Identification and molecular mapping of indica high-tillering dwarf mutant htd4, a mild phenotype allelic mutant of D14 in rice (Oryza sativa L.)
Abstract
- Metabolism of strigolactones (SLs) can improve the efficiency of nutrient use by regulating the development of roots and shoots in crops, making them an important research focus for molecular breeding. However, as a very important plant hormone, the molecular mechanism of SL signal transduction still remains largely unknown.
- In this study, we isolated an indica high-tillering dwarf mutant 4 (htd4), a spontaneous mutant of rice, from the restorer line Gui99.
- Mapping and sequencing analysis showed that htd4 was a novel allelic mutant of D14, in which a single base substitution forms a premature termination codon. Quantitative RT-PCR analyses revealed that expression levels of the genes D10, D17, D27, D3 and D14 increased significantly, while expression of D53 decreased in htd4, compared with the wild type. A subcellular localisation assay showed that the mutant of D14 in htd4 did not disturb the normal localisation of D14 proteins. However, a BiFC assay suggested that the mutant-type D14 could not interact with D3. Additionally, compared with other D14 allelic mutants, htd4 was the first mutant of D14 discovered in indica, and the differences in many yield traits such as plant height, seed-setting rate and grain sizes between htd4 and the wild type were less than those between other D14 allelic mutants and the wild type.
- Therefore, htd4 is considered a mild phenotype allelic mutant of D14. We conclude that the absence of functional D14 caused the high-tillering dwarf phenotype of htd4. Our results may provide vital information for research on D14 function and the application of htd4 in molecular breeding.
Introduction
Rice is one of the most important crops for human consumption, and thus improving rice yield has great significance pertaining to global food security (Khush 2005). The number of panicles is one of the main yield factors in rice; an increase in quantity of panicles increases rice yield (Huang et al. 2003). Generally, the tiller number positively correlates with the effective number of panicles, and therefore tiller number is the key factor regulating rice yield (Xing et al. 2002).
The rice tiller is a special type of branch at the vegetative growth stage, and forms three types of branches, together with the branched spike and spikelet (Gao et al. 2009a). Tillers originate from the lateral meristem of the leaf axil, and the tillering process involves formation, dormancy and outgrowth of axillary buds (Liang & Wang 2016). In general, the majority of tiller buds are dormant, but a few of them can grow into tillers. Recently, carotenoid-derived plant hormones, strigolactones (SLs), have been reported to play a vital role in inhibiting shoot branching (Al-Babili & Bouwmeester 2015). SLs are the long-sought branching-repressing hormones, which inhibit shoot branches by repressing the outgrowth of axillary buds. The function of SLs is highly conserved in both monocots and dicots (Gomezroldan et al. 2008; Mikihisa et al. 2008). Mutants deficient in SLs have been reported to have a high branching phenotype in several plant species (Sorefan et al. 2003; Hamiaux et al. 2012; Zhou et al. 2013).
In rice, previous studies found that the D3, D10, D14/D88/HTD2, D17/HTD1, D27 and D53 genes are involved in either the biosynthesis or signalling pathway of SLs (Ishikawa 2005; Arite et al. 2007, 2009; Zou et al. 2007; Zhou et al. 2013). Among these genes, D17/HTD1, D10 and D27 encode carotenoid cleavage dioxygenase 7 (CDD7), CCD8 and β-carotene isomerase, respectively, and are involved in continuous cleavage of β-carotene and synthesis of SLs (Alder & Al-Babili 2012). The interactions of D14, D3 and D53 play an important role in signal transduction of SLs: D14, which encodes a protein of the α/β-fold hydrolase superfamily, has a conserved three-dimensional structure and contains a highly conserved catalytic serine–histidine–aspartic acid triad (Arite et al. 2009); D3 is a leucine-rich-repeat F-box protein, and participates in an skp, cullin and F-box containing (SCF) complex (Ishikawa 2005; Zhao 2014); and D53 protein, which is similar to the I class Clp ATP enzyme in structure, is substrate of the SCF-D3 ubiquitin complex. D14 perceives and hydrolyses SLs through the highly conserved catalytic serine–histidine–aspartic acid triad, and combines with F-box (D3) protein and D53, then D53 is degraded by the proteasome system to ensure success of SL signal transduction (Jiang et al. 2013; Zhou et al. 2013).
As the receptor of SLs, D14 is the key component of the D14–D3/MAX2–D53/SMXLs complex. Recently, through studies of the Arabidopsis allelic mutant d14-5, D14 has been identified as a non-canonical hormone receptor with dual functions of generating and sensing the active form of SLs. D14 combines with active SLs, and then hydrolyses it into a covalently linked intermediate molecule (CLIM), leading to a conformational change of D14 to promote interaction with D3 (Yao et al. 2016). Although the function of D14 has been reported in a wide range of species, the sequence of interaction among D14, D3/MAX2 and D53/SMXLs remains unclear. Furthermore, the regulatory mechanism of D14 is still largely unknown. Additionally, for Oryza sativa, it is also not clear whether there is a functional difference of D14 between indica and japonica cultivars.
Here, we report a novel rice high-tillering dwarf mutant htd4 derived from the indica cultivar Gui99. The results of molecular mapping and sequencing show that htd4 is a novel allelic mutant with a premature termination mutation in exon 1 of D14. Compared with other allelic mutants of D14, htd4 has weaker phenotypic changes in plant height, grain shape, 1000-grain weight and seed-setting rate. In addition, htd4 is derived from the well-known restorer line Gui99, which has been widely used for breeding. Therefore, mutant htd4 may have high potential value in studies of D14 function and its applications in hybrid breeding.
Material and Methods
Plant material and growth conditions
Mutant htd4 was obtained from natural variation of indica Gui99, and Gui99 was used as the wild type (WT). Molecular mapping material was the F2 genetic population derived from htd4 crossed with the indica variety Peiai 64. The mutant htd4 and WT material used for phenotype investigation, qRT-PCR and the F2 genetic population were all cultivated in the experimental field of Jiangxi Agricultural University during the natural growing season.
Phenotypic observation
After germination, tiller number and plant height were measured in ten WT plants and ten htd4 plants per week. In week 8, the length and width of the top four blades were measured in WT and htd4. At the heading stage, the length and diameter of internodes in WT and htd4 were measured. At the maturing stage, the yield traits of WT and htd4 were investigated based on ten biological replicates. All data were processed in Microsoft Excel 2016.
Molecular mapping of HTD4
The bulked segregant analysis (BSA) method (Zhang et al. 1994) was used to identify linkage of simple sequence repeat (SSR) markers of the target gene. Then, 179 mutant plants obtained in the F2 population were used for rough mapping of the candidate gene. The genetic distance of linkage markers was calculated using MAPMAKER/EXP3.0 software (Abrahamson 1987). For fine mapping, 41 new SSR markers were developed between the rough mapping region utilising SSRHunter (Qiang Li 2005), according to the reference sequence of ‘Nipponbare’ (japonica), and the number of mutant plants in the F2 population was expanded to 875. The predicted genes and genomic sequences were found in the Rice Genome Annotation Project (http://rice.plantbiology.msu.edu/cgi-bin/gbrowse/rice/), and all predicted genes were amplified and then sequenced by Invitrogen (Shanghai, China). The important primers used for mapping are provided in Table S1.
Quantitative RT-PCR
Mutant htd4 and WT seedlings at the six to seven leaf stage were used to study expression of related SLs. The second leaves were ground into fine powder in liquid nitrogen, and total RNA was extracted using the Tiangen RNA Extraction Kit [RNAprep Pure Plant Kit (DP441)] according to the manufacturer's instructions. Then, 20-μl cDNA was obtained with the Qiagen (Shanghai, China) reverse transcription kit (QuantiTect Reverse Transcription Kit) from 1 μg RNA according to the manufacturer's instructions. Next, qRT-PCR was conducted using a 20 μl reaction volume, including 0.4 μl cDNA, 0.2 μm specific primers for each gene and the SYBR Premix Ex Taq Kit (TaKaRa, Liaoning, China) in an ABI PRISM 7500 system (Applied Biosystems, Beijing, China), according to the manufacturer's instructions. The procedure was carried out as follows: initial polymerase activation for 30 s at 95 °C, followed by 40 cycles of 95 °C for 5 s and 60 °C for 34 s. The rice ubiquitin gene was used as internal control. There were three biological replicates for each sample in qRT-PCR. The 2−▵▵CT method was used to analyse the relative expression of each gene in the mutant and WT (Livak & Schmittgen 2001). The primers used for qRT-PCR are given in Table S2.
Subcellular localisation
To generate the 35S::D14WT-Green fluorescent protein (GFP) and 35S::D14MT-GFP constructs, full-length D14 cDNA from WT and the coding sequence of D14MT from htd4 were amplified, and the resulting PCR products were cloned into the binary vector PAN580 using the In-Fusion Advantage PCR Cloning Kit (Clontech, Beijing, China) and sequenced (Invitrogen). The recombinant vectors and nuclear marker D53-mcherry (Zhou et al. 2013) were then transformed into rice protoplasts according to protocols described previously (Bart et al. 2006). GFP signals were detected using a Leica TCS-SP4 confocal microscope. The primers used for subcellular localisation are given in Table S3.
Bimolecular fluorescence complementation (BiFC) analysis
The streamlined double open reading frame (ORF) expression (pDOE) BiFC system was used for the BiFC assay (Gookin & Assmann 2014). For BiFC analysis, the D14WT::NmVen210, D14MT::NmVen210, D3::CVen210, D14WT::NmVen210–D3::CVen210 and D14MT::NmVen210–D3::CVen210 vectors were constructed. The recombinant vectors and nuclear marker H2B (Kanda et al. 1998) were then introduced into Agrobacterium tumefaciens (strain GV3101) and transiently expressed in 5–6-week-old Nicotiana benthamiana leaves according to a previously described protocol (Walter et al. 2004). Fluorescence signals were visualised using a Leica TCS-SP4 confocal microscope 48–72 h after infiltration. The primers used for BiFC analysis are given in Table S4.
Results
Morphological characteristics of the htd4 mutant
Compared with the WT, the htd4 mutant showed a high-tillering dwarf phenotype (Fig. 1a). In detail, plant height in htd4 mutants decreased by about 24.73% compared with WT, while tiller number increased about four-fold in htd4 mutants (Fig. 1b and c). To investigate how tillering and plant height changed over time, the tiller number and plant height of WT and htd4 were measured each week after germination. At the early tillering stage, htd4 plant height was slightly shorter than that of WT, but the start time of tillering was advanced and tiller number was higher than WT (Fig. 1d and e). Over time, the difference in height between htd4 and WT increased gradually, and reached a stable state after 7 weeks. The tiller number of htd4 sharply increased after week 5, and differences in tiller number between WT and mutant htd4 increased gradually. The tiller number of htd4 increased for 10 or more weeks, but remained stable in WT after week 7 (Fig. 1f and g). These results indicate that plant height in htd4 increased significantly more slowly than in WT, but the increase in tiller number was much faster compared with WT.
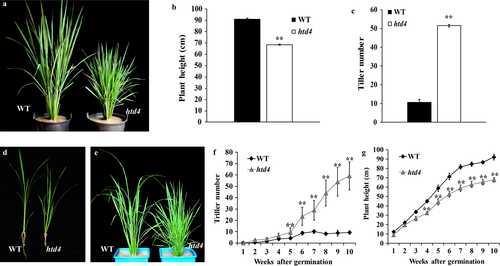
We also compared blade traits between htd4 and WT. The lengths and widths of htd4 leaves in different positions were all significantly smaller than those of the WT (Fig. 2a–c). To investigate the reason for the thin, dwarf phenotype of htd4, each internode length and diameter in htd4 and WT were measured during the heading stage. Compared with WT, each internode length of htd4 was shorter, except for the fifth internode, and the diameter of each internode was significantly reduced (Fig. 2d–f).
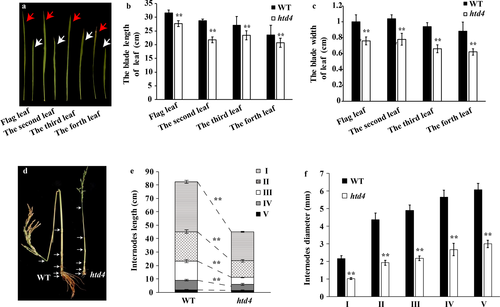
To investigate the impact of htd4 on rice yield, the major yield traits were inspected. The number of effective panicles per htd4 plant was significantly larger than in WT; the grain lengths and grain widths were not significantly different; however the panicle lengths, number of grains per panicle, primary branch numbers, seed-setting rates and 1000-grain weights decreased by 24.23%, 47.94%, 62.08%, 6.25%, and 8.78%, respectively; Fig. 3a–h).
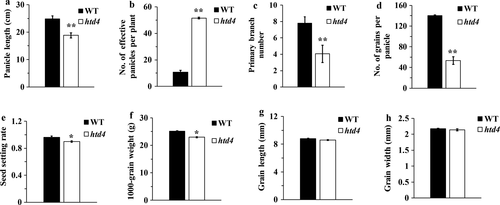
Molecular mapping and characterisation of HTD4
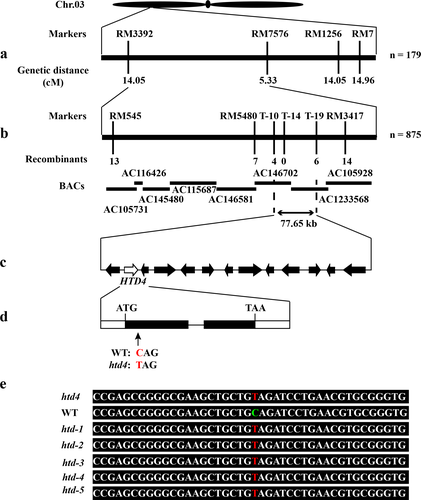
In order to map HTD4, F1 and F2 populations were produced through crossing htd4 with the indica variety ‘Peiai 64’. All F1 plants showed normal plant heights and tiller numbers; while the ratio of normal plants to mutant plants in the F2 population was about 3.55 (χ2C = 1.05 < χ20.05 = 3.84), a ratio of 3:1. This result indicates that the high-tillering dwarf phenotype of htd4 is controlled through a single, recessive nuclear gene. HTD4 was roughly mapped to a region between SSR markers RM3392 and RM7576 on chromosome 3 using 179 mutant plants obtained in the F2 population (Fig. 4a). To fine-map HTD4, 41 SSR markers were developed between RM3392 and RM7576. Furthermore, the number of mutant plants in the F2 population was increased to 875. Finally, HTD4 was fine-mapped to a region between SSR markers T-10 and T-19. The marker interval between T-10 and T-19 was about 77.65 kb on the O. sativa japonica physical map, including 13 predicted genes annotated in the Rice Genome Annotation Project (Fig. 4b and c). To determine the candidate gene, all 13 genes were amplified and sequenced with specific primers in htd4 and WT. The result was that LOC_Os03g10620 had a single-nucleotide substitution in base pair 172 of the first exon in htd4, which caused the codon CAG to change to TAG (Fig. 4d). Remarkably, the substitution of C to T could lead to premature termination of translation. Not surprisingly, this base substitution occurred in other mutant plants in the F2 population (Fig. 4e). In addition, there was no other gene sequence that would cause a transformation in htd4 and WT; therefore, LOC_Os03g10620 was confirmed as the candidate gene of HTD4.
The mutant D14 in htd4 cannot interact with D3
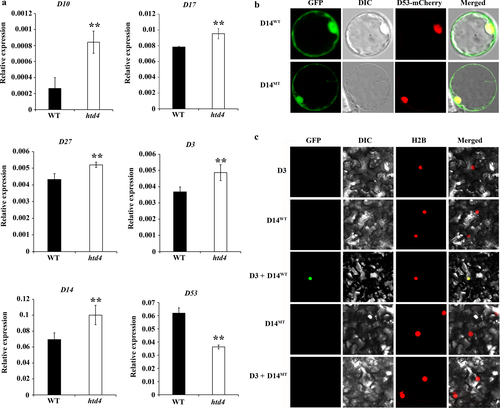
To explore the functional defect of D14 in htd4, we first analysed the relative expression of D10, D17, D27, D3, D14 and D53, which are all involved in metabolism of SLs. The results revealed that the relative expression levels of D10, D17, D27, D3 and D14 were all significantly up-regulated in htd4, while D53 was significantly down-regulated (Fig. 5a). We inferred that the premature translation termination of D14 in htd4 might influence the normal subcellular localisation of the D14 protein. However, subcellular localisation results showed that the mutant-type D14 (D14MT) and wild-type D14 (D14WT) were all located in the cytomembrane and nucleus, which means that the mutant of D14 in htd4 did not disturb the normal localisation of D14 proteins (Fig. 5b). Previous studies showed that D14 can interact with D3 in the nucleus (Zhao 2014). Therefore, we carried out a BiFC assay to confirm whether the mutant D14 can interact normally with D3. Interestingly, D14WT can interact with D3 in nuclei while D14MT cannot interact with D3 (Fig. 5c). This result indicated that the mutant of D14 in htd4 influences the interaction between D14 and D3.
Discussion
In this study, we found a novel allelic mutant of D14. There are three allelic mutants of D14 that have previously been reported. Compared with WT, the most obvious phenotypes of mutant htd4 were higher tiller number, dwarf stature, shorter internodes and smaller panicle and leaf. These phenotypes are consistent with other D14 allele mutants. However, htd4 has some characteristics that distinguish it from these other mutants. First, the mutational pattern of htd4 was different from that of d14/htd2/d88. In the d14 mutant, a 615 bp DNA fragment caused endogenous active transposition of nDart1, which was inserted into the intron of the D14 gene and stopped translation of the D14 gene (Arite et al. 2009). In the htd2 mutant, T-DNA was inserted into HTD2, resulting in a 463 bp deletion between the promoter and the first exon of the HTD2 gene (Liu et al. 2009). For the d88 mutant, a single nucleotide substitution occurred in nucleotide 235 (GGG→AGG) in the first exon of the D88 gene, which changed glycine into arginine at this site, disrupting the N-myristoylation site in the protein (Gao et al. 2009b). For the htd4 mutant, a single nucleotide substitution in nucleotide 172 (CAG→TAG) of the HTD4 gene leads to premature termination of HTD4 protein translation. The htd4 mutant was obtained from the indica cultivar ‘Gui99’, while the d14, htd2 and d88 mutants were obtained from japonica cultivars. Additionally, the differences in many yield traits, such as plant height, seed-setting rates and grain sizes, between htd4 and WT were less than those between d14/htd2/d88 and their WT. Therefore, htd4 is a mild phenotype, allelic mutant of D14 in rice.
The mechanism of feedback regulation is ubiquitous in hormone metabolism (Lin et al. 2015; Maier et al. 2015; Tang et al. 2016). In the SL metabolic pathway, D14 perceives SLs and then binds to the SCF-D3 complex to form D14–SCF-D3 ubiquitin ligase, which interacts with D53 and promotes degradation of D53, so as to suppress the repression of downstream target genes (Jiang et al. 2013; Zhou et al. 2013). If SL signalling cannot occur normally, the downstream target genes will induce synthesis of SLs, and this mechanism has been verified as higher concentrations of SLs detected in root exudates of d14 and d53 (Arite et al. 2009; Zhou et al. 2013). In this study, the transcript levels of SLs biosynthesis-related genes D10, D17 and D27, and the signalling promoting factors D14 and D3 were up-regulated, while D53 was down-regulated (Fig. 5a). It is probable that the synthesis of SLs and the expression of D14 and D3 were induced by the downstream target genes in SL signalling, while the signal suppression of D53 was inhibited.
As the receptor of SLs, D14 can bind SLs in the cytomembrane, and then enter the nucleus to interact with D3. Thus, D14 should be distributed both in the cytomembrane and the nucleus. In this study, subcellular localisation analysis showed that D14MT and D14WT were both located in the cytomembrane and nucleus (Fig. 5b), which is consistent with previous reports (Zhou et al. 2013; Zhao 2014). This result indicated that D14MT remained the signal peptide so that it could be accurately distributed. D14 can bind and hydrolyse SLs, and the hydrolytic activity is essential for protein–protein interaction of the D14-SLs complex with D3 (Al-Babili & Bouwmeester 2015). In the crystal structure of D14, a highly conserved catalytic serine–histidine–aspartic acid triad is the prerequisite for binding and hydrolysing SLs (Hamiaux et al. 2012; Kagiyama et al. 2013; Nakamura et al. 2013). In this study, compared with WT, the D14 protein of htd4 retains only 57 amino acids because of a premature termination mutation in exon 1, and the truncating protein does not include the catalytic domain of hydrolase, especially the highly conserved catalytic serine–histidine–aspartic acid triad (Fig. 6). Therefore, SLs cannot be hydrolysed in htd4, which may lead to a lack of interaction of D14-SLs-D3. Taken together, these results suggest that SL signalling is disturbed in htd4, leading to the high-tillering dwarf phenotype of htd4.
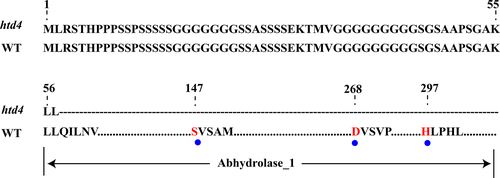
Because of the ability to coordinate the capture of nutrients from the soil and carbon from the atmosphere through regulating the development of the root and shoot in crops, SLs may have broad applications for molecular breeding (Smith 2013). In addition, the regulatory mechanism of transcription and post-translational modifications of D14 are largely unknown, and polymorphism of the D14 allele mutant might be one of the key factors to study these unknowns. According to our results, the mutant D14 protein in htd4 has only the first 57 amino acids, but this does not affect the precise localisation of the protein. Therefore, our results have shown that the signal peptide of D14 protein is located in the first 57 amino acids, which provides an important supplement to functional studies of D14. Additionally, htd4 is the first D14 allelic mutant from the indica background, and the phenotype of htd4 is milder than other allelic mutants. This could mean that there is a functionally similar D14 gene in the indica background that partly compensates for its protein function in htd4, which is important for the functional study of D14 in the indica background.
During the last 20 years, the restorer line Gui99, having the characteristics of high combining ability with other varieties, good cold tolerance, abundant pollen and high seed production, has been widely used in hybrid rice production (Qin et al. 1994; Zhang et al. 2006). htd4 contains the fertility restoring gene because of the well-known restoring line Gui99 background. In this study we found that plant height of htd4 was about 70 cm, and seed-setting rate was about 85%. The plant height of some japonica rice varieties, such as kitaake, is about 70 cm. Therefore, the fertility-restoring gene htd4 could be utilised in breeding indica–japonica hybrid rice, thus incorporating high tillering and good seed-setting rates.
Acknowledgements
The research was supported by the National Natural Science Foundation of China (Grant Nos. 31560350 and 31760350) and the Science and Technology Program of Jiangxi, China (Grant No. 20171ACF60018).




