Clonal integration facilitates spread of Paspalum paspaloides from terrestrial to cadmium-contaminated aquatic habitats
Abstract
- Cadmium (Cd) is a hazardous environmental pollutant with high toxicity to plants, which has been detected in many wetlands. Clonal integration (resource translocation) between connected ramets of clonal plants can increase their tolerance to stress. We hypothesised that clonal integration facilitates spread of amphibious clonal plants from terrestrial to Cd-contaminated aquatic habitats.
- The spread of an amphibious grass Paspalum paspaloides was simulated by growing basal older ramets in uncontaminated soil connected (allowing integration) or not connected (preventing integration) to apical younger ramets of the same fragments in Cd-contaminated water.
- Cd contamination of apical ramets of P. paspaloides markedly decreased growth and photosynthetic capacity of the apical ramets without connection to the basal ramets, but did not decrease these properties with connection. Cd contamination did not affect growth of the basal ramets without connection to the apical ramets, but Cd contamination of 4 and 12 mg·l−1 significantly increased growth with connection. Consequently, clonal integration increased growth of the apical ramets, basal ramets and whole clones when the apical ramets were grown in Cd-contaminated water of 4 and 12 mg·l−1. Cd was detected in the basal ramets with connection to the apical ramets, suggesting Cd could be translocated due to clonal integration. Clonal integration, most likely through translocation of photosynthates, can support P. paspaloides to spread from terrestrial to Cd-contaminated aquatic habitats.
- Amphibious clonal plants with a high ability for clonal integration are particularly useful for re-vegetation of degraded aquatic habitats caused by Cd contamination.
Introduction
Due to increased human activities such as mining and application of herbicides, chemical fertilisers and pesticides, cadmium (Cd) has become one of the most widespread and hazardous environmental pollutants (Lux et al. 2011; Gallego et al. 2012; Rizwan et al. 2016). Cd can be readily taken up by plants and causes damage to thylakoid membranes, and denaturation of photosynthetic enzymes and proteins (Janik et al. 2010; Lysenko et al. 2015; Chen et al. 2016). It also can decrease stomatal conductance and cause leaf chlorosis and withering, leading to strong impacts on plant morphological, structural, physiological and biochemical attributes even at low concentrations (Ci et al. 2009; Anjum et al. 2016; Chen et al. 2016). Cd has been detected in water and sediments of many wetlands (Deng et al. 2004; Jacob et al. 2013; Bai et al. 2014), causing great damage to ecosystem functions and services (Javadi et al. 2010; Wang et al. 2011; Hechmi et al. 2014; Xiao et al. 2015). To restore such degraded wetlands, it is essential to restore vegetation and select Cd-tolerant plant species.
Amphibious plants can complete their life cycle in both terrestrial and aquatic habitats, and many of them can spread quickly through clonal growth (Wang et al. 2009; Xiao et al. 2010; Sosnova et al. 2011; Luo et al. 2014; Dong et al. 2015). The growth characteristics enable amphibious clonal plants to spread their asexual individuals (ramets) from terrestrial to aquatic habitats and vice versa (Wang et al. 2009). The inter-ramet vascular connections allow translocation of resources such as carbohydrates, water and nutrients between connected ramets through clonal integration (Song et al. 2013; Roiloa et al. 2014). Clonal integration has been shown to improve growth and spread of ramets suffering from various environmental stresses in both terrestrial and aquatic habitats (Amsberry et al. 2000; Yu et al. 2004; Wang et al. 2009; Xu et al. 2010; He et al. 2011; Song et al. 2013; Luo et al. 2014; Roiloa et al. 2014). However, relatively few studies have examined the role of integration in clonal plants growing in the transition between terrestrial and aquatic habitats (Wang et al. 2009; Luo et al. 2014; Liu et al. 2016).
If aquatic habitats are heavily contaminated with Cd, then it is nearly impossible for any vascular plant species to establish initially establish directly in such habitats. Because clonal integration enables amphibious clonal plants to survival and grow across terrestria–-aquatic transition zones (Wang et al. 2009; Luo et al. 2014; Elgersma et al. 2015; Glover et al. 2015), we can speculate that established ramets of amphibious clonal plants in terrestrial habitats can support young ramets to initially establish in aquatic habitats (e.g. wetlands) heavily contaminated with Cd. Thus, amphibious clonal plants with a high ability for clonal integration may be suitable to revegetate degraded wetlands having heavy contamination with Cd. To date, however, few studies have examined the ability of amphibious clonal plants to expand from terrestrial habitats to aquatic habitats contaminated with heavy metals such as Cd or the roles of clonal integration during the expansion (Guo & Hu 2012; Roiloa & Retuerto 2012; Yan et al. 2013).
Wang et al. (2009) showed, for the first time, that clonal integration facilitated Alternanthera philoxeroides growth from terrestrial to aquatic conditions at different water depths, and in this study neither terrestrial nor aquatic conditions were contaminated with heavy metals. Later, another two studies showed that clonal integration enabled A. philoxeroides and Vallisneria natans to spread from terrestiral to Cu-contaminated aquatic conditions (Guo & Hu 2012; Yan et al. 2013). However, A. philoxeroides is an invasive species and not suitable for wetland restoration. We need to test and select native species that can be potentially useful for wetland restortation.
Paspalum paspaloides is an amphibious clonal grass native to China, which grows vigorously at transition areas and can spread rapidly from terrestrial to aquatic habitats (Bhattacharya et al. 2010; Johns et al. 2014). This species is reported to have high ability to tolerate Cd (Chen et al. 2005; Bhattacharya et al. 2010), but it is unknown how clonal integration contributes to this ability. We simulated the spread of P. paspaloides from terrestrial to Cd-contaminated aquatic habitats by growing basal (relatively old) ramets in uncontaminated soil and apical (relatively young) ramets in water contaminated with Cd of different concentrations. Stolons connecting the apical and basal ramets were either intact – to allow clonal integration – or severed – to prevent integration. Specifically, we tested the hypothesis that Cd contamination will decrease the growth and photosynthetic capacity of P. paspaloides and that clonal integration will alleviate such negative effects.
Material and Methods
Plant species
Paspalum paspaloides (Michx.) Scribn is an amphibious, stoloniferous, perennial grass (Bhattacharya et al. 2010; Chen et al. 2015a). It is widely distributed in China, as well as in tropical and subtropical regions worldwide. P. paspaloides can produce creeping stolons that bear leaves, roots and axillary buds at each node. Each node along the stolon with its leaves and roots forms a ramet. This species grows frequently in riparian areas, and can spread quickly by asexual propagation of stolon fragments to occupy both terrestrial and aquatic areas.
Experimental material
Plants of P. paspaloides were collected from four different locations at least 10 m apart, in each of four riparian areas in Chengdu, Sichuan Province, China. We mixed the plants from the different locations and areas, and vegetatively cultivated them in a greenhouse at the Forest Science Company of Beijing, Forestry University, Beijing.
In August 2015, we selected clonal fragments of P. paspaloides, each having eight ramets and an apex. These fragments were at the same developmental stage and propagated from stolon cuttings. For each clonal fragment, the four older ramets were termed ‘basal part’, and the other four younger ones with the apex ‘apical part’. Each clonal fragment was grown in a plastic box (50 cm × 36 cm × 17.5 cm, long × wide × high) that was physically separated by a plastic divider into two equal sections to simulate the transition zone of the riparian region. The left section of the box was filled with a 1:1 (v:v) mixture of riverbank soil and sand (containing 0.33 ± 0.03 mg total N·g−1 and 0.75 ± 0.04 mg total P·g−1, mean ± SE, n = 6) to a depth of 10 cm to simulate terrestrial habitats, and was grown with the basal part of a clonal fragment. The right section was filled with a medium level of eutrophic water (1.76 mg total N·l−1 and 0.16 mg total P·l−1) to a depth of 10 cm to simulate aquatic habitats, and was grown with the apical part of the clonal fragment. Because of the divider, nutrients, water and plant roots in the two sections did not interfere with one another other. After 2 weeks for recovery (on 15 August 2015), 102 clonal fragments of P. paspaloides were selected and used in the experiment described below.
Experimental design
The experiment used a factorial design with four levels of Cd (0, 4, 8 and 12 mg·l−1) crossed with two levels of stolon connection (connected or not connected), resulting in a total of eight treatments (Fig. 1). Six clonal fragments were randomly selected and harvested to evaluate the initial dry mass of apical and basal ramets on day 0 (0.22 ± 0.02 g for apical ramets; 0.16 ± 0.03 g for basal ramets). The remaining 96 fragments were randomly assigned to each of the eight treatments, with 12 replicates for each treatment. For the treatments with Cd levels of 4, 8 and 12 mg·l−1, CdCl2 (Sigma-Aldrich, Shanghai, China) solution was added to the right section of the boxes with the apical parts, and for the treatment without Cd, the same volume of distilled water was added. For the disconnected treatment, the stolon connecting the basal and apical parts was severed, and for the connected treatment the stolon connecting the two parts remained untreated (intact).
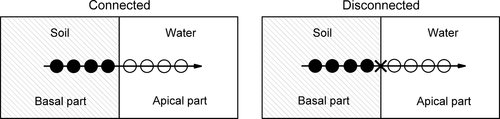
The experiment lasted 60 days. The daily maximum air temperature ranged between 25 and 33 °C during this period. The photosynthetically active radiation measured at water surface at noon was 520–1000 μmol photons m−2·s−1 (Li-250A photometer; Li-Cor Biosciences, Lincoln, NE, USA). Distilled water was added to the boxes to compensate for loss due to evaporation. All plants survived to harvest.
Growth measurements
Six replicates were randomly selected and harvested on day 30, and the other six were harvested on day 60. Before harvest, the ramet number of the apical and basal parts was measured separately. Then, the leaves, stolons and roots of the apical and basal parts were harvested separately, washed thoroughly with tap water, rinsed with distilled water, and dried in an oven at 80 °C for at least for 72 h. The oven-dried samples were weighed, and then ground to fine powder to analyse Cd2+.
Chlorophyll fluorescence measurements
On days 30 and 60, the maximal quantum yield of PSII (Fv/Fm) was determined on the youngest fully expanded leaves (i.e. mature leaves closest to the growth zone of the shoot) of both apical and basal parts using a portable modulated fluorometer (PAM-2500, Walz, Effeltrich, Germany) at 09:00–12:00 h. The maximum and minimum fluorescence in dark-adapted leaves (Fm and Fo, respectively) were measured after 30 min of dark adaptation using leaf clips. The intensity and duration of the saturation pulse applied to determine Fm were 3500 μmol photons m−2·s−1 and 1 s, respectively. Fv/Fm was calculated as Fv/Fm = (Fm−Fo)/Fm (Lysenko et al. 2015). The leaves were then exposed to white actinic light at an intensity of 800 μmol·m−2·s−1 for 3 min. The same process was conducted to obtain the steady-state value fluorescence (Fs) and the maximal light-adapted fluorescence level (Fm′). The effective quantum yield of PSII in the light were calculated as Yield = (Fm′−Fs)/Fm′, and non-photochemical energy quenching in PSII (NPQ) as NPQ = (Fm−Fm′)/Fm′ (Lysenko et al. 2015).
Tissue Cd2+ analysis
About 200 mg dried powder of leaf, stolon and root samples was digested with HNO3/HClO4 (4:1, v/v) for 3 h in heat-resistant glass tubes on a heating block at 180 °C. The digests were diluted in deionised water and the Cd concentration determined using an atomic absorption spectrophotometer (AA-7000, Shimadzu, Kyoto, Japan), according to the method described by Guo et al. (2016). A standard curve of CdCl2 (Sigma-Aldrich) from 0 to 20 mg·l−1 was used for calculation.
Statistical analyses
Before analyses, data were checked for normality and homogeneity of variance. To increase normality and homogeneity of variance, data for Cd concentrations of both apical and basal parts were square root-transformed. Three-way anova was used to examine the effects of stolon connection, Cd and experiential duration (day 30 versus 60) on growth parameters of the apical part, the basal part and the whole clonal fragment, and on chlorophyll fluorescence and Cd concentrations of the apical and basal parts. Tukey multiple comparison tests were conducted to examine differences in growth, chlorophyll fluorescence and Cd concentrations among four Cd treatments (Kim 2015). When significant effects of stolon connection and/or stolon connection × Cd were detected, linear contrasts were followed to examine differences between the connected and the disconnected treatments at each Cd level, using LMATRIX subcommands to control the family-wise error rate (Howell & Lacroix 2012). Data of Cd concentrations in leaves or roots of a few samples were not available due to the low amounts of the samples that were below the instrument detection. All analyses were done using SPSS 16.0 (SPSS, Chicago, IL, USA). Effects were considered significant if P < 0.05.
Results
Growth performance of the apical and basal parts
Cadmium, stolon connection and their interaction had significant effects on all growth variables except root mass of the apical part of P. paspaloides (Table 1a). With increasing Cd concentration, these variables decreased markedly, except stolon mass and ramet number on day 30 (Fig. 2A–J). Without Cd contamination, no significant differences were found in these variables between the connected and the disconnected treatment (Fig. 2A–D, G–J). However, when plants were subjected to Cd contamination, the connected treatment had markedly higher values than the disconnected treatment, except for stolon mass and total mass on day 30 (Table 1a, Fig. 2A–D, G–J).
| Cd | C | D | Cd × C | Cd × D | C × D | Cd × C × D | |
|---|---|---|---|---|---|---|---|
| (a) Apical part | |||||||
| Leaf mass | 7.37*** | 30.69*** | 1.40ns | 3.35* | 0.04ns | 1.51ns | 0.78ns |
| Stolon mass | 7.43*** | 5.09* | 14.23*** | 7.14*** | 2.14ns | 7.00* | 1.23ns |
| Root mass | 2.65# | 0.66ns | 2.31ns | 2.43# | 1.91ns | 2.18ns | 2.06ns |
| Total mass | 8.74*** | 9.08** | 10.18** | 7.42*** | 1.69ns | 7.41** | 0.47ns |
| Ramet number | 2.76* | 31.58*** | 1.04ns | 13.32*** | 2.14ns | 3.99* | 1.70ns |
| (b) Basal part | |||||||
| Leaf mass | 4.69** | 0.01ns | 40.15*** | 1.29ns | 0.78ns | 1.01ns | 0.95ns |
| Stolon mass | 9.32*** | 1.80ns | 107.43*** | 2.49# | 3.51* | 3.86# | 1.63ns |
| Root mass | 10.49*** | 19.12*** | 53.58*** | 4.77** | 5.77** | 1.41ns | 1.32ns |
| Total mass | 9.09*** | 2.14ns | 91.99*** | 2.65# | 2.82* | 3.02# | 1.45ns |
| Ramet number | 7.69*** | 1.35ns | 103.72*** | 0.82ns | 1.63ns | 1.57ns | 1.08ns |
| (c) Clonal fragment | |||||||
| Leaf mass | 5.41** | 2.12ns | 35.44*** | 1.85ns | 0.70ns | 1.68ns | 0.88ns |
| Stolon mass | 10.92*** | 4.97* | 127.73*** | 4.25** | 2.92* | 8.88** | 1.07ns |
| Root mass | 8.58*** | 15.09*** | 43.66*** | 6.40** | 3.71* | 3.48* | 1.41ns |
| Total mass | 11.25*** | 6.46* | 109.20*** | 4.65** | 2.69# | 7.29** | 1.21ns |
| Ramet number | 8.86*** | 10.55** | 103.73*** | 3.31* | 2.04ns | 3.85* | 0.94ns |
- Values are F and symbols show P (***P < 0.001, **P < 0.01, *P < 0.05, #0.05 < P < 0.1 and nsP ≥ 0.1). Degrees of freedom (3, 80) for effects of Cd, Cd × C, Cd × D and Cd × C × D and (1, 80) for other effects.
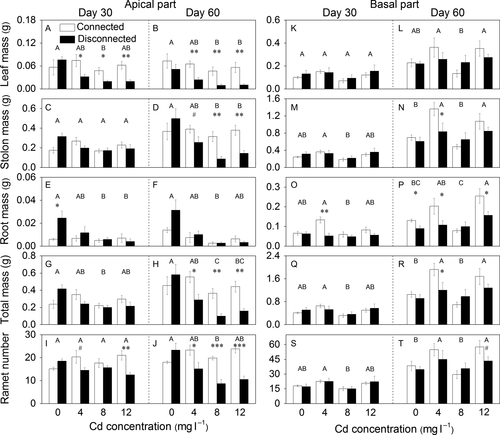
For the basal part, Cd significantly affected all growth variables (Table 1b), and these variables were higher at the Cd levels of 4 and 12 mg·l−1 than at the other levels on day 60 (Fig. 2K–T). The interaction effects of stolon connection and Cd significantly affected root mass and marginally affected stolon mass and total mass (Table 1b). At the Cd levels of 4 and 12 mg·l−1, the connected treatment had higher values of these variables than the corresponding disconnected treatment, except for stolon mass and total mass at the Cd level of 12 mg·l−1 on day 60 (Fig. 2M–R).
Growth performance of the whole fragments
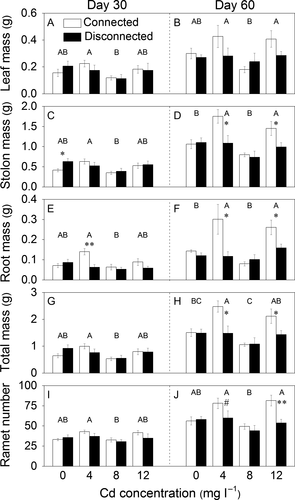
Cadmium, stolon connection and their interaction had significant effects on all growth variables except leaf mass of the whole fragments of P. paspaloides (Table 1c). The whole fragments had significantly higher stolon mass and root mass and marginally higher total mass at the Cd levels of 4 and 12 mg·l−1 than at the other levels on day 60 (Fig. 3D, F, H). Without Cd contamination, stolon mass, root mass, total mass or ramet number did not differ significantly between the connected and the disconnected treatment on day 60 (Fig. 3D, F, H, J). However, at the Cd levels of 4 and 12 mg·l−1, these variables were markedly higher in the connected treatment than in the disconnected treatment (Fig. 3D, F, H, J).
Photosynthetic capacities of the apical and basal parts
Cadmium did not significantly affect any of photosynthetic parameters of apical and basal ramets except Fv/Fm of apical ramets (Table 2a); however, no significant differences were found in Fv/Fm of apical ramets among Cd treatments on days 30 and 60 (Fig. 4A, B). The interaction effects of Cd, stolon connection and duration markedly affected Fv/Fm and ΔF/Fm′ (Table 2a). Without Cd contamination, stolon connection did not significantly affect Fv/Fm and ΔF/Fm′ except Fv/Fm on day 60 (Fig. 4A–D). With Cd contamination, however, the connected treatment had markedly higher Fv/Fm and ΔF/Fm′ than the disconnected treatment (Fig. 4A–D).
| Cd | C | D | Cd × C | Cd × D | C × D | Cd × C × D | |
|---|---|---|---|---|---|---|---|
| (a) Apical part | |||||||
| Fv/Fm | 2.83* | 32.80*** | 4.44* | 4.40** | 1.11ns | 0.52ns | 2.84* |
| ΔF/Fm′ | 0.47ns | 61.09*** | 93.25*** | 2.51# | 1.37ns | 8.07** | 3.58* |
| NPQ | 1.14ns | 0.38ns | 6.25* | 1.85ns | 0.44ns | 0.26ns | 0.61ns |
| (b) Basal part | |||||||
| Fv/Fm | 0.71ns | 0.72ns | 3.31# | 0.83ns | 0.58ns | 0.60ns | 0.78ns |
| ΔF/Fm′ | 0.26ns | 1.97ns | 144.54*** | 0.06ns | 0.34ns | 0.24ns | 1.34ns |
| NPQ | 2.15ns | 28.95*** | 3.56# | 0.66ns | 1.41ns | 0.45ns | 0.53ns |
- Values are F and symbols show P (***P < 0.001, **P < 0.01, *P < 0.05, #0.05 < P < 0.1 and nsP ≥ 0.1). For the basal part, degrees of freedom (3, 80) for effects of Cd, Cd × C, Cd × D and Cd × C × D and (1, 80) for other effects; for apical part df either (3, 73) or (1, 73) because leaves of some ramets died back.
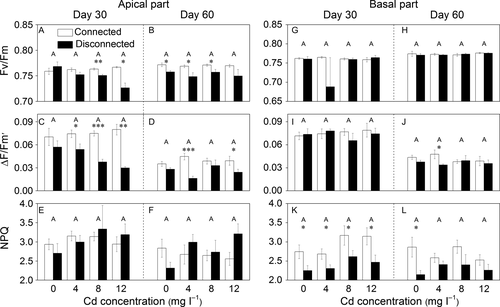
For the basal part, stolon connection significantly influenced NPQ (Table 2b). The connected treatment had markedly higher NPQ than the disconnected treatment (Fig. 4K, L).
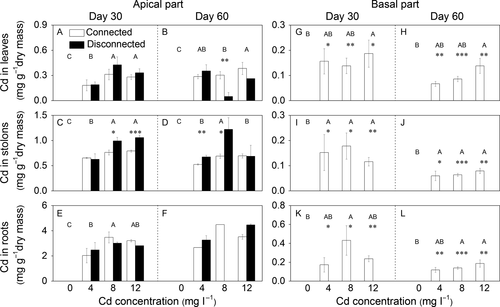
Cadmium concentration in the apical and basal parts
Generally, the Cd concentration was highest in roots, lowest in leaves, and intermediate in stolons (Fig. 5). For the apical part, increasing Cd concentration in water significantly increased Cd concentrations of leaves, stolons and roots (Table 3a, Fig. 5A–F), and stolon connection significantly decreased the Cd concentration of stolons at the Cd levels of 8 and 12 mg·l−1 on day 30, and at levels of 4 and 8 mg·l−1 on day 60 (Table 3a, Fig. 5C, D).
| Cd | C | D | Cd × C | Cd × D | C × D | Cd × C × D | |
|---|---|---|---|---|---|---|---|
| (a) Apical part | |||||||
| Leaf Cd | 29.71*** | 0.36ns | 0.15ns | 0.77ns | 6.73*** | 4.53* | 3.38* |
| Stolon Cd | 83.32*** | 10.11** | 1.18ns | 3.48* | 2.19# | 0.34ns | 1.95ns |
| Root Cd | 105.46*** | 0.66ns | 9.33** | 0.67ns | 2.02ns | 1.25ns | 0.82ns |
| (b) Basal part | |||||||
| Leaf Cd | 11.10*** | 86.81*** | 5.28* | 11.10*** | 0.78ns | 5.28* | 0.78ns |
| Stolon Cd | 5.57** | 48.60*** | 6.87* | 5.57** | 1.26ns | 6.87* | 1.26ns |
| Root Cd | 6.97*** | 48.88*** | 4.69* | 6.97*** | 2.04ns | 4.69* | 2.04ns |
- Values are F and symbols show P (***P < 0.001, **P < 0.01, *P < 0.05, #0.05 < P < 0.1 and nsP ≥ 0.1). Degrees of freedom either (3, 80) or (1, 80) for effects on leaf, stolon and root Cd concentrations of basal part, and stolon Cd concentration of apical part, either (3, 72) or (1, 72) for leaf Cd concentration of apical part, and either (3, 62) or (1, 62) root Cd concentration of apical part.
With stolon connection, Cd was detected in leaves, stolons and roots of the basal part in the three Cd contamination treatments, but Cd concentrations were not significantly different among these treatments (Table 3b, Fig. 5G–L). Without stolon connection, Cd was not detectable in leaves, stolons or roots of the basal part (Fig. 5G–L).
Discussion
When the apical ramets of P. paspaloides were disconnected from the uncontaminated basal ramets, their growth was reduced by Cd contamination, agreeing with previous findings (Zhang et al. 2010; Bankaji et al. 2015; Chen et al. 2015a; Guo et al. 2016). When they were connected to the uncontaminated basal ramets, however, Cd contamination, even the very high concentration of Cd (i.e. 12 mg·l−1), had no impact on their growth. Thus, clonal integration benefited the growth of the apical ramets in the aquatic conditions contaminated by Cd, most likely by importing photosynthates translocated from the connected basal ramets growing in the uncontaminated soil. A benefit of clonal integration has also been reported in other amphibious clonal species such as Ipomoea aquatica, Typha angustifolia, Spartina alterniflora, Ludwigia hexapetala and Alternanthera philoxeroides, when ramets are exposed to nutrient deficiency, shading or flooding (Wang et al. 2008; Xiao et al. 2010; Elgersma et al. 2015; Glover et al. 2015; Liu et al. 2016). The results support our hypothesis and suggest that clonal integration enables P. paspaloides to expand from uncontaminated terrestrial habitats to aquatic habitats that are heavily contaminated with Cd.
While clonal integration was reported to reduce the growth of ramets exporting resources in some cases (Pauliukonis & Gough 2004; Wang et al. 2009; Glover et al. 2015), a recent meta-analysis showed that it generally has no significant costs (Song et al. 2013). In our study, we found that clonal integration generally had no negative effects on growth of the basal ramets and even had positive effects on the growth of basal stolons at the Cd level of 4 mg·l−1 and growth of basal roots at the Cd levels of 4 and 12 mg·l−1 on day 60. Similarly, benefits to the basal ramets have also been found in previous studies on Solidago canadensis (Hartnett & Bazzaz 1983), Fragaria vesca (Roiloa & Retuerto 2007) and Fragaria orientalis (Zhang et al. 2009), showing increased photosynthates and growth in basal ramets in favourable habitats when connected to apical ramets in stressed habitats. The result was likely because bidirectional translocation of resources has been reported in previous studies, which allows translocation of resources from donor ramets to recipient ramets and vice versa (Stuefer et al.1994; Roiloa & Retuerto 2007; Chen et al.2015b). Because the apical ramets in this study were growing in high nutrient conditions to simulate natural eutrophication, the enhanced growth of the basal ramets with connection to the apical ramets might be due to translocation of nutrients from the apical ramets to the basal ramets, which is in line with the findings of Song et al. (2013). Meanwhile, the basal ramets might transport only surplus resources to the apical ramets (Yu et al. 2001; Liu et al. 2016). Because there were significant benefits to both apical and basal ramets, clonal integration significantly increased growth of the whole clonal fragment. This result is consistent with finding of a meta-analytical study (Song et al. 2013) and suggests that clonal integration greatly improves tolerance of the whole fragments of P. paspaloides to Cd contamination in aquatic habitats.
When connected to the uncontaminated basal ramets, the apical contaminated ramets had significantly higher photosynthetic capacities (Fv/Fm and ΔF/Fm′) than the disconnected ones. The high photosynthetic capacities of the apical ramets might fulfil their resource demand for the initial establishment and production of new ramets to extend the population, which might be another reason why we did not find apparent costs to the basal ramets (Yu et al. 2001; Liu et al. 2016). Irrespective of the Cd level, clonal integration significantly up-regulated NPQ of the basal leaves to protect the photosynthetic apparatus and minimise photoinhibition from excess light energy injury (Li et al. 2015; Lysenko et al. 2015). These results suggest that clonal integration can greatly affect photosynthetic capacity of P. paspaloides to adapt to Cd contamination.
Clonal integration also allowed Cd to be translocated from the contaminated apical ramets to the uncontaminated basal ramets. In the aquatic habitats contaminated by Cd, Cd was taken up directly by the roots of the apical ramets of P. paspaloides, which was then partly moved upward to the stolons and leaves and partly translocated via interconnected stolons to the basal ramets growing in the uncontaminated terrestrial habitats. The translocation of heavy metals such as copper among interconnected ramets has also been observed in some other clonal species (Roiloa & Retuerto 2012; Yan et al. 2013). If such translocation is substantial, then there will be a potential risk for the basal ramets to be poisoned by Cd translocated from the apical ramets so that their growth would be greatly reduced by clonal integration (i.e. significant costs to the basal ramets). There is a further risk of clonal integration through spreading heavy metals from contaminated aquatic habitats to uncontaminated terrestrial habitats. However, the Cd concentration of the apical ramets in the contaminated water was ca. 48 times higher than that of the basal ramets in the uncontaminated soil. The concentration of Cd in the basal ramets was apparently not high enough to become toxic so that growth of the basal ramets was not reduced by clonal integration.
In summary, clonal integration, most likely through the translocation of photosynthates, enables amphibious clonal plants to expand from terrestrial habitats to aquatic habitats contaminated by heavy metals such as Cd. Through clonal integration, amphibious clonal plants are likely to be able to establish their ramets and expand their population in aquatic habitats contaminated by exceptionally high concentration of heavy metals, where non-clonal plants may not be able to establish. Thus, amphibious clonal plants with a high ability for clonal integration may be potentially useful for revegetation of degraded wetlands that are heavily polluted by heavy metals such as Cd.
Acknowledgements
We thank Qi Shu, Lin Liu, Ya-Nan Mu and Xiao-Ya Zhang for assistance during plant cultivation and harvesting. We are grateful to Dr. Yong-Jian Wang and two anonymous referees for providing valuable comments, and Dr. John Kershaw for linguistic corrections on an earlier version of the manuscript. This research is supported by the Fundamental Research Funds for the Central Universities (2015ZCQ-BH-0), and the National Natural Science Foundation of China (31670428, 31200314, 31570413).




