Changes in floral biology and inbreeding depression in native and invaded regions of Datura stramonium
Abstract
- Plant populations invading new environments might compromise their fitness contribution to the next generation, because of the lack of native specialist pollinators and/or potential mates. Thus, changes in plant mating system and traits linked to it are expected in populations colonising new environments where selection would favour selfing and floral traits that maximise reproductive output. To test this, we studied native (Mexico) and non-native (Spain) populations of the obligate sexual reproducing annual weed Datura stramonium.
- Flower size, herkogamy, total number of seeds per plant, number of visits by and type of pollinators, and inbreeding depression were assessed in native and non-native populations. Finally, we measured phenotypic selection on corolla size and herkogamy in each population.
- Flower size and herkogamy showed wide and similar variation in both ranges. However, the largest average flower size was found in one non-native population whereas the highest average positive herkogamy was detected in one native population. On average, flowers in the native range received more visits by pollinators. Hawkmoths were the main visitors in the native populations while only bees were observed visiting flowers in Spain's populations. Only in the native range was inbreeding depression detected. Selection to reduce herkogamy was found only in one native population.
- Absence of both inbreeding depression and selection on floral traits suggest a change in mating system of D. stramonium in a new range where generalist pollinators may be promoting high reproductive success. Selection against deleterious alleles might explain the reduction of inbreeding depression, promoting the evolution of selfing.
Introduction
In flowering plants, long-distance dispersal could result in colonisation of novel environments, following establishment and range expansion (Pannell 2015). This process, which generally involves single or few individuals, largely depends on the genetic constitution of colonisers and on their effective reproduction in the novel environment (Baker 1955; Barrett et al. 2008).
After dispersal, populations in the new range are subject to severe reductions in population size associated with founder events, bottlenecks and genetic drift. These processes have detrimental effects on introduced plant populations, reducing their genetic diversity and outcrossing rates, increasing homozygosity within populations and limiting the adaptive evolution of traits under new ecological conditions (Charlesworth 2003; Dlugosch & Parker 2008; Charlesworth et al. 2009; Eckert et al. 2010). However, despite negative effects expected by the reduction of genetic diversity, some introduced species perform successfully in the new distribution ranges (i.e. ‘the paradox in invasion biology’; Sax & Brown 2000), although not all species become invasive in a new environment.
In order to understand the invasion paradox it is necessary to analyse changes brought about by factors of the new environments and the target characters related to the ability to colonise; for instance, increased growth rate, competitive ability or changes in the mating and/or reproductive systems (van Kleunen et al. 2015). Particularly, in animal-pollinated flowering plants, a main limiting barrier for successful reproduction during the colonisation process is the scarcity or lack of potential mates as well as the absence of effective pollinators in the new environment (Moodley et al. 2016). Baker (1955) noted that compatible species capable of self-fertilisation could be more successful colonisers than self-incompatible ones; this is the so-called Baker's law (Stebbins 1957). He suggested that during dispersal and establishment phases, colonising populations are small and would suffer a reduction in services of their native pollinators. Contrary to obligate outcrossers (i.e. self-incompatibility systems, SI), self-compatible species can maximise their fecundity through self-fertilisation. Hence, uni-parental reproduction can be advantageous, enabling demographic growth in alien populations (Baker 1955; Barrett et al. 2008; Richardson & Pyšek 2012; Barrett 2015). Empirical evidence supports Baker's law, suggesting that self-compatible species are more prone to be invasive than self-incompatible species (Pannell 2015).
Following Baker's ideas, in the absence of mates or pollen limitation scenarios, selfing can confer ‘reproductive assurance’ to colonising plants, and hence, will be selected over outcrossing. The ‘reproductive assurance’ (Schoen & Lloyd 1992) and the ‘automatic advantage of selfing’ (Fisher 1941) are two main hypotheses to explain the evolution of selfing in plant populations. Further, since selfing increases homozygosity, it may promote the maintenance of superior homozygote genotypes to particular combinations of environmental conditions (Pannell 2015). However, selfing evolution may depend on other factors, like behaviour and quality of pollinators (Moeller & Geber 2005; Karron et al. 2012; Rodger et al. 2013; Pannell 2015), and on the magnitude of the inbreeding depression (Lande & Schemske 1985).
Inbreeding depression (ID), the reduction of fitness of offspring derived from selfing relative to those derived from outcrossing due to genetic load and/or over-dominance in loci linked to fitness, is considered a main selective limit for the evolution of selfing (Lande & Schemske 1985). However, selection against homozygote individuals through generations is expected to reduce genetic load and hence ID (genetic purge; Lande & Schemske 1985; Yahara 1992; Dart & Eckert 2013). In highly selfing populations, a negative correlation between selfing rate and ID is expected (Lande & Schemske 1985). During the colonisation event, the dynamics of ID may initially exacerbate the negative effects associated with bottlenecks, founder events and genetic drift, reducing the genetic and phenotypic variation, compromising the potential adaptive response to the new environment.
The evolution of self-fertilisation is generally accompanied by correlated changes in flower features (i.e. ‘selfing syndrome’; Lloyd 1979). Flower traits can be modified in different ways: by selection on characters related to self-fertilisation, e.g. herkogamy (Takebayashi et al. 2006; Bodbyl-Roels & Kelly 2011), dichogamy (Webb & Lloyd 1986), or by differential selection on resource allocation to female, male and attractiveness functions (e.g. flower size and display; Cruden 1977; Lloyd 1987; Charlesworth & Morgan 1991). This may occur in new environments where pollination by generalists could relax prior selection on flower traits exerted by the native pollinators, or where different abiotic conditions impose severe limitations for pollinators (Memmott & Waser 2002; Goulson & Hanley 2004; Morales & Aizen 2006). However, changes in flower traits can also occur through pleiotropic effects of genes associated directly with the size of the floral organs when selfing increases, as has been demonstrated for petal size during the evolution of selfing in Capsella (Sicard et al. 2016). Besides changes in floral design, floral display can evolve intimately linked to mating system; large floral displays can incur a cost by reducing opportunities for outcrossing (Harder & Barrett 1995; Barrett & Harder 1996).
In this study with Datura stramonium L., an annual plant native to North America (Symon & Haegi 1991), we aim to determine whether changes in floral traits and inbreeding depression associated with the plant mating system have occurred during the colonisation of a new and different environment (Mediterranean climate areas in Spain that the species encountered early in its Old World spread; Sanz Elorza et al. 2004). Many Mediterranean plants behave as aggressive weeds in other parts of the world, while the Mediterranean region is less prone to be invaded, especially on the mainland (Hulme et al. 2008; Arianoutsou et al. 2013). However, in some cases aliens weeds have become noxious in the Mediterranean region, as has occurred with D. stramonium. This species is becoming more frequent in natural habitats in other parts of the invaded range (Weaver & Warwick 1984; van Kleunen et al. 2007). In the study region, in particular Andalusia, riverbanks of some natural parks are being massively invaded, thus subject to strong efforts to eliminate it in these critical Mediterranean habitats (Sanz Elorza et al. 2004). This is unexpected because natural pristine habitats in the Mediterranean Basin are considered relatively resistant to invasions in comparison with temperate and other Mediterranean regions (Bresch et al. 2013). Specifically, we assess if populations from the native and non-native ranges differ in: (i) flower size and herkogamy; (ii) number of visits by pollinators to flowers and the type of pollinators; (iii) direction and intensity of selection on flower size and herkogamy; and (iv) magnitude of inbreeding depression, related to the amount of genetic variation in populations. We hypothesise that native populations will show larger corolla size, positive or approach herkogamy (stigma protruding beyond anthers) and more phenotypic variation on these characters than non-native populations. On the other hand, in the native range, we expect higher visitation to flowers by specialised pollinators. Further, reduced herkogamy and flower size due to selection is expected in populations in the new range (Ollerton et al. 2012). Finally, we expect higher genetic variation in the native populations than in the new range and thus higher inbreeding depression in the former.
Material and Methods
Study species
Datura stramonium (Solanaceae) is an annual weed native to North America (Mexico and southern United States; Weaver & Warwick 1984). In Spain it was introduced after the conquest of Mexico between 1540–1577 (Sanz Elorza et al. 2004), probably through the harbours in Spain, which had extensive trade with America. D. stramonium is self-compatible and its flowers are fragrant and produce nectar associated with its native pollinators, hawkmoths (Manduca sexta, M. quinquemaculata and Hyles lineata; Grant 1983), although it is also visited by honeybees (Apis mellifera; Sharma 1972). The variation in outcrossing rates in D. stramonium could be affected by plant spatial arrangement and, by the stigma position (i.e. herkogamy): positive or approach herkogamy (stigma above anthers) increases outcrossing rates (Motten & Antonovics 1992; Motten & Stone 2000). Also, herkogamy is positively related to inbreeding depression (Stone & Motten 2002). Moreover, van Kleunen et al. (2007) found that plants’ outcrossing rates were not affected by population size. Flowers of D. stramonium are tubular, white (recessive) or purplish (dominant) corollas. They open at dusk, last for one night only, are large (ca. 10 cm; Stone & Motten 2002) and herkogamy has moderate heritability (h2 = 0.3; Motten & Stone 2000). Flowering time occurs in the summer months, both in native and in non-native (Spain) ranges, despite a different climate regime – wet and dry, respectively. Since in D. stramonium flowers are produced in successive metamers as the plant develops, the number of open flowers per plant is variable and depends on plant size (Camargo et al. 2017). Because D. stramonium is an annual herb and many generations had passed since its introduction to Spain, it is possible that modifications in flower traits, which may affect mating system, have occurred in invasive populations.
Study sites
The study was conducted during two summer seasons (2015–2016). In Spain we sampled five populations in the southern region (Table 1). Five populations were sampled in the region of Andalusia with a typical Mediterranean climate, characterised by a moist winter and hot and dry summer. Most populations inhabit cultivated and waste areas, except in Cardeña, where the population is on a riverbank in a protected natural area. In Mexico, four populations we sampled in the central region (Table 1), with summer rains and a dry season in winter and spring; all populations are ruderal.
| range | locality (province) | geographic coordinates | altitude (m a.s.l.) | mean annual precipitation (mm) | mean annual temperature (°C) |
|---|---|---|---|---|---|
| native |
Pedregalc (Mexico City) |
19°19′03.72″ N 99°11′26.67 W |
2,323 | 803 | 15.6 |
|
(State of Morelos) |
19°00′33.93″ N 99°03′40.46 W |
2,050 | 1463.2 | 19.9 | |
| Taxcob (State of Guerrero) |
18°33′26.35″ N 99°35′55.84″ W |
1,746 | 1417.6 | 21.6 | |
| Teotihuacána (State of Mexico) |
19°40′57.78″N 98°50′34.50″ W |
2,294 | 563.3 | 14.9 | |
| Texcocoa (State of Mexico) |
19°29′19.67″ N 98°51′57.72″ W |
2,353 | 710.3 | 15.9 | |
| Ticumána (State of Morelos) |
18°42′37.62″ N 99°07′00.66″ W |
1,210 | 850 | 24.1 | |
| Tulab (State of Hidalgo) |
20°02′29.70″ N 99°19′50.08″ W |
2,081 | 699.4 | 17.6 | |
| non-native | Alamilloa (Seville) |
37°25′14.59″ N 05°59′35.86″ W |
10 | 576 | 18.6 |
| Cardeñaa (Cordoba) |
38°14′47.51″ N 04°12′57.15″ W |
342 | 645 | 17 | |
| Dos Hermanasa (Seville) |
37°16′19.60″ N 05°56′16.90″ W |
42 | 591 | 18.1 | |
| Hinojosa,b,c(Huelva) |
37°18′29.31″ N 06°23′56.55″ W |
81 | 515 | 18 | |
| La Zubiaa,b,c(Granada) |
37°08′14.532″ N 03°36′4.73″ W |
681 | 462 | 15.2 |
- a Populations used for phenotypic variation and selection analysis.
- b Pollinators recorded.
- c Used to estimate inbreeding depression.
Phenotypic variation in flower traits
In each population, we sampled all reproductive plants available (Table 2). From each plant we collected two to five flowers, which were longitudinally cut, pressed and dried in an electric stove at 50 °C and stored for further measurements. We measured the length of the corolla (i.e. flower size) and the minimum distance between anthers and stigma. When stigma was above anthers, herkogamy was considered positive (approach), negative (reverse) when the stigma is below the anthers, or zero when contacting each other. In order to explore the phenotypic variation in herkogamy (i.e. positive or negative), we estimated the percentage of plants and flowers of each type in each population.
| range | population | N | corolla length (mm) | range corolla length (mm) | herkogamy (mm) | range herkogamy (mm) |
|---|---|---|---|---|---|---|
| native | Santo Domingo | 29 | 88.63 ± 6.64 | 68.63 – 102.44 | −1.77 ± 2.77 | −7.19 – 3.02 |
| Teotihuacán | 18 | 67.48 ± 9.24 | 50.50 – 84.50 | −0.04 ± 0.09 | −0.27 – 0.00 | |
| Texcoco | 29 | 79.29 ± 7.63 | 66.16 – 94.84 | −1.45 ± 1.72 | −5.55 – 1.28 | |
| Ticumán | 25 | 95.58 ± 8.78 | 73.01 – 112.35 | 4.07 ± 2.91 | −1.99 – 9.44 | |
| total average | 83.90 ± 12.53 | 0.44 ± 4.24 | ||||
| non-native | Alamillo | 33 | 79.52 ± 7.13 | 62.06 – 89.77 | −0.37 ± 1.70 | −8.58 – 4.10 |
| Cardeña | 29 | 86.47 ± 9.50 | 64.61– 107.32 | −1.84 ± 1.71 | −6.13 – 0.00 | |
| Dos Hermanas | 35 | 95.21 ± 6.64 | 73.42 – 109.40 | −0.83 ± 1.60 | −6.66 – 0.78 | |
| Hinojos | 28 | 109.89 ± 5.62 | 98.50 – 121.29 | 1.14 ± 1.35 | 0.00 – 5.32 | |
| La Zubia | 30 | 101.32 ± 5.08 | 89.78 – 112.24 | 0.81 ± 0.81 | 0.00 – 2.99 | |
| total average | 94.07 ± 12.62 | −0.16 ± 2.17 |
To assess the difference in corolla and herkogamy between ranges, we conducted linear mixed effects analysis of each character, with range as a fixed effect and populations nested within range, and plants nested within population and range, as random effects. The significance of random factors was evaluated from a likelihood-ratio χ2 test (LRT) between a model that included populations as random effects (function lme) and one that does not (LRT1; function gls; Zuur et al. 2009). In order to correct negative variances and to test a correct alternative hypothesis (σ2 > 0; one-tailed test), we divided the P-value by two (Zuur et al. 2009). Then a second LRT2 was performed to test the plants within populations effect without correction for the P-value.
In order to estimate the variance components accounted for in populations, plants within populations and flowers within plants (as residual variance), we performed linear mixed effects analysis separately for each range and trait. The significance of random effects was evaluated through a likelihood-ratio χ2 test, following the same procedure as described above. All linear mixed effects analyses and estimates of variance components were conducted using R (R Foundation for Statistical Computing 2015) and the nlme package (Pinheiro et al. 2017).
Selection on flower traits
To estimate the magnitude and direction of selection gradients on herkogamy and flower size, in each population, in each range, we performed a multiple regression analysis of the total number of seeds per individual as a fitness measure (w;Lande & Arnold 1983; Brodie et al. 1995). First, we obtained the average values of each floral trait per each individual plant. Because absolute maternal fitness (total seed number per individual) varies with plant size (Núñez-Farfán & Dirzo 1994), we included basal stem diameter (as a proxy for plant size; Valverde et al. 2015) of each individual plant in the selection analyses. Directional selection gradients (βi) were estimated as the standardised partial linear regression coefficients of relative fitness (wr) as a function of flower size, herkogamy and stem size. In each population, predictor variables were standardised (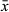 = 0 and s = 1) and maternal plant fitness was relativised as the total number of seeds per individual, divided by the corresponding mean fitness of each population (Lande & Arnold 1983). Due to small sample size per population (n ≤ 35 plants), only directional selection gradients were estimated.
= 0 and s = 1) and maternal plant fitness was relativised as the total number of seeds per individual, divided by the corresponding mean fitness of each population (Lande & Arnold 1983). Due to small sample size per population (n ≤ 35 plants), only directional selection gradients were estimated.
To obtain the absolute maternal fitness of each individual, we collected all fruits of marked plants at the end of the reproductive season. Fruits were kept in individual paper bags before they had opened, labelled and dried in an electric stove at 50 °C, and stored. Because D. stramonium can produce tens of fruits with hundreds of seeds each, we calculated the number of seeds of each fruit, using the relationship between the total numbers of seeds and fruit volume, from a sample of fruits per population. Fruit volume was calculated with the formula of a prolate spheroid as 4/3π(a2b), where a is the equatorial ratio (width) and b the polar ratio (length) of a fruit.
Inbreeding depression
To assess the magnitude of inbreeding depression (δ) in native and non-native populations, we conducted controlled pollination experiments in three natural populations, two in Spain (Hinojos and La Zubia) and one in Mexico (Pedregal; data from Núñez-Farfán et al. 1996). In each population we selected another sample of individual plants (20–35) with open flowers to perform crosses. In each individual plant, we randomly allocated flowers to: (i) hand self-pollination or (ii) hand cross-pollination, emasculating the receptor flowers prior to anthesis. Pollen was applied onto the stigma ad libitum. Flowers were bagged using fine-mesh nylon bags. Given that D. stramonium flowers last is only one night, both treatments were applied on the same night to all individuals of each site. At maturity, all fruits were harvested before opening and releasing the seeds (1.5 months later). We counted the total number of seeds produced per fruit with ImageJ version1.50d software (National Institutes of Health, Bethesda, MD, USA). To test differences in seed production between hand cross-pollination versus hand self-pollination treatments, we used a t-test for each population separately.
Inbreeding/outbreeding depression (δ) was calculated per plant for each population as δ = [1 - (ws/wx)] if ws < wx; and δ = [(wx/ws) - 1] when ws > wx; where ws corresponds to seed production by self-pollination, and wx to seed production by outcrossing (Agren & Schemske 1993). The differences between populations in δ were evaluated by means of an ANOVA.
Floral visitors
Pollinator visitation rates were quantified in two populations in Spain and three in Mexico (data from 2003; Table 1) as the number of visits per flower every 15 min over 3 h, with an interval of 5 min between observation times. Observations were done from 19:00 h, before flowers open, up to 22:30 h when the activity of pollinators ceased. The type of pollinator was also recorded for each visit.
Results
Phenotypic variation in flower traits
Linear mixed effects analysis revealed that corolla length and herkogamy did not differ between ranges (corolla: t = 1.44, df = 7, P = 0.1931; herkogamy: t = −0.28, df = 7, P = 0.7897). For corolla, the fraction of variance explained by populations within range was 63.14%, plants within populations 16.08%, and variance within plants 20.78%. In the case of herkogamy, the population effect explained 33.23% of variance, plants within populations 12.79%, and variance within plants 54.89%. The likelihood ratio χ2 test indicated that both random effects (populations nested in ranges and individuals nested in populations) were significant for both corolla (LTR1: L = 476.868, df = 1, P < 0.0001 and LTR2: L = 125.556, df = 1, P < 0.0001) and herkogamy (LTR1: L = 236.175, df = 1, P < 0.0001 and LTR2: L = 29.212, df = 1, P < 0.0001).
The non-native population Hinojos had the higher average corolla length, and the native population Teotihuacán had the shortest (Table 2). On the other hand, most populations had negative (reverse) average herkogamy (Table 2). The higher average value of positive (approach) herkogamy was found in the Ticumán population, which is also the population with the largest average corolla length in the native range (Table 2). An analysis of herkogamy revealed that in two populations, one native (Teotihuacán) and one non-native (Cardeña), all individual plants and flowers had negative herkogamy. The Ticumán population had the largest percentage of plants with positive herkogamy (64.52%) and it also has the largest range of corolla size and the second largest range of herkogamy (Table 2). Likewise, at the flower level, 80% of flowers of the Ticumán population had positive herkogamy, whereas most flowers showed negative herkogamy (from 50% to 100%) in the remaining populations.
The linear mixed effects analyses conducted within each range indicate that the largest fraction of variation in corolla is explained by the population effect (Table 3), revealing phenotypic population differentiation within each range (Table 3). A low but significant fraction of the variance in corolla was explained by variation among plants within populations, suggesting genetic variance given that environmental conditions within populations are apparently very homogeneous (Table 3). The likelihood ratio χ2 tests indicated that population (LTR1) and plants within population (LTR2) were significant in the native range (LTR1: L = 188.313, df = 1, P < 0.0001 and LTR2: L = 76.959, df = 1, P < 0.0001), as well in the non-native range (LTR1: L = 307.872, df = 1, P < 0.0001 and LTR2: L = 47.781, df = 1, P < 0.0001).
| effect | corolla | herkogamy | |||
|---|---|---|---|---|---|
| var. comp. | % variance | var. comp. | % variance | ||
| native | Population | 145.74 | 58.09 | 6.87 | 37.05 |
| Plant (Pop.) | 46.51 | 18.54 | 2.63 | 14.18 | |
| Residual | 58.60 | 23.36 | 9.05 | 48.76 | |
| non-native | Population | 141.37 | 68.32 | 1.41 | 27.85 |
| Plant (Pop.) | 31.70 | 15.32 | 0.69 | 13.59 | |
| Residual | 33.83 | 16.35 | 2.97 | 58.54 | |
In contrast, although the fraction of variance in herkogamy explained by population and plants within population effects were significant within each range, we found that the largest fraction of variance in herkogamy is accounted for by flowers within individual plants level (Table 3). The likelihood ratio χ2 tests indicated that population (LTR1) and plants within population (LTR2) were significant in the native range (LTR1: L = 130.185, df = 1, P < 0.0001 and LTR2: L = 19.874, df = 1, P < 0.0001) and in the non-native range (LTR1: L = 74.085, df = 1, P < 0.0001 and LTR2: L = 5.944, df = 1, P = 0.015).
Floral visitors
The likelihood of a flower being visited by pollinators varied between native and non-native populations of D. stramonium (Table 4). An ANOVA showed significant differences among populations (Table 4A). On average, flowers received ca. threefold more visits in Mexico than in Spain (Table 4A). However, population variation was observed, as one native population had an average value similar to that of plants in Spain (Tula population; Table 4A). Moreover, bees of different sizes are the main flower visitors in Spain, whereas sphingid moths are the main flower visitors in the native populations studied (Table 4B). Notwithstanding, pollinators are almost absent or very scarce in some native populations (A. López-Velázquez and J. Núñez-Farfán, unpublished data). It is remarkable that in non-native populations the average of open flowers per night per individual was 2.08 (range: 2–5 flowers) in Hinojos and, 7.24 (range: 1–15 flowers) in La Zubia; while in the native range, Santo Domingo, an average of 2.77 (range: 1–5) open flowers was recorded.
| (A) range | population | N | #visits/flowera |
|---|---|---|---|
| native | Santo Domingo | 164 | 3.83 ± 0.21b |
| Tula | 50 | 1.18 ± 0.38a | |
| Taxco | 110 | 4.04 ± 0.25b | |
| non-native | Hinojos | 158 | 1.15 ± 0.19a |
| La Zubia | 210 | 0.91 ± 0.18a |
| (B) range | identity of pollinators |
|---|---|
| native |
Hawkmoths (Manduca sexta, Hyles sp.) Bees (Apis mellifera) |
| non-native | Bees (Apis mellifera, Anthophora sp.) |
| Carpenter bees (Xylocopa sp.), | |
| Bumblebees (Bombus sp.) |
- a ANOVA executed using log-transformed values.
Inbreeding depression
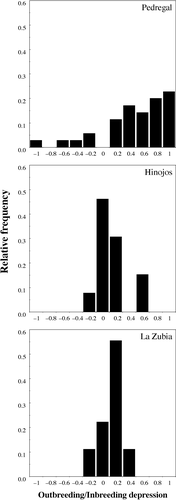
Controlled self- and outcross pollinations carried out in native and non-native populations, show that both types of crosses differed in the total number of sound seeds produced only in the native population (Table 5A). Estimation of outbreeding/inbreeding depression per plant in each population revealed no differences in average seed production between selfing and outcrossing in the two Spanish populations studied (Table 5B), but significant inbreeding depression was detected in the native population (Table 5B). The distribution of δ at plant level shows outbreeding as well inbreeding depression in each population (Fig. 1). However, the highest frequency of plants with positive δ was found in the native population (Fig. 1).
| (A) range | population | Nf | cross-pollination | Nf | self-pollination | t | P |
|---|---|---|---|---|---|---|---|
| native | Pedregal | 36 | 95.19 ± 6.5 | 40 | 58.32 ± 6.37 | 4.03 | 0.0001 |
| non-native | Hinojos | 19 | 470.52 ± 18.72 | 14 | 470.28 ± 34.99 | 0.01 | 0.9949 |
| La Zubia | 16 | 548.06 ± 25.54 | 14 | 520.42 ± 27.30 | 0.75 | 0.4560 |
| (B) population | N | δ |
|---|---|---|
| Pedregal | 35 | 0.371 ± 0.081a |
| Hinojos | 13 | 0.058 ± 0.133a,b |
| La Zubia | 9 | −0.032 ± 0.160b |
- Different letters indicate significant differences (P < 0.05).
Selection on flower traits
In most Spanish populations of D. stramonium phenotypic selection analyses showed that stem diameter was positive and significantly related to plant fitness (Table 6). Plant size explained between 29%–53% of variance in plant fitness in each population (Table 6). In contrast, only in one out of four native populations, was this relationship significant (Table 6). Selection on corolla size and herkogamy was not significant for most populations (Table 6). Only in the native population of Santo Domingo was negative directional selection on herkogamy detected, implying that plants with low or negative herkogamy had higher seed output (Table 6). The same pattern of selection on herkogamy was detected in the native population of Ticumán, although the selection gradient was marginally significant (Table 6).
| range | population | trait | β (±SE) | t | P | F | R2 |
|---|---|---|---|---|---|---|---|
| native | Santo Domingo | Stem | 0.19 (0.03) | 1.03 | 0.314 | 3.05* | 0.19 |
| Corolla length | 0.16 (0.02) | 0.89 | 0.381 | ||||
| Herkogamy | −0.41 (0.07) | −2.20 | 0.037 | ||||
| Teotihuacán | Stem | 0.27 (0.03) | 1.05 | 0.317 | 1.43NS | 0.08 | |
| Corolla length | −0.43 (0.03) | −1.67 | 0.124 | ||||
| Herkogamy | 0.04 (2.49) | 0.15 | 0.880 | ||||
| Texcoco | Stem | 0.75 (0.02) | 4.67 | 0.000 | 9.21*** | 0.48 | |
| Corolla length | 0.03 (0.02) | 0.20 | 0.842 | ||||
| Herkogamy | −0.06 (0.08) | −0.36 | 0.721 | ||||
| Ticumán | Stem | 0.27 (0.05) | 1.22 | 0.238 | 2.54NS | 0.19 | |
| Corolla length | 0.06 (0.03) | 0.28 | 0.780 | ||||
| Herkogamy | −0.41 (0.09) | −1.97 | 0.065 | ||||
| non-native | Alamillo | Stem | 0.74 (0.02) | 6.01 | 0.000 | 12.6*** | 0.52 |
| Corolla length | 0.09 (0.01) | 0.71 | 0.486 | ||||
| Herkogamy | 0.05 (0.04) | 0.43 | 0.673 | ||||
| Cardeña | Stem | 0.57 (0.03) | 3.45 | 0.002 | 4.94** | 0.31 | |
| Corolla length | −0.16 (0.02) | −0.96 | 0.346 | ||||
| Herkogamy | −0.03 (0.08) | −0.16 | 0.877 | ||||
| Dos Hermanas | Stem | 0.47 (0.01) | 3.08 | 0.004 | 6.63** | 0.29 | |
| Corolla length | −0.00 (0.02) | −0.01 | 0.989 | ||||
| Herkogamy | −0.27 (0.08) | −1.48 | 0.148 | ||||
| Hinojos | Stem | 0.69 (0.03) | 5.56 | 0.000 | 12.8*** | 0.53 | |
| Corolla length | 0.16 (0.02) | 1.31 | 0.202 | ||||
| Herkogamy | −0.15 (0.08) | −1.24 | 0.226 | ||||
| La Zubia | Stem | −0.12 (0.03) | −0.72 | 0.478 | 1.93NS | 0.08 | |
| Corolla length | −0.39 (0.02) | −2.16 | 0.039 | ||||
| Herkogamy | 0.09 (0.16) | 0.46 | 0.650 |
- Full model significance: ***P < 0.0005; **P < 0.01; *P < 0.05; NS: not significant.
Discussion
During the invasion of novel environments, plant populations may face new physical and biotic factors in relation to their native environments (Eckert et al. 2010). In the case of animal-pollinated plants, two plausible scenarios in a new environment are expected: an absence of specialised pollinators or a different set of generalist, opportunistic pollinators (Aigner 2006). This, coupled with the reduced availability of potential mates, predicts changes in plant mating system and flower morphology, among other factors (Baker 1955; Stebbins 1957; Sicard & Lenhard 2011; Ollerton et al. 2012; Pannell 2015). These changes may result from selection for selfing and/or relaxed selection exerted by pollinators on flower traits (e.g. reduced size and herkogamy). Our results indicate partial agreement with expectations. First, populations of both ranges of D. stramonium did not differ in the average size of flowers and herkogamy, and there is broad phenotypic variation in these two traits within the native and non-native ranges. Second, while Spanish populations of D. stramonium were visited mainly by bees (mostly honeybees), Mexican populations were visited by legitimate pollinators as expected from the flower syndrome (e.g. hawkmoths), and visitation rates were higher for Mexican populations; however, given that pollinators were recorded in only three populations in which phenotypic measures were taken, these results must be interpreted with caution. Third, inbreeding depression was detected in a Mexican population whereas it was nil for all the non-native populations examined. Finally, negative selection on herkogamy was detected in only one Mexican population. Thus, results suggest that an evolutionary change in mating system is underway in populations of D. stramonium in Spain. We should admit that we have not currently obtained estimates of selfing/outcrossing rates in natural conditions, but it is reasonable to infer that differences in inbreeding depression should be due to differences in mating systems.
In general, the higher visit rate by pollinators in the native range does not correspond with a higher seed set in comparison with the non-native range. However, one must be bear in mind that seed set represents a proxy for maternal fitness and the contribution of pollinators to male fitness is not known. Thus, a first account would determine pollination efficiency of legitimate pollinators in terms of outcrossing rate. However, low visit rate and very high fruit and seed set in the non-native range probably means that most seeds are produced by selfing, as already determined for honeybees visiting Mediterranean native flowers (González-Varo et al. 2009, 2010). In addition, we cannot exclude some maternal effects and plasticity if non-native populations grow in richer or more humid soils. Furthermore, in the case of one Spanish population (La Zubia), the high number of open flowers per night and per individual could promote higher levels of geitonogamy, which may enhance the selfing rate and therefore low levels of inbreeding depression, as observed. This scenario was probably very common during the colonisation of the Mediterranean in agricultural and waste areas, where honeybees are most frequent and behave in such an opportunistic manner. In native areas, however, there seems to be pollen-limited seed set, with pollinators producing a highly variable selfed-outcrossed seed output, and correlated variation in seed quality. In these circumstances, outcrossing would still be advantageous, and hence genetic load has not been purged. To obtain nectar, hawkmoths visit many flowers in long flights between plants. In contrast, we observed that all bees collected both nectar and pollen, spending a long time in each flower before visiting another flower, usually in the same plant. As a result, pollen transfer will be very different between these two types of pollinator and hence in promoting outcrossing or selfing (Herrera 1987; Barluenga et al. 2011).
During the transition to selfing, morphological and physiological changes in reproductive traits are expected, such as reduced flower size and level of herkogamy (Bodbyl-Roels & Kelly 2011; Sicard & Lenhard 2011). Sicard & Lenhard (2011) have synthesised four possible, non-mutually exclusive, scenarios for the changes in flower size and herkogamy. These include changes in resource allocation to individual flowers, selection for more efficient pollination, selection for rapid plant and flower maturation in marginal habitats, and florivory. In this study, native and non-native populations did not differ in average flower size; in fact, the Spanish population Hinojos had a higher average flower size of D. stramonium. Hence, data do not agree with the above first and third scenarios. Regarding the fourth scenario, a previous study showed that herbivory levels in Spain are too low (Valverde et al. 2015) to affect flower size. In theory, there is a possible fifth scenario, which considers that changes in flower traits, specifically those related to flower size, are a consequence of the genetic modulation that occurs in selfing populations, as observed in Capsella rubella (Sicard et al. 2016). However, in the case of D. stramonium, it seems that flower size is very variable even within populations, which makes it unlikely to be associated with selfing rates. Despite within-population differences among individuals in flower size explaining a significant amount of the variance, suggesting genetic variation of this character, a similar amount of variance is explained from flowers within individuals (Table 3). It seems that besides ‘noise’ plasticity, there is evidence of developmental phenotypic plasticity in flower size and herkogamy in D. stramonium (Camargo et al. 2017). Thus, selection for efficient self-pollination (in terms of number of seeds) is a plausible scenario, since populations from Spain show reduced herkogamy. It must be noted, however, that herkogamy varies widely within and among populations, but the highest positive herkogamy was found in a native population (Table 2). On the other hand, since flowers are larger than in native populations, is this trait maintained through selection by pollinators? This is probably not the case. Flowers with deep corolla tubes are thought to have evolved in close associations with pollinators, namely, but not exclusively, hawkmoths (Nilsson 1988). According to this, it is not expected that large flowers would be maintained in Spain by bees, as in the native range. However, as Aigner (2006) pointed out, in fine-grained environments (e.g. two types of pollinator) an optimal flower phenotype can be considered generalised when it functions well with both specialised and generalised pollinators, or when a broad symmetrical trade-off occurs. It remains to be determined if floral phenotypes in the non-native range experience coarse- or fine-grained pollinator environments (see also Devaux et al. 2014).
Recent experimental evidence from native populations of D. stramonium indicates that (i) there is genetic variation, plasticity, and genetic variation in plasticity of floral traits, including herkogamy, in response to variation in the soil nutrient environment; (ii) there is also an interaction between flower position (i.e. ontogeny) and nutrient conditions; and (iii) a systematic trend to reduce herkogamy along flower position in the low nutrient environment, thus increasing the likelihood of self-fertilisation (Camargo et al. 2017). Thus, phenotypic plasticity appears to be a plausible explanation for the maintenance of large flower sizes in Spain. Most populations of D. stramonium from Spain inhabit cultivated, irrigated fields and riverbeds, which are very rich in water and nutrient resources. In fact, evidence in Datura wrightii demonstrates that environmental factors (i.e. water availability) affect floral phenotype, like corolla size and herkogamy, and thus within-population variation in mating system (Elle & Hare 2002).
During colonisation, inbreeding depression can limit the evolution of selfing in the new environments (Lande & Schemske 1985). However, selfing permits the expression of deleterious recessive alleles that can be purged from colonising populations, thus reducing the magnitude of inbreeding depression (Crnokrak & Barrett 2002; Pannell 2015). Furthermore, range expansion may involve the depletion of additive genetic variance necessary for adaptive potential in new environments (Pujol & Pannell 2008); this reduction can be a consequence of a genetic bottleneck and further exacerbated by changes in mating system (Pannell 2015). We did not detect inbreeding depression in seed production in the two Spain populations studied. This suggests that populations in Spain may have passed through a genetic bottleneck and lack genetic variation, and/or that selection to purge deleterious alleles has occurred (Pujol & Pannell 2008). Ongoing studies on within-population genetic variation will determine such a possibility. In contrast, we found strong inbreeding depression for this fitness component in a Mexican population (δ = 0.37), which indicates that the population maintains a genetic load of deleterious alleles. Ample evidence of inbreeding depression in D. stramonium, in fitness-related characters (Sosenski 2004) and resistance to herbivores, has been detected in other Mexican populations, together with variation among families in the magnitude of inbreeding depression (Bello-Bedoy & Núñez-Farfán 2010). Moreover, evidence derived from multiple studies suggests that inbreeding depression increases in stressful as compared with benign environments (Armbruster & Reed 2005). The latter argument reinforces our conclusion, given that the detection of inbreeding depression in the native population derived from experiments in a benign environment (greenhouse). Thus, this evidence further supports our hypothesis for the change in mating system of D. stramonium in Spain.
In plants, success in colonising new environments might depend on the ability to respond to selection rather than physiological plasticity and/or tolerance to the environmental conditions (Lee 2002). Natural selection could proceed in response to environmental climatic factors and biotic interactions. However, response to selection, in turn, could be limited by additive genetic variation. In line with this, it is expected that selection, during and after colonisation, might influence plant mating for different reasons (see Pannell 2015), but one important factor is the absence of native pollinators. While D. stramonium is pollinated by specialised pollinators (i.e. sphingid moths) in its native range, our data revealed that it is visited by generalist flower visitors in Spain. Thus, no selection on floral traits that favour outcrossing, such as flower size and positive herkogamy, is expected. In agreement with this, no selection on floral traits was detected in the non-native range. However, in the non-native population La Zubia the selection gradient on corolla size was negative and significant, an expected result, but the whole model was not statistically significant. In contrast, in the native population Santo Domingo, we detected negative directional selection on herkogamy (impliying that plants with low or negative herkogamy had higher seed output). Also, in the native population Ticumán, selection to reduce the distance between anthers and stigma was marginally significant, but the whole model was not. In contrast with non-native range, in the native range there might be enough genetic variation for selection to proceed. One must be bear in mind, however, that selection on these floral traits was assessed considering fitness as seed output. Thus, selection to reduce herkogamy may increase seed output but not necessarily the quality of produced progeny. Independent evidence derived from controlled crosses, selfing and outcrossing clearly indicated that in the native population outcrossing outperforms selfing (i.e. inbreeding depression; cf. Table 5), whereas both types of mating produce equivalent amount of seeds (i.e. no inbreeding depression) in non-native populations. These results suggest that in Spain, selection for selfing has occurred, perhaps due to the purging of deleterious alleles. In addition, these results suggest that genetic variation is lower in the non-native populations. Although the amount of phenotypic variation in flower traits could be similar in both ranges, it is likely that it contains different amounts of phenotypic plasticity. In this study and in a previous one (Valverde et al. 2015), we detected positive phenotypic selection on plant size in D. stramonium in Spain. There, plants grow tall, flower and set seeds during the hottest and driest period, when native Mediterranean plants are inactive and cannot compete for space and resources, in comparison with smaller temperate European plants, whose seed output is much lower (J. Arroyo, personal observations). Whether this phenotypic variation to produce larger plants in D. stramonium is genetically based or due to plasticity remains to be answered in future studies.
Acknowledgements
We thank Alejandra de Castro, Andrés Barea, R. Tapia-López, S. Velázquez, L. Martínez García, R. Torres and A. Pérez Salas for field and lab assistance. We are grateful to the reviewers for suggestions that improved this manuscript. Thanks to the staff of the Nature Park “Sierra de Cardeña-Montoro” and “Alamillo” Park in Spain. Financial support was provided by MINECO (grant 2013 CGL2013-45037-P to JA), PAPIIT and UNAM (grant IN212214) to JNF and a post-doctoral fellowship from CONACYT, Mexico, to VJL (grant 252042). Parts of these results constitute the MSc Thesis of EMB in “Advanced Biology” at the University of Seville.




