermoScientific, MA, USA) and gene-specific oligonucleotides (Table S1). The PCR products were cloned into the pGEM-T Easy vector system (Promega). The cloned products were verified by sequencing, following which, the attB1 and attB2 sites were ligated to the 5′ and 3′ ends of the cDNA. Subsequently, the BP-clonase (Invitrogen) reaction was performed to produce the Gateway-entry clone pDONR-Zeo-PdDJ-1. Additionally, the genes were cloned into the bacterial expression vector pET-DEST42 (Invitrogen), the yeast expression vector pYES-DEST52 (Invitrogen), the nEYFP tagged expression vector pSITE- nEYFP-C1 (TAIR stock ID-CD3-1648) and the plant expression vector pEarleyGate 203 (TAIR stock ID-CD3-689) using the LR-clonase reaction (Invitrogen). The empty bacterial vector (negative control) was generated using the BsrGI restriction enzyme (ThermoScientific) to excise the Gateway cassette of pET-DEST42 vector followed by recircularization using T4 ligase (New England Biolabs). qPCR was performed using Fast-SYBR kit (Invitrogen) and gene-specific primers (Table S1). The expression data were analyzed using the previously published 2−ΔΔCT method (Livak and Schmittgen, 2001), and ACTIN as a reference gene (Patankar et al., 2016).
2.5 Abiotic stress tolerance assay in E. coli
The bacterial expression cassettes pET-DEST42-PdDJ-1 and the empty pET-DEST42 vector were introduced into BL21(DE3) E. coli cells and cultured until mid-exponential phase (OD600 0.4–0.6) in liquid Luria-Bertani (LB) medium containing 1 mg/ml ampicillin at 37°C. The genes were induced by adding 0.5 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) in the presence of different stress conditions, including, 250 mM NaCl, 5 mM H2O2 and 0.5 mM MG. The growth rate was quantified by recording the optical density (OD600) at 2 h intervals for a total time of 12 h. Subsequently, the cells were harvested for MG estimation.
2.6 Abiotic stress tolerance assay in yeast cells
The yeast expression cassettes pYES-DEST52-PdDJ-1 and the empty pYES-DEST52 vector (negative control) were mobilized into a yeast hsp31Δ knockout cell line of Saccharomyces cerevisiae (YDR533c; hsp31 MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0) (GE Healthcare Dhar Macon). The transformed cells were cultured until OD600 of 0.6–0.8 was reached in synthetic defined (SD) medium supplemented with glucose. Cells were spotted on Ura− SD-galactose agar plates supplemented with 7 mM MG and 1.5 mM H2O2. The cells were incubated at 30°C for 72 h. Additionally, the cells were inoculated into Ura− SD galactose liquid medium in the presence of different stress conditions i.e. 7 mM MG, 700 μM H2O2, and 500 mM NaCl. The growth rate was monitored at 6 h intervals for 30 h.
2.7 MG estimation
MG was quantified in the cells using a previously described methodology (Jain et al., 2018). Briefly, bacterial cells were harvested by centrifugation, lysed in 250 μl of 5 M perchloric acid for 15 min on ice and centrifuged at 11000 g for 10 min. Next, the supernatant was collected, neutralized with 1 M Na2HPO4 and centrifuged again at 11000 g for 10 min. 10 μl of NaN3 was then added to 1 ml of the obtained supernatant. Lastly, a reaction mixture containing 250 μl of 7.2 mM 1,2-diaminobenzene, 100 μl of 5 M perchloric acid, and 650 μl of the final supernatant was prepared and incubated in the dark for 3 h. The absorbance of the reaction mixture was then measured at 336 nm. The concentration of MG in the cell samples was estimated from a standard curve constructed using a 1–100 μM MG solution.
2.8 ROS accumulation in yeast cells
The effect of MG on ROS accumulation was determined in the hsp31Δ knockout yeast mutant cell line transformed with the different expression cassettes of pYES-DEST52-PdDJ-1. The yeast cells were grown until an early exponential stage (OD600 ~ 0.6) and harvested by centrifugation at room temperature. The cells were then treated with 7 mM MG for 7 h and 1 mM H2O2 for 1 h at 30°C. A peroxide-specific dye, 50 mM 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFAD) (Invitrogen), and a mitochondria-specific superoxide dye, 5 mM MitoSOX™ (Invitrogen), were used to stain the cells for 30 min at 30°C in the dark. The cells were then washed with phosphate buffer saline to remove the excess stain. The mean fluorescence intensity (MFI) of the stained cells (5000 events) was acquired using the BD FACSAria flow cytometer to estimate the ROS accumulation, and the results obtained from this analysis were plotted using the FlowJo software (Becton, 2019).
2.9 Subcellular localization of the PdDJ-1 genes in planta
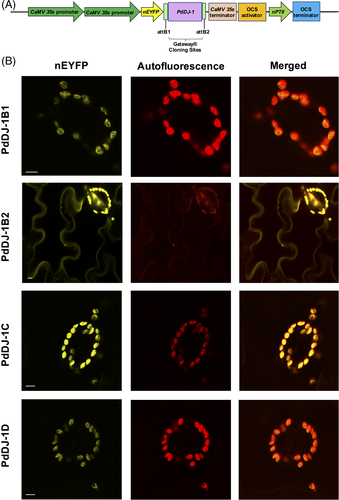
To determine the subcellular localization of the PdDJ-1 proteins in plant cells, the pSITE- nEYFP-C1-PdDJ-1 expression cassettes were first introduced into Agrobacterium tumefaciens GV3101:pMP90 cells by electroporation. Leaves of 3- to 4-week-old Nicotiana benthamiana plants were then co-infiltrated with Agrobacterium tumefaciens harboring various pSITE-nEYFP-C1-PdDJ-1 expression cassettes. Leaf epidermal cells were analyzed for expression using YFP filter set with ex-514 nm, em-520-560 nm for nEYFP-C1-PdDJ-1B1, nEYFP-C1-PdDJ-1B2, nEYFP-C1-PdDJ-1C, and nEYFP-C1-PdDJ-1D using a confocal laser scanning microscope (Fv3000 Olympus). The chloroplast autofluorescence was also imaged. The images were acquired using 60× objective lens magnification.
2.10 Functional screening of PdDJ-1C in Arabidopsis
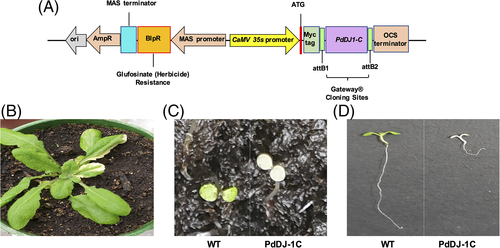
The binary plant expression cassette pEarleyGate 203-PdDJ-1C was first introduced into Agrobacterium tumefaciens LBA4404 by electroporation. The transformed cells were selected on YEB medium (5 g beef extract, 1 g yeast extract, 5 g peptone, 5 g sucrose, 300 mg MgSO4, 20 g agar and 1-l water) supplemented with 10 mg l−1 rifampicin and 50 mg l−1 kanamycin. The selected colonies were confirmed by PCR using the 35S promoter forward (5′- AAGGGTCTTGCGAAGGATAG-3′) and the OCS terminator reverse (5′- CGCATATCTCATTAAAGCAC-3′) primer sets. For the generation of transgenic Arabidopsis lines, 45-day-old wild type A. thaliana Columbia (Col-0) plants were transformed by the floral dip method (Clough and Bent 1998) and the resulting seeds (T0) were harvested and dried. These seeds were then screened for the PdDJ-1C transgenic plants. Firstly, the seeds were surface sterilized using 70% ethanol, rinsed in sterile distilled water twice, and then stratified at 4°C for 5 days. Then, the seeds were germinated in potting compost (Bulrush, United Kingdom). The germinated seedlings were sprayed with 0.01% Basta® (Bayer, Germany) herbicide solution on the 7- and 14th-day post-germination. The surviving transgenic plants (T1) were confirmed by PCR using phire plant direct PCR master mix (ThermoScientific) with the 35S promoter forward and the OCS terminator reverse primer sets. The resulting T2 seeds were harvested and dried. The T2 seeds were surface sterilized, stratified, and germinated on ½ Murashige and Skoog (MS) agar supplemented with 10 mg L−1 of Basta®.
2.11 Statistical analysis
The statistical significance was verified using one-way anova univariant analysis and Tukey's post hoc test, in SPSS software version 21 (Brosius, 2013).
3 RESULTS
3.1 Identification and sequence analysis of the DJ-1 gene family in P. dactylifera L.
BLASTP search on the NCBI database revealed the presence of four putative DJ-1 orthologs in the genome of P. dactylifera L., designated in this study as PdDJ-1B1, PdDJ-1B2, PdDJ- 1C, and PdDJ-1D with the largest being PdDJ-1C with 1322 bp and the smallest being PdDJ- 1D with an 1166 bp size (Table S2). Multiple sequence alignment of the retrieved PdDJ-1 genes revealed that PdDJ-1B1 and PdDJ-1B2 are the most similar with an alignment score of 96.1929, whereas PdDJ-1C and PdDJ-1D are the most dissimilar with an alignment score of 22.4507.
In silico analysis of the PdDJ-1 proteins indicated that PdDJ-1C has the largest molecular weight (47.17 kDa) and is the most alkaline protein (pI = 9.01), whereas PdDJ-1D has the lowest molecular weight (41.22 kDa) and is the most acidic protein (pI = 5.32). Further subcellular localization prediction of the PdDJ-1 proteins using the CELLO v.2.5 prediction tool, which uses a two-level support vector machine (SVM) system predicted that PdDJ-1B1 and PdDJ-1C were localized in the chloroplast whereas PdDJ-1B2 and PdDJ-1C in the cytoplasm.
Protein secondary structure prediction using the SOPMA tool indicated the dominance of alpha helices followed by random coils in the PdDJ-1B1, PdDJ-1B2, and PdDJ-1C proteins whereas random coils are more dominant than the alpha-helix in the PdDJ-1D protein (Table S3).
Promoter sequence analysis signified the presence of various cis-acting regulatory elements within 1.5 kb upstream of the PdDJ-1 cDNA, including potential environmental stress and plant hormone activated transcription factor binding sites that may regulate the expression of the PdDJ-1 genes (Table S4).
HMM profiling of the PdDJ −1 proteins, using the Pfam database, helped to identify the presence of two DJ-1/PfpI domains (PF01965.24) belonging to the Class-I glutamine amidotransferase superfamily (CL0014) within all the retrieved PdDJ-1 protein sequences (Table S5). Moreover, conserved domain analysis of the PdDJ-1 proteins in comparison to the DJ-1 proteins from various plant species denoted the two DJ-1/PfpI domains, boxed in red for the N-terminal and blue for the C-terminal, in addition to the conserved catalytic triad of aspartate/glutamate, cysteine, and tyrosine/histidine residues, which are boxed in green (Figure 1).
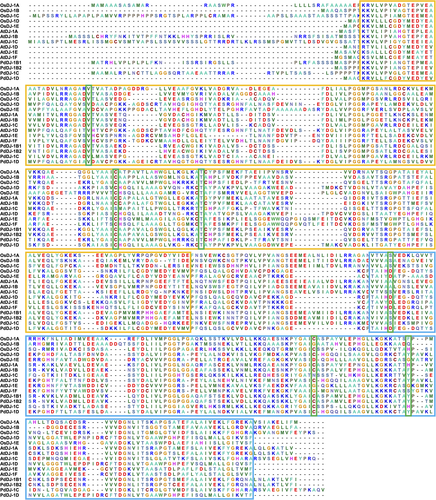
Further, pairwise alignment score of the P. dactylifera L. DJ-1 proteins to the homologs from O. sativa, and A. thaliana, showed that PdDJ-1B1 and PdDJ-1B2 have the highest alignment score to AtDJ-1B and OsDJ-1A, whereas PdDJ-1C is highly similar to AtDJ-1C and OsDJ-1D. In addition, PdDJ-1D is very similar to AtDJ-1D and OsDJ-1D (Table S6).
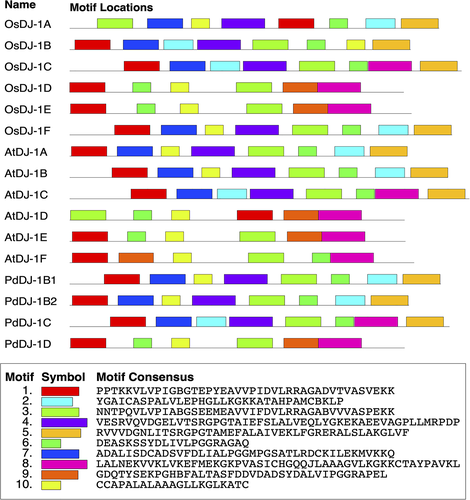
The two conserved DJ-1/PfpI domains are boxed in red for the N-terminal and blue for the C-terminal. The conserved catalytic triads aspartate/glutamate, cysteine, and tyrosine/histidine residues are boxed in green. The DJ-1 proteins from other well-characterized plant models include O. sativa OsDJ-1A-1 (LOC4324581), OsDJ-1B (LOC4324580), OsDJ-1C (LOC4339342), OsDJ-1D (LOC4337361), OsDJ-1E (LOC4350819) OsDJ-1F (LOC4341205), and A. thaliana AtDJ-1A (OAP06772.1), AtDJ-1B (OAP14691.1), AtDJ-1C (OAO96833.1), AtDJ-1D (OAP06941.1), AtDJ-1E (NP_030434.1), AtDJ-1F (OAP06168.1). Motif prediction using the MEME tool revealed the presence of various conserved motifs in the retrieved protein sequence from P. dactylifera L., O. sativa, and A. thaliana (Figure 2) showed that OsDJ-1B, OsDJ-1C, AtDJ-1A, AtDJ-1B, AtDJ-1C, PdDJ-1B1, PdDJ-1B2 and PdDJ-1C has seven identical motif sites each, whereas OsDJ-1D, AtDJ-1D, and PdDJ-1D has seven identical motifs sites each.
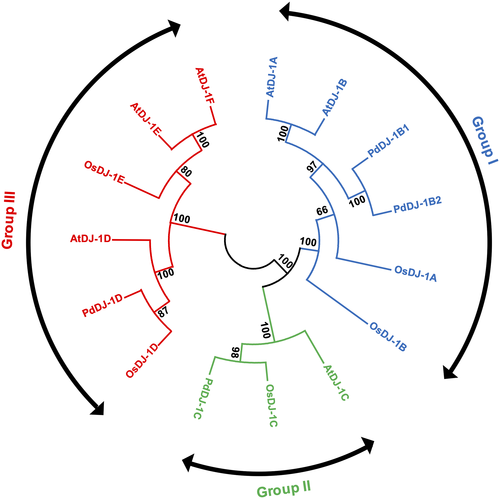
Phylogenetic analysis of the DJ-1 proteins from P. dactylifera L., A. thaliana, and O. sativa showed that the DJ-1 proteins are clustered into three groups. In Group I, PdDJ-1B1 and PdDJ-1B2 are grouped along with AtDJ-1A, AtDJ-1B, OsDJ-1A and OsDJ-1B. In Group II, PdDJ-1C is grouped along with AtDJ-1C and OsDJ-1C. In Group III, PdDJ-1D is grouped along with AtDJ-1D, OsDJ-1D, AtDJ-1E, AtDJ-1F, and OsDJ-1E (Figure 3).
3.2 Gene expression analysis of the PdDJ-1 genes under different stress conditions using qPCR
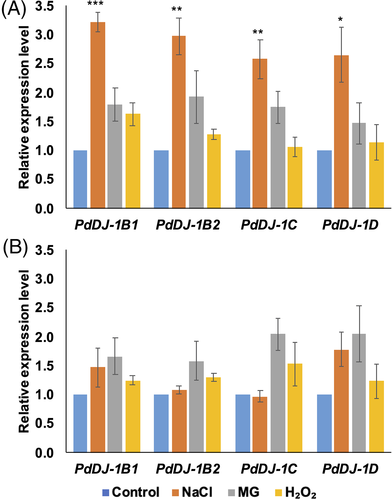
To examine the effect of different environmental stimuli on the expression of the PdDJ-1 genes, qPCR expression analyzes of the PdDJ-1 genes in the date palm tissues from the seedlings grown under control (distilled water), salinity (300 mM NaCl), oxidative (10 mM H2O2) and MG stress (10 mM MG) was conducted. The results showed that PdDJ-1 gene expression was altered in a stress- and tissue-dependent manner (Figure 4). Under NaCl stress, all the PdDJ-1 genes were upregulated, with PdDJ-1B1 (P ≤ 0.001), PdDJ-1B2 (P ≤ 0.01), PdDJ-1C (P ≤ 0.01) and PdDJ-1D (P ≤ 0.05) indicating significant upregulation in leaf when compared to the control leaves. There was also a trend of upregulation of all the PdDJ-1 genes in the leaves of the MG treated plants. In H2O2 treated leaves, only the PdDJ-1B1 gene showed a trend of upregulation (Figure 4(A)). A similar trend of non-significant upregulation of PdDJ-1B1 and PdDJ-1D gene expression was observed in the roots of the salinity treated plants. In addition, all the PdDJ-1 genes were non-significantly upregulated in the MG, and H2O2 treated roots (Figure 4(B)).
3.3 Heterologous expression of PdDJ-1 in E. coli confers tolerance to abiotic stresses
BL21(DE3) cells transformed with the expression cassettes pET-DEST42-PdDJ-1B1, pET- DEST42-PdDJ-1B2, pET-DEST42-PdDJ-1C and pET-DEST42-PdDJ-1D (Figure 5(A)) or with the control empty vector pET-DEST42 were subjected to different abiotic stresses, and their growth rate and MG accumulation were estimated. The results showed that overexpression of the PdDJ-1 genes have significantly (P ≤ 0.001) enhanced the growth in the E. coli cells under MG stress (0.5 mM MG), oxidative stress (5 mM H2O2) and salinity stress (250 mM NaCl) in comparison to control cells transformed with the empty vector (Figure 5(B)). In addition, quantification of the MG cellular concentration after 12 h showed that the PdDJ-1 overexpressed BL21(DE3) cells had a significantly (P ≤ 0.001) lower MG accumulation as compared to the empty vector control (Figure 5(C)).
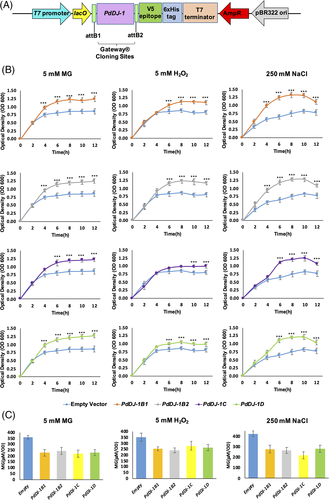
3.4 Functional complementation of S. cerevisiae knockout hsp31Δ cells
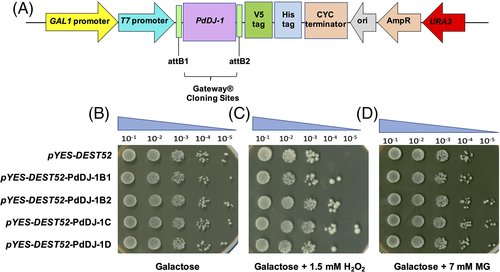
Hsp31Δ knockout yeast cells were transformed with expression cassettes pYES-DEST52- PdDJ-1B1, pYES-DEST52-PdDJ-1B2, pYES-DEST52-PdDJ-1C, and pYES-DEST52-PdDJ-.
1D and the control empty vector pYES-DEST52 (Figure 6(A)). The cells were then serially diluted and spotted on the control plate Ura− SD-galactose agar as a reference (Figure 6(B)). The results showed that overexpression of the PdDJ-1 genes enhanced the growth of hsp31Δ knockout yeast cells spotted on Ura− SD-galactose agar supplemented with 1.5 mM H2O2 (Figure 6(C)) and 7 mM MG (Figure 6(D)).
In addition, the cells were cultured in liquid SD-galactose along with different stresses, and their growth was monitored at 6 h intervals. The growth of the PdDJ-1-overexpressing hsp31Δ knockout yeast cells was significantly (P ≤ 0.001) enhanced compared to the empty vector cells under the abiotic stress conditions of 7 mM MG, 700 μM H2O2 and 500 mM NaCl (Figure 7).

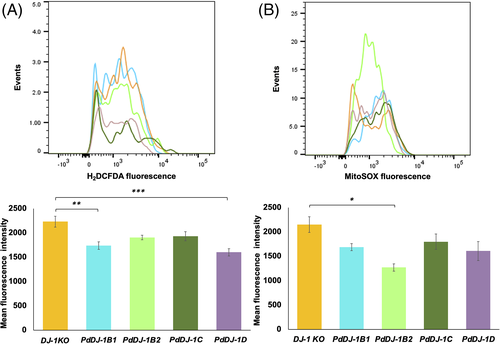
3.5 Overexpression of the PdDJ-1 genes reduced the accumulation of ROS
In this experiment, the extent of ROS accumulation was determined in the hsp31Δ knockout yeast cell line in response to 7 mM MG stress. The results showed a significant reduction of cytosolic ROS accumulation in PdDJ-1B1 (P ≤ 0.05) and PdDJ-1D (P ≤ 0.001) overexpressing lines as compared to the control empty vector cells (Figure 8(A)). In addition, PdDJ-1B2 transformed cells indicated a significant (P ≤ 0.05) reduction in the accumulation of superoxide molecules in the mitochondria (Figure 8(B)).
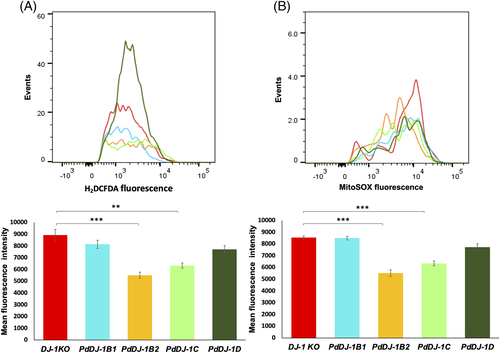
In response to oxidative stress treatment using 1 mM H2O2, the hsp31Δ knockout yeast mutant cells transformed with PdDJ-1B2 (P ≤ 0.001) and PdDJ-1C (P ≤ 0.001) showed a significant reduction of cytosolic ROS accumulation (Figure 9(A)). In addition, the mitochondrial superoxide molecules accumulation was also significantly (P ≤ 0.001) reduced as compared to the cells harboring the empty vector (Figure 9(B)).
3.6 Subcellular localization of the PdDJ-1 proteins
Confocal microscopy was used to localize the PdDJ-1 proteins upon the expression of the different pSITE-nEYFP-C1-PdDJ-1 cassettes (Figure 10(A)) in N. benthamiana leaf epidermal cells. The results indicated that PdDJ-1B1, PdDJ-1C and PdDJ-1D were localized to the chloroplast, whereas PdDJ-1B2 was localized to the cytoplasm and chloroplasts (Figure 10(B)).
3.7 Functional screening of PdDJ-1C in A. thaliana
Genetic transformation of Arabidopsis with most PdDJ-1 genes failed to produce viable transgenic plants. However, it was possible to transform Arabidopsis with PdDJ-1C genetically. Transgenic overexpression of the PdDJ-1C gene using the pEarleyGate203 expression cassette (Figure 11(A)) in Arabidopsis resulted in the generation of a variegation phenotype in the leaves of T1 plants (Figure 11(B)). Furthermore, selfing of the T1 transgenic lines resulted in non-viable albino homozygous plants (Figure 11(C)), which showed significant growth inhibition as compared to the wild type A. thaliana Columbia (Col-0) seedlings of the same growth stage (Figure 11(D)).
4 DISCUSSION
Soil salinization, a major limiting factor affecting crop growth and yield, is increasing with climate change and rising sea levels. Salinity stress leads to morphological, cellular, physiological, and biochemical changes in the plants. Numerous reports have shown that salinity leads to impaired water uptake (Isayenkov and Maathuis, 2019), reduced photosynthetic activity (Chaves et al., 2009; Yaish et al., 2017), ionic imbalance and toxicity (Zhu, 2003; Munns, 2005; Rengasamy, 2010), accumulation of ROS (AbdElgawad et al., 2016; Jana and Yaish, 2020b), upregulation of antioxidant genes (Dionisio-Sese and Tobita, 1998; Abogadallah, 2010), alteration of the biosynthesis and accumulation of phytohormones (Fahad et al., 2015), and accumulation of RCS (Mano, 2012; Filippou et al., 2014; Al Kharusi et al., 2019; Patankar et al., 2019).
Among the RCS, accumulation of MG, a highly reactive cytotoxic metabolite under salinity stress, has been widely reported (Yadav et al., 2005; Mostofa et al., 2018). In order to overcome the toxic effects of MG hyperaccumulation in plant cells, such as inactivation of various cellular macromolecules (Jain et al., 2018), plants have evolved multiple MG detoxification pathways (Mostofa et al., 2018), one of which is the GSH-independent glyoxalase III/DJ-1 pathway (Zhao et al., 2014; Ghosh et al., 2016).
In this study, four PdDJ-1 genes were identified from the genome of P. dactylifera, which was facilitated by the presence of the two conserved DJ-1/PfpI domains on the N- and C-terminals of the protein sequences along with the conserved catalytic triads (aspartate/glutamate, cysteine, and tyrosine/histidine residues) as previously described (Ghosh et al., 2016). The central cysteine residue is directly involved in the catalysis and, therefore, it is a key component for the DJ-1 enzymatic activity (Lin et al., 2012). Furthermore, motif analysis showed that the DJ-1 proteins are highly conserved, except for the DJ-1D homologs that have a variable number of motifs present in their sequences from different species.
The concentration of H2O2, MG, and NaCl used in these experiments was optimized empirically. It was observed that the effective nonlethal concentration of each of these chemicals varies based on the organism used (date palm, S. cerevisiae, or E. coli).
The significant upregulation of all the PdDJ-1 genes under salinity stress in the date palm leaves (Figure 4(A)), suggests that the genes are important in MG detoxification, which has been shown to be hyperaccumulated under salinity stress conditions (Yadav et al., 2005). Moreover, this observation is consistent with previous studies reporting the upregulation of the DJ-1 gene family under abiotic stress in crops such as Glycine max (Islam and Ghosh, 2018), Medicago truncatula (Ghosh, 2017) and Erianthus arundinaceus (Manoj et al., 2019). In addition, the DJ-1 transgenic plants exhibited biotic and abiotic stress tolerance along with a reduced MG accumulation in Arabidopsis (Xu et al., 2010) and Nicotiana tabacum (Melvin et al., 2017). These observations provide an indication that the PdDJ-1 genes may potentially enhance the abiotic stress tolerance of the date palm as well under specific circumstances.
The accumulation of MG in E. coli under various stress conditions has been previously documented (Chakraborty et al., 2014), and it leads to inhibition of growth (Zhu et al., 2001; Lee and Park, 2017). Therefore, heterologous expression of PdDJ-1 in our study would have enhanced the glyoxalase activity of the E. coli cells, thereby significantly reducing MG accumulation and improving the growth of the overexpressing E. coli cells under different stress conditions (Figure 4).
Hsp31 is a DJ-1/ThiJ/Pfp protein, which has previously been shown to have antioxidant and MG detoxification activities (Wilson et al., 2004; Skoneczna et al., 2007; Natkańska et al., 2017). Therefore, the MG- and antioxidant-detoxification ability of the PdDJ-1 protein family was tested using the closely related homologous knockout mutant of the yeast cells. The S. cerevisiae heat shock protein hsp31 is a stress-induced member of the ThiJ/DJ-1/PfpI protein family and has been reported to play an important role in the diauxic-shift reprogramming and cell survival in the stationary phase (Miller-Fleming et al., 2014). Hsp31 has GSH-independent methylglyoxalase activity and is important in regulating redox homeostasis (Bankapalli et al., 2015). Thus, the loss-of-function hsp31Δ knockout yeast mutant YDR533C cell line resulted in oxidative and MG hypersensitive phenotypes (Martinat et al., 2004; Hasim et al., 2014). Here we report that the overexpression of the PdDJ-1 genes in the yeast knock out YDR533C cell lines was able to complement the loss of function of the Hsp31/DJ-1 gene, thereby enhancing the growth of the cells under MG, oxidative and salinity stress conditions (Figure 5). Furthermore, the accumulation of cytosolic ROS and mitochondrial superoxide molecules was significantly reduced with the overexpression of the PdDJ-1 genes in the yeast knockout cells under MG and oxidative stress conditions, thus indicating the antioxidative activity of the PdDJ-1 genes. These findings are in agreement with previous studies reporting the critical role of DJ-1 in oxidative stress tolerance and mitochondrial dynamics in S. cerevisiae (Bankapalli et al., 2020).
Overexpression of most PdDJ-1 genes in Arabidopsis failed to produce transgenic plants, potentially since MG is a critical signaling molecule in plants (Hasanuzzaman and Fujita, 2011; Hoque et al., 2016). In addition, it has also been reported that higher plants produce glycating agents such as MG, which is the main precursor for the formation of advanced glycation end products (AGE) during photosynthesis in the chloroplast (Takagi et al., 2014). Moreover, it has been suggested that glycating agents produced by plants are essential for the photosynthetic metabolism (Rabbani et al., 2020). Therefore, overexpressing the PdDJ-1 genes in Arabidopsis may negatively impact MG homeostasis, thereby inhibiting the photosynthetic machinery and resulting in non-viable plants. However, overexpression of only one gene (PdDJ-1C) in Arabidopsis resulted in the production of non-viable albino plants. Phylogenetic analysis showed that PdDJ-1C is the most similar ortholog to the Arabidopsis AtDJ-1C genes (Figure 3). Therefore, overexpression of PdDJ-1C may alter the level of endogenous AtDJ-1C, thereby interfering with the photosynthetic machinery. This may explain why the transgenic plants had an albino phenotype. Consistently, a previous study has also reported that transgenic silencing of the AtDJ-1C genes resulted in the generation of non-viable albino Arabidopsis plants (Lin et al., 2011). This observation, along with the results from the in vivo localization experiment of the PdDJ-1C in the chloroplast (Figure 10(B)), may suggest that the PdDJ-1C gene is vital for MG homeostasis and chloroplast development. However, further experimental work is required to describe the comprehensive mechanism of action of the PdDJ-1 gene family in plants.
Computational prediction of the subcellular location of the PdDJ-1B2 showed that this protein is cytoplasmic, however experimental in vivo localization using N. benthamiana leaf epidermal cells showed that PdDJ-1B2 was localized to the cytoplasm and chloroplasts. This observation may open the door to further investigations leading to the identification of other functions controlled by this PdDJ-1 protein member.
In conclusion, PdDJ-1 plays an essential role in abiotic stress tolerance in the date palm. This was clear from the in vivo studies, which showed an enhanced abiotic stress tolerance in the transgenic bacteria, yeast, and a pleiotropic phenotype in transgenic Arabidopsis plants. Members of the PdDJ-1 gene family identified in this study could be good candidates for breeding programs aimed at identifying date palm varieties with high abiotic stress tolerance features.
ACKNOWLEDGMENTS
This study is supported by the generous grant number RC/RG-SCI/BIOL/18/01 from the research council (TRC), Oman to MWY. Authors would also like to thank the Central Analytical and Applied Research Unit (CAARU) and Dr. Sirin A. Adham, Department of Biology, SQU, for the help in the flow cytometry experiments.
AUTHOR CONTRIBUTIONS
Gerry A. Jana and Mahmoud W. Yaish conceived and designed the experiments. Mahmoud W. Yaish supervised the work, provided the required supplies, and performed the final editing of the manuscript. Gerry A. Jana analyzed the data and wrote the manuscript. Pannaga Krishnamurthy performed the subcellular localization experiment and revised the manuscript, Prakash P. Kumar revised and edited the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




