The karrikin ‘calisthenics’: Can compounds derived from smoke help in stress tolerance?
Abstract
Karrikins (KARs) are unique butenolides derived as a by-product of incomplete combustion during wildfire. Some receptive plant species respond to KARs in the form of accelerated germination. These molecules originate from stress to mediate tolerance against different sub-optimal conditions like oxidative stress, drought and low-light intensity (shade stress). KARs promote seed germination, seedling establishment and ecological diversity by accelerating the abundance of selective communities of plants. The signaling pathway is closely related, yet unique from strigolactones (SLs). Due to the structural relatedness with SLs, KARs have potential roles in mediating abiotic stress tolerance in plants. In addition, the KAR directly/indirectly interact with crucial phytohormones like abscisic acid, gibberellic acid, auxins and ethylene. This article is a summarized update on KAR research in recent times. The exhaustive discussions would be beneficial for understanding the extraordinary strategy devised by nature to protect plants from stress using a molecule which is itself generated out of stress.
Abbreviations
-
- ABA
-
- abscisic acid
-
- ABRE
-
- ABA responsive element
-
- AsA-GSH
-
- ascorbate-glutathione
-
- CAT
-
- catalase
-
- GA
-
- gibberellic acid
-
- GA3ox1
-
- GA-3-oxidase 1
-
- GAs
-
- gibberellic acids
-
- GR
-
- glutathione reductase
-
- GST
-
- glutathione S-transferase
-
- HSP101
-
- HEAT SHOCK PROTEIN 101
-
- IAA
-
- auxins
-
- KAI2/HTL
-
- KAR insensitive 2/hyposensitive to light
-
- KARs
-
- karrikins
-
- LHCB1
-
- light harvesting chlorophyll binding protein B1
-
- PhyA
-
- phytochrome A
-
- PIN1
-
- PINFORMED1
-
- ROS
-
- reactive oxygen species
-
- SLs
-
- strigolactones
-
- SMAXL
-
- SUPPRESSOR OF MAX2-LIKE
-
- SOD
-
- superoxide dismutase
-
- STH7
-
- salt tolerance homolog 7
-
- TF
-
- transcription factor
-
- UV
-
- ultraviolet
Introduction
Seed germination is a vulnerable and crucial physiological process. Unfavorable environmental conditions like drought, salinity, extremes of temperature, heavy metal toxicity, low exposure to light (shade), etc. negatively regulate normal seed development and germination (Banerjee and Roychoudhury 2016a, Banerjee et al. 2017, Banerjee and Roychoudhury 2018a). The process of germination involves the dormant seeds to respond to a series of endogenous and abiotic cues. Successful physiological responses during this process result in the establishment of seedlings (Imran et al. 2013, Paul et al. 2017). The entire process of dormant seed strengthening and germination is mediated via a crucial interplay among phytohormones like gibberellic acids (GAs), abscisic acid (ABA) and auxin [indoleacetic acid (IAA)] (Miransari and Smith 2014). The GA represses the DELLA transcription factors (TFs) and stimulates the transcription of GAMyb. This TF in turn induces α-amylase in the aleurone layer cells and breaks the seed dormancy (Liu and Hou 2018). ABA on the contrary represses GA-signaling and promotes seed dormancy (Banerjee and Roychoudhury 2017). IAA also regulates seed dormancy and the plant shade avoidance syndrome (Procko et al. 2014). Such low-light stress is responsible for retarded growth rate and lower crop yields (Gommers et al. 2013). Karrikins (KARs) are by-product compounds produced during pyrolysis. These are closely associated with the strigolactone (SL)-signaling pathway and stimulate several plant species to germinate en masse (Waters et al. 2012, Banerjee and Roychoudhury 2018b). KARs also regulate plant responses during shade avoidance (Flematti et al. 2015). Thus, in spite of not being classified as a ‘phytohormone,’ KAR exhibit interesting interactions among the conventional plant hormones during the above mentioned physiological processes (Meng et al. 2017).
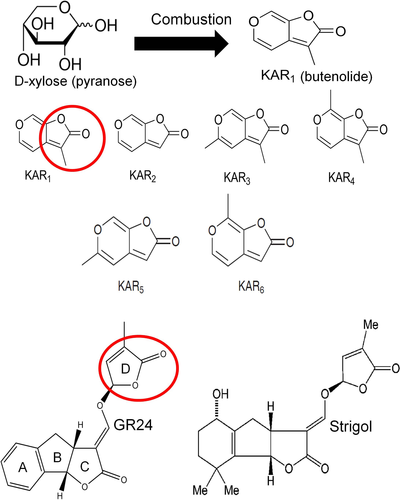
KAR has its origin from an environmental hazard, viz., forest fire (Nelson et al. 2012). The smoke produced by fire when passed through water formed ‘smoke water’ (De Lange and Boucher 1990). Liquid chromatography was used to separate the compounds found in smoke water into several fractions. Germination bioassays using the fractions and their further fractionations led to the isolation of a group of butenolides fused to a pyran ring. One of the lactones were systematically named as 3-methyl-2H-furo[2,3-c]pyran-2-one. Detailed investigations identified many more closely associated organics which were together designated as KAR (Flematti et al. 2004). 3-methyl-2H-furo[2,3-c]pyran-2-one is also referred to as KAR1. Fig. 1 illustrates a possibility of KAR1 synthesis via the combustion of D-xylose (a pyranose sugar molecule). It also highlights the various structures of KAR. In the ecosystem, KAR formed by forest fires remain bound to soil particles. Once washed by rainfall, these lactones stimulate the germination of fire ephemerals and accelerate their community abundance (Flematti et al. 2015). Several studies have shown the positive effects of these stress-generated compounds to induce germination in other economically important plant species and even regulate abiotic stress tolerance via the interaction with crucial phytohormones. Thus, KAR research in abiotic stress physiology is a novel avenue awaiting novel findings.
Understanding the KAR dynamics
The strategy adopted by the fire ephemerals to respond to KAR for germination has remained specialized yet successful. During forest fires, the nutrients bound to the plants are released and an open habitat is created. One cycle of rainfall under such circumstances provides an edge to the KAR-responding seeds over other competing plant species capable of colonizing the landscape (Flematti et al. 2015). These seeds remain viable in the soil for several cycles of wetting and drying till a forest fire precedes atmospheric precipitation (Flematti et al. 2015). Interestingly, KAR do not immediately stimulate the germination of seeds that fall on a charred landscape. This is because a period of ‘after-ripening’, i.e., incubation of seeds buried within the soil is quintessential for responding to the KAR induction (Nelson et al. 2012). However, the chances of KAR degradation in the soil during after-ripening are reduced by the presence of several aromatic compounds in smoke, which act as ‘organic sunscreens’ by absorbing the harmful ultraviolet (UV) rays. Otherwise, the KARs are decayed in direct exposure to UV rays from natural sunlight (Scaffidi et al. 2012). Active KARs are retained in the soil for about seven years (Flematti et al. 2015) .
The KAR response is hypothesized to have evolved independently in different plant groups. Several plant species including trees, herbs, shrubs, annuals and even agricultural weeds and horticultural plants respond to KAR (Stevens et al. 2007, Nelson et al. 2012). This illustrates that the KAR-induction is a basic physiological response fundamentally imbibed within the plant genome, which the fire ephemerals have intelligently converted to their advantage in post-charred landscapes. KAR have been reported to promote the growth rate of maize, tomato and Arabidopsis thaliana (Arabidopsis) seedlings and alter photomorphogenesis to produce seedlings with reduced stature and larger leaves (Waters et al. 2014). The effectiveness of KAR can be compared to that of phytohormones, as the seeds of some fire ephemerals can sense and respond to very low concentrations (10−10 M) of the most potent KAR, viz., karrikinolide (KAR1) (Flematti et al. 2015).
KAR induces seed germination and seedling development
KAR accelerates the germination of after-ripened seeds which are well conditioned and also receptive to KAR induction. The Ler ecotype of Arabidopsis initially remains dormant in the freshly harvested state and so these seeds were assayed for KAR-induced germination. Since nitrates aid in overcoming the primary dormancy of Arabidopsis seeds, the bioassay was performed using water. Interestingly, it was observed that compared to GA4, epi-brassinolide and the ethylene precursor, 1-aminocyclopropane-1-carboxylic acid, KAR exhibited higher stimulatory effect on the germination of Arabidopsis seeds (Nelson et al. 2009). However, this stimulation did not alter the endogenous levels/balance of GA and ABA (Nelson et al. 2009). Arabidopsis seeds were more responsive to KAR2 though KAR1 and KAR3 have been recorded as the most active KARs (Flematti et al. 2007). KAR4 containing a 7-methyl group has minimal activity in Arabidopsis. The lactone in KARs has close similarity with the D-ring in SLs (Fig. 1) (Waters et al. 2012, Waters et al. 2017). Application of KAR has been found to have more profound stimulatory effects on Arabidopsis seed germination in comparison to equivalent concentrations of the SL analogue GR24 (Nelson et al. 2010). However, the SL biosynthetic mutants of Arabidopsis do not exhibit delayed germination. This shows that SLs play less prominent roles in altering the period of seed dormancy (Scaffidi et al. 2013). KARs also play crucial roles in improving seed sensitivity to low-fluence rates of light, thus promoting light-dependent germination (Nelson et al. 2010).
In spite of the close structural similarities between KAR and SL, plants like Arabidopsis can distinguish between the two. Elongation of the hypocotyl is inhibited by both KAR and GR24. However, cotyledon expansion with subsequent chlorophyll accumulation is induced only by KAR (Nelson et al. 2010, Waters et al. 2012). Exogenous application of KAR under continuous red light of up to 20 μmol m−2 s−1 promoted seedling photomorphogenesis. The requirement of continuous red light is crucial, as weak blue light can also inhibit the elongation of hypocotyls and mask the effects of KAR (Waters et al. 2014). The hypocotyl elongation is suppressed by KAR1 and KAR2 in a dose-dependent manner. The extent of inhibition is so evident that the hypocotyl length of Arabidopsis plants treated with 1 μM KAR2 was half that of control seedlings (Waters and Smith 2013). Similar kind of responses was observed in the Lactuca sativa and Brassica tournefourtii seedlings (Nelson et al. 2010). Thus, KAR stimulates seed germination and promotes seedling vigor under a continuous intensity of light to improve seedling establishment in charred landscapes. KAR-induced photomorphogenesis is dependent on both phytochrome A (PhyA) and PhyB. Hence, KAR requires a constant source of light for altering the morphology of Arabidopsis seedlings (Nelson et al. 2010). Both GR24 and KAR1 were found to induce the expression of salt tolerance homolog 7 (STH7) in Arabidopsis (Thussagunpanit et al. 2017). Application of GR24 and KAR1 also induced genes associated with photosynthesis like light harvesting chlorophyll binding protein B1 (LHCB1) and ribulose bisphosphate carboxylase small subunit (Rbcs). Thus, it has been proposed that KAR and SL co-ordinate photomorphogenesis in an STH7-dependent fashion (Thussagunpanit et al. 2017).
The central role of the ABA-dependent TF, HY5 is well characterized under both blue and red light-mediated responses (Banerjee and Roychoudhury 2016a). Interestingly, a G-box cis element [ABA responsive element (ABRE)] has been identified in the promoters of a large number of KAR-responsive genes like KAR insensitive 2/hyposensitive to light (KAI2/HTL) (Waters et al. 2014). This ABRE might be a binding site for HY5. Thus, this juncture can be a convergence point of KAR response and light-mediated signaling during seedling development.
Biochars in determining community diversity
KARs are formed from charred biological materials or biochars which have potential roles in carbon sequestering, reforestation, edaphic remediation and re-vegetation. These are also used as beneficial soil additives during agriculture (Lehmann 2007, Beesley et al. 2011, Spokas et al. 2012, Van De Voorde et al. 2014, Cernansky 2015, Drake et al. 2015, Jeffery et al. 2015). Soil treated with biochars exhibit high-ion exchange capacity and organic carbon content. These factors promote plant growth (Crane-Droesch et al. 2013). However, the growth-inducing property of biochars is biphasic and species-specific, since the plant-biochar relation can range from highly positive (+200%) to highly negative (−85%) (Mukherjee and Lal 2014). Jeffery et al. (2017) used a global-scale meta-analysis to show that biochars had minimal effect on crop yield in the temperature latitudes, whereas the growth of crops was accelerated by 25% in the tropics under similar treatments. Application of biochars on native species of Australia resulted in the change in the composition of plant community and density (Drake et al. 2015). The soil microenvironment and structure of the soil microbial community is also altered after biochar application (Liao et al. 2016). In an experiment, it was observed that addition of cotton straw biochar promoted the edaphic microbial biomass, utilization of carbon substrate and enzyme activities associated with carbon and nitrogen transformation in drip-irrigated desert soil (Liao et al. 2016).
Biochars contain compounds like KAR (promote germination) and even trimethylbutenolide (inhibit germination). Subtle balance between the two compounds determine species-dependent seed establishment after rainfall (Light et al. 2010). Kochanek et al. (2016) identified and quantified KAR1 in biochars and determined its unique roles in regulating community diversity. The effects of KAR1 from biochars on germination were experimented on Solanum orbiculatum and Brassica tournefortii. Plasticity to KAR1 was visualized in Lactuca sativa and Lycopersicon esculentum (Kochanek et al. 2016). Germination of dormant seeds and seedling establishment was induced by the KAR- abundant biochars in a way similar to post-fire incidences (Kochanek et al. 2016). Thus, KAR influences community composition and diversity after forest fires by physiologically accelerating the abundance of receptive plant species. Nature has formulated the production of the ‘smoke-compound’, viz., KAR from an environmental disaster to initiate a new generation of KAR-sensitive plants to predominate the barren landscape. The occurrence of such ecological succession is highly significant in the global phytogeographical context.
KAR signaling in plants
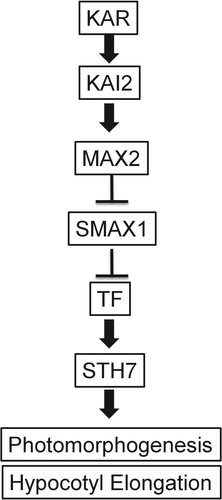
The KAR signaling cascade has been mainly deciphered in the model plant Arabidopsis thaliana. Fig. 2 presents a pictorial representation of KAR signaling, whereas Table 1 compares the molecular components of KAR signaling pathway with those of the SL signaling cascade. The gene named more axillary growth 2 (MAX2) was found to be instrumental in KAR responses. This gene identified by a forward genetic screen of kai mutants encodes an F-box protein (Nelson et al. 2011). The functional F-box protein associates with the components of the E3 ubiquitin protein ligase complexes like ASK1 and Culin (Stirnberg et al. 2007).
| Inter-relation among signaling components | Characteristics | Karrikin signaling | Strigolactone signaling |
|---|---|---|---|
| Signal | Analog | KAR | SLs |
| Receptor | Homolog | KAI2 | D14 |
| E3 ligase | — | MAX2 | MAX2 |
| Repressor | Homolog | SMAX1 (inhibited by MAX2) | D53 (inhibited by MAX2) |
| Transcription factor | — | Active TF due to inhibition of SMAX1 | Active TF due to inhibition of D53 |
| Responses | — | Activation of downstream genes | |
Homologs of MAX2 have been identified in Oryza sativa and Pisum sativum and these mediate SL responses along with seed germination (Waters et al. 2014). Thus, molecular components involved in KAR-specific responses might also be related with SL signaling. The Arabidopsis and rice dwarf 14 (D14) gene involved in repressing secondary shoot outgrowth is also linked to SL signaling. Interestingly, both KAI2 (receptor for KAR) and D14 (receptor for SLs) represent the α/β-fold hydrolases (Zhao et al. 2013, Zhou et al. 2013, Waters et al. 2014). Genetic data predicts that similar to D14, KAI2 also interacts with MAX2 for proteasomal degradation (Morffy et al. 2016). Overlapping phenotypes have been observed in the kai2, d14 and max2 mutants of Arabidopsis. Abnormal leaf morphology was reported for both the d14 and kai2 mutants (Waters et al. 2012). The d14 and kai2 mutants share partial phenotypes of max2 plants. Similar to the SL biosynthetic mutants, both d14 and max2 exhibit abnormal shoot branching. The kai2 plants have normal development in this physiological process. Thus, it was inferred that KAI2 mediated the SL-independent functions of MAX2 (Scaffidi et al. 2013, De Cuyper et al. 2017). External cues and ligands promote KAI2 or D14 to synchronize and regulate the action of MAX2 (Hamiaux et al. 2012). Treatment of Arabidopsis plants with KAR up regulated the expression of d14-like 2 (DLK2), thus highlighting the close proximity between KAR and SL signaling (Sun et al. 2016). The hypocotyls of d14 kai2 double mutants were unresponsive to both SLs and KAR (Scaffidi et al. 2013). The eight membered SUPPRESSOR OF MAX2-LIKE (SMAXL) protein family, having sequence similarities with HEAT SHOCK PROTEIN 101 (HSP101) were identified as potential inhibitors of KAR signaling. This was because smax1 single and smax1/max2 double mutants exhibited a phenotype, similar to that of wild-type seedlings treated with KAR. Thus, due to inactivation of these suppressor genes, the KAR signaling was constitutively activated (Stanga et al. 2013). SMAX1 also possibly regulates only the KAR-responsive pathway, since the smax1 alleles did not suppress the abnormal shoot branching phenotype in max2 (Stanga et al. 2013).
Some of the molecular components of KAR signaling have been identified in rice as well. D53 was identified as the homolog of SMAX1 in rice (Zhou et al. 2013). A high-tillering dwarf phenotype was observed in rice plants harboring a single dominant mutant d53 allele. The influence of the SL-analogue, GR24 is required for the interaction of D53 with D14 and the rice MAX2 orthologue, D3. The GR24-mediated ubiquitination and degradation of D53 occurs only in the presence of functional D3 and D14 proteins. In the rice system, D53 is regarded as a key component of SL signaling, involved in developing the shoot architecture (Jiang et al. 2013). Thus, differential responses to KAR and SLs are mediated during varying developmental stages by the members of the SMAX1/D53 family, which might also control multiple gene expression by post-transcriptional regulation (Shinohara et al. 2013, Li and Phan Tran 2015).
The structural mechanism of KAR interaction with their receptors is still not clear. Interestingly, both D14 and KAI2 contain a conserved catalytic triad consisting of Ser-His-Asp within a hydrophobic pocket. This region is quintessential in the binding and hydrolysis of GR24 (Hamiaux et al. 2012, Kagiyama et al. 2013). However, X-ray crystallographic analyses demonstrated that though KAR1 remains positioned at the opening of this hydrophobic pocket via aromatic-aromatic interactions, its localization does not favor a nucleophilic attack by the activated Ser of the catalytic triad (Guo et al. 2013). It was also speculated that the aromatic-aromatic interactions with KAR1 were sufficient to induce conformational change of KAI2 and that the catalytic triad had no role in KAR signaling (Guo et al. 2013). This conserved sequence plays important role in SL signaling, since it is mediated by both D14 as well as KAI2 (Waters and Smith 2013). A very interesting structural insight of ligand specificity in KAI2 has been recently reported. Xu et al. (2016) identified an intermediate-evolving KAI2, KAI2iB in Striga hermonthica. This receptor specifically bound KAR and not SL. Crystal structures of the unbound and KAR-bound receptors revealed positional differences in a helix, located at the entry of the ligand binding cavity (Xu et al. 2016). This resulted in the formation of a smaller closed pocket solely responsible for the selective binding of KAR. Furthermore, some non-conserved amino acids were also found to mediate the unique ligand binding profile of KAI2iB in S. hermonthica and the exertion of evolutionary forces on these residues possibly led to the diversification of KAI2 receptors (Xu et al. 2016). Bythell-Douglas et al. (2017) phylogenetically analyzed 339 members of the D14/KAI2 family from land plants and charophyte algae. The study suggested that SL perception actually evolved via neo-functionalization of the D14/KAI2 family, and the transition from KAI2-like to D14-like proteins was induced by protein interactions rather than by the requirement of SL perception (Bythell-Douglas et al. 2017).
Interaction of KAR with phytohormones
Seed germination is a critical physiological process which involves the participation of abiotic stress-associated phytohormones like ABA, GAs, IAA, ethylene and SLs. The intricate relations between KAR and SL signaling have already been highlighted in the previous section.
Interactions of KAR with ABA and GA
Since KARs are also inducers of seed germination, they exhibit interactions with other phytohormones for promoting species establishment (Meng et al. 2017). Nelson et al. (2009) initially reported that ABA decelerates the action of KAR on seed germination probably by down regulating the GA biosynthetic genes. It was also shown that GA biosynthesis is required by KAR to regulate germination in Arabidopsis (Nelson et al. 2009). The expression of the GA anabolic genes, viz., GA-3-oxidase 1 (GA3ox1) and GA3ox2 were elevated in Arabidopsis seeds treated with KAR (Nelson et al. 2010). In Avena fatua grasses, the caryopsis sensitivity to KAR1 and GA3 significantly increased during dry storage and the smoke-induced germination was inhibited after the application of paclobutrazol, an inhibitor of GA synthesis (Cembrowska-Lech and Kępczyński 2017). Along with paclobutrazol, other inhibitors of GA anabolism like ancymidol and flurprimidol also antagonized the germination-promoting effects of KAR1, and this inhibition was released on treatment with GA3 (Kępczyński et al. 2013). Recent data also revealed that KAR1 works in synchronization with GA3 to degrade ABA to phaseic acid in order to induce dormancy release in the seeds of A. fatua (Kępczyński 2018).
However, another study in soybean seeds revealed that the application of KAR inhibited GA biogenesis by inducing ABA production (Meng et al. 2016). Thus, the relationship of KAR with GA and the universal stress hormone, ABA stands out to be contradictory in a species-specific manner. Hormone quantification showed lowered GA4/ABA ratio, thus delaying germination. Further analyses showed that the transcripts in the ABA-dependent pathway were also up regulated in the soybean seeds, thus making them more hardened to abiotic stress combinations (Meng et al. 2016). Interestingly, such observations were recorded only under shaded conditions. Other species exhibiting similar KAR-induced hormonal alterations include Capsella bursapastoris, Bromus sterilis and Alopecurus myosuroide (Daws et al. 2007). The KAR-induced repression of seed germination can be attributed to the Mediterranean origin of soybean. It has been suggested that the altered climatic origin triggered differential mechanisms of evolution among the crop species (Meng et al. 2016). Artificial breeding of soybean might also have resulted in the alteration/mutation of critical genes which now yield suppressive effects on germination in response to KAR (Meng et al. 2016). Combined environmental stresses like high humidity and elevated temperatures negatively affect the yield and nutritional quality of soybean by inducing pre-harvest sprouting (Quinhone and Ida 2015, Shu et al. 2015). The initial inhibitory effects of KAR on soybean germination actually help in by-passing pre-harvest sprouting. After an initial delay, the seeds germinate normally with enhanced stress-tolerating capability due to high endogenous ABA content (Meng et al. 2017).
Interactions of KAR with IAA
In another study, the application of KAR suppressed the transcription of an IAA response gene, IAA1 (Meng et al. 2016). Thus, in Arabidopsis, KAR promotes seed germination by reducing the endogenous levels of IAA. SLs, the close relatives of KARs, also induce germination by blocking IAA transport, via the degradation of PINFORMED 1 (PIN1) transporters (Shinohara et al. 2013). It is understood that IAA maintains seed dormancy and germination by stimulating the ABA-dependent pathways (Nagpal et al. 2000). However, the intricate tripartite interactions among KAR, IAA and ABA are yet to be unraveled in Arabidopsis.
Probable interactions of KAR with ethylene
The interactions between ethylene and KAR are yet to be clearly deciphered. However, some published reports indicated towards the close relationship between these growth stimulators during germination of A. fatua seeds. It was observed that unlike KAR1, ethylene alone could not induce the germination of dormant caryopses. This was because the application of ethylene, 1-aminocyclopropane-1-carboxylate (ACC) and even ethylene liberating compound, ethephon did not aid in seed germination (Kępczyński and Van Staden 2012). However, the stimulatory effects of KAR1 were suppressed in the presence of 2, 5-norbornadiene (NBD: competitive and reversible inhibitor of ethylene reception) and 1-methylcyclopropene (1-MCP: a non-reversible ethylene inhibitor), thus indicating the necessity of ethylene for the release of dormancy. Application of ethylene after NBD treatment again restored the germination potential in A. fatua caryopses (Kępczyński 2018). However, treatment of the dormant caryopses with ethylene biosynthetic inhibitors like α-aminoisobutyric acid and aminoethoxyvinylglycine did not affect the activity of KAR1 to a notable extent (Kępczyński and Van Staden 2012). Though the inter-relation between KAR1 and ethylene is still unclear, the above experiments clearly highlight the possible crosstalk of ethylene in mediating KAR1-induced germination of the dormant caryopses of A. fatua.
Roles of KAR under variable environmental factors
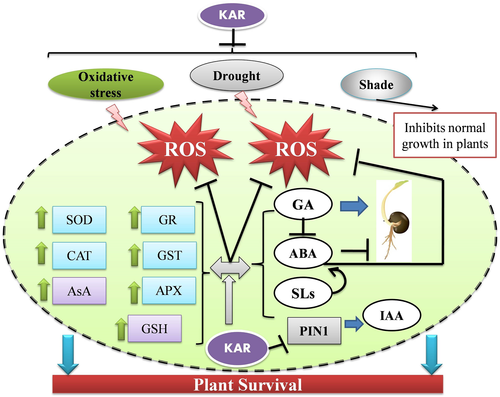
KARs are deployed by the plant system to confer protection against different combinations of abiotic factors like oxidative stress, drought and low-light intensities. The increasing evidences of KAR in regulating abiotic stress responses have promoted the onset of novel research works in this field.
KARs and the cellular oxidative homeostasis
The possible inter-relation among KAR1, GA3 and hydrogen peroxide (H2O2) in promoting seed germination has been reported in A. fatua (Kępczyński 2018). Like KAR1 and GA3, endogenous production of H2O2 reduced the ABA content (Cembrowska-Lech et al. 2015). Furthermore, the application of diphenyleneiodonium (DPI: inhibitor of apoplastic H2O2 production from NADPH oxidase) diminished the germination-inducing effects of KAR1 and GA3 (Cembrowska-Lech and Kępczyński 2016). However, KAR1 also stimulated the activities of antioxidative enzymes like superoxide dismutase (SOD) and catalase (CAT) in the embryos of A. fatua caryopses. This is necessary to limit oxidative injuries due to uncontrolled overproduction of reactive oxygen species (ROS) during dormancy release (Cembrowska-Lech and Kępczyński 2016). The importance of the ascorbate-glutathione (AsA-GSH) cycle in the maintenance of ROS homeostasis is well established in any kind combination of abiotic stresses (Banerjee and Roychoudhury 2018a). KAR directly regulates this core ROS-scavenging cycle by increasing the contents of ascorbate and dehydroascorbate. This replenishes the AsA-GSH cycle, as more molecules of starting materials are fed in. The activities of ascorbate peroxidase (APX) and glutathione reductase (GR) are also increased (Cembrowska-Lech and Kępczyński 2016). Thus, excess H2O2 is scavenged and the reduced to oxidized glutathione ratio (GSH:GSSG) within the cell is restored (Fig. 3).
Role of KARs in drought stress tolerance
Drought is the most prevalent type of abiotic stress causing drastic crop losses around the world. Soil water deficiency seriously limits germination and affects the life cycle of crops and other plants (Banerjee and Roychoudhury 2016b, Banerjee and Roychoudhury 2018c). Thus, due to physiological deterioration, some species succumb to death, while some produce compromised quantities of yield (Banerjee and Roychoudhury 2015).
The stress ameliorative roles of exogenous KAR treatment was first reported in three medicinal plants, viz., Trachyspermum copticum, Foeniculum vulgare and Cuminum cyminum (MousaviNik et al. 2016). Application of KAR1 at a stimulating concentration of 10 μM maximally promoted the germination rate, germination index, seedling vigor and radicle length in T. copticum seedlings among the three species exposed to drought. Highest improvement in shoot length was observed in F. vulgare plants treated with KAR (MousaviNik et al. 2016). The mechanism of such tolerance has been preliminarily attributed to the fact that KAR reduces the rate of lipid peroxidation and the activities of oxidative enzymes (Sunmonu et al. 2016). KAR can also mobilize the accumulation of starch from the cotyledons or endosperm by inducing amylase activity (Sunmonu et al. 2016). Recently, another report highlighted the involvement of KAI2 receptor during plant responses to drought stress (Li et al. 2017). The Arabidopsis mutants of KAI2 were observed to be hypersensitive to water deficient conditions. These mutants exhibited enlarged stomatal aperture due to ABA-hyposensitivity which resulted in uncontrolled water loss. Low-anthocyanin contents along with high rates of membrane damage were also recorded (Li et al. 2017). The reported data indicated towards an interesting crosstalk between KAI2 and ABA, operative during drought stress in Arabidopsis. KAI2 was found to induce ABA catabolism and simultaneously promote ABA-mediated responses (Li et al. 2017). Thus, the question now arises whether the reception of KAR by KAI2 generates a signal that is similar to the ABA-dependent pathway. Microarray analysis revealed differentially expressed genes like DLK2, BZS1/STH7, KUF1, GA3ox1, SMAX1, SMXL2 and 2OG-Fe(II) oxidoreductase which were down regulated in the kai2 mutants exposed to drought (Li et al. 2017). In another experiment, Wang et al. (2018) observed that KAR triggered the expression of abiotic stress-inducible genes like dehydration-responsive element binding 2A (DREB2A), WRKY33 and ethylene-responsive factor 5 (ERF5) in Arabidopsis exposed to mild osmotic stress. Germination of the kai2 mutant seeds was strikingly inhibited by salinity, mannitol-mediated osmotic stress and high temperature (Wang et al. 2018). Bai et al. (2017) performed whole transcriptome sequencing in Phormium tenax exposed to drought stress. Interestingly, the ‘response to KAR’ node was found to be one of the ten most significant nodes in the gene ontology (GO) enrichment results in the biological processes (BP) (Bai et al. 2017). Thus, KARs are involved in the drought-induced responses in P. tenax. The exact mechanism is however yet to be elucidated.
Role of KARs during low-light intensity
Plant growth under shade is the most prevalent type of low-light intensity stress. The red/far red light ratio and intensity reaching the lower vegetation are decreased due to the existing upper vegetation canopy (Casal 2012). This affects seed germination, development and seedling growth (Casal 2013). It is reported that plants growing under shade exhibit lower resistance to both abiotic and biotic stresses (Wit et al. 2013). KARs have been recently classified as the endogenous group of molecules which can attenuate the shade avoidance syndrome in plants by increasing the chlorophyll content and elongating the hypocotyls (Nelson et al. 2010).
Interestingly, Arabidopsis mutants with impaired IAA biosynthesis were not sensitive to shade stress (Cole et al. 2011). The accumulation of IAA in the cotyledons and its transport to the hypocotyls was reported in Brassica rapa seedlings grown under shade (Procko et al. 2014). Nelson et al. (2011) observed the repression imposed by KAR on IAA1 expression. Thus, it is hypothesized that KAR aids in seedling establishment under shade stress by suppressing IAA anabolism (Meng et al. 2017). Waters et al. (2012) showed that similar to max2 mutants of Arabidopsis, the kai2 mutants exhibited a phenotype characterized by an elongated hypocotyl. The hy5/kai2 double mutant possessed an even longer hypocotyl compared to that of hy5 and kai2 mutants. This phenotype was similar to that of the hy5/max2 mutants (Waters and Smith 2013). This illustrates the HY5-independent regulation of seedling establishment by KAI2, though its interactions with other regulators of photomorphogenesis like PHYTOCHROME INTERACTING FACTORs (PIFs) and CONSTITUTIVE PHOTOMORPHOGENIC 1 (COP1) are largely unknown.
Conclusions
KARs are smoke-derived compounds which promote the germination of receptive seeds after a period of after-ripening and atmospheric precipitation. These are unique molecules produced by forest fires in order to stimulate seed and seedling establishment along with abiotic stress tolerance. Since the past few years, significant progress has been achieved in decoding the KAR biosynthesis, reception and roles in diversifying ecosystem communities. KARs are closely associated with crucial phytohormones like ABA, GA, IAA and ethylene which altogether regulate seed germination (Fig. 3). Interestingly, KARs have been shown to induce ABA-dependent responses like stomatal closure, via the KAI2 receptor. Simultaneously, KAR suppresses ABA biosynthesis to accelerate seed germination in a species-specific manner. In spite of the involvement of ROS in germination, KAR activates the antioxidant machinery and maintains oxidative homeostasis within the cell. This is more evident during drought where exogenous KAR ameliorates stress-induced damages in cultivated plants. Loss of the KAI2 receptor resulted in hypersensitivity to drought due to delayed stomatal closure. This indicates towards a clear crosstalk between KAR and ABA, since stomatal closure in the mesophyll tissue is a physiological process regulated by ABA. KAR also confers tolerance against low-light intensity during shade stress by down regulating IAA biosynthesis. Though largely involved in photomorphogenesis, the functions of KAI2 have been observed independent of the crucial TF, viz., HY5.
Future perspectives
KARs are possibly the only class of molecules which are produced directly from a devastating environmental phenomenon only to facilitate ecological community diversity and tolerance against a number of abiotic stresses. Though this field of research is rapidly evolving, very little information is available till date regarding its exact roles in optimizing plant development during its life cycle. Future endeavors should involve genome-wide investigations which can reveal novel molecular regulators involved in KAR signaling. Further experiments highlighting the effects of KAR as a protective agent during multiple abiotic stresses can be pursued. Epigenetic data in this field is also lacking. The involvement of chromatin factors in regulating the nucleosomal architecture on application of KAR during sub-optimal conditions can unearth interesting and valuable outcomes. The effects of KAR and its signaling components under other abiotic stresses like salinity, extremes of temperatures, heavy metal toxicities, etc. should be carefully documented in order to truly popularize KARs as agents promoting multiple stress tolerance.
Author contributions
A.B. drafted the overall manuscript. A.R. supervised the entire work, critically analyzed the manuscript and made modifications. D.K.R. provided his valuable suggestions during the drafting of the manuscript.
Acknowledgements
Financial assistance from Council of Scientific and Industrial Research (CSIR), Government of India, through the research grant [38(1387)/14/EMR-II] to Dr A.R. is gratefully acknowledged. The authors are grateful to University Grants Commission, Government of India, for providing Junior Research Fellowship to Mr. A.B.




