Convergence beyond flower morphology? Reproductive biology of hummingbird-pollinated plants in the Brazilian Cerrado
Abstract
Convergent reproductive traits in non-related plants may be the result of similar environmental conditions and/or specialised interactions with pollinators. Here, we documented the pollination and reproductive biology of Bionia coriacea (Fabaceae), Esterhazya splendida (Orobanchaceae) and Ananas ananassoides (Bromeliaceae) as case studies in the context of hummingbird pollination in Cerrado, the Neotropical savanna of Central South America. We combined our results with a survey of hummingbird pollination studies in the region to investigate the recently suggested association of hummingbird pollination and self-compatibility. Plant species studied here differed in their specialisation for ornithophily, from more generalist A. ananassoides to somewhat specialist B. coriacea and E. splendida. This continuum of specialisation in floral traits also translated into floral visitor composition. Amazilia fimbriata was the most frequent pollinator for all species, and the differences in floral display and nectar energy availability among plant species affect hummingbirds' behaviour. Most of the hummingbird-pollinated Cerrado plants (60.0%, n = 20), including those studied here, were self-incompatible, in contrast to other biomes in the Neotropics. Association to more generalist, often territorial, hummingbirds, and resulting reduced pollen flow in open savanna areas may explain predominance of self-incompatibility. But it is possible that mating system is more associated with the predominance of woody hummingbird plants in the Cerrado plant assemblage than to the pollination system itself.
Introduction
Pollinators have an active role in the selection of specific reproductive traits of plants, including inflorescence and floral features (e.g. Castellanos et al. 2004; Dudash et al. 2011; Temeles et al. 2013), flowering dynamics and phenology (e.g. Aizen & Vázquez 2006) and even breeding systems (Devaux et al. 2014). The initial impulse that led to the proposal of the pollination syndrome concept was the observation of convergent morphological traits in flowers, showing adaption of non-related plants towards the same functional group of pollinators (Faegri & van der Pijl 1979; Fenster et al. 2004; Rosas-Guerrero et al. 2014). This convergence seems to involve reproductive attributes at physiological and molecular level (Schemske & Bradshaw 1999; Cronk & Ojeda 2008), and in some cases it may affect plant reproductive strategy as a whole (e.g. Linhart et al. 1987; Kudo et al. 2008; Temeles et al. 2013). Therefore, comparative studies involving unrelated species associated with the same pollinator group remain an interesting approach to reveal such patterns (e.g. Wolowski et al. 2013; Amorim et al. 2014).
Hummingbird-pollinated plants often have contrasting reddish coloured and narrow tubular corolla, lack scent as attractant and produce copious amount of dilute nectar (Faegri & van der Pijl 1979; Cronk & Ojeda 2008). Many plants rely on hummingbirds for pollination in the New World, especially in the rain forests, where their community-wide importance as pollinators is considerable (Bawa 1990; Buzato et al. 2000; Martín González et al. 2015). In these areas, phenological patterns, plant distribution and nectar offer affect the attraction of hummingbirds and have an impact on pollen flow and pollination success (Stiles 1975; Linhart et al. 1987; Temeles et al. 2013).
Flowering pattern and plant breeding system are not usually comprised within the pollination syndrome concept, which is restricted to floral traits. Thus, it is noteworthy that the majority of hummingbird-pollinated plants in the Neotropics were found to be self-compatible in a recent survey (Wolowski et al. 2013). This overall trend provides some valuable insights on the reproduction of plants associated with the same group of pollinators; and implies that the association with a specific group of pollinators might go beyond the convergence of floral traits. Moreover, some flowering patterns, especially the floral display size, have been associated with pollination by distinct hummingbird groups, as well as changes in the foraging behaviour of associated hummingbird pollinators (Stiles 1975; Feinsinger 1976; Garcia-Meneses & Ramsay 2012; Justino et al. 2012). For instance, plants associated with traplining hummingbirds often produce few flowers per day, while non-hermit hummingbirds might engage in territorial behaviour in highly rewarding plants with a large floral display (Stiles 1975; Feinsinger 1976; Justino et al. 2012). This difference in the foraging behaviour of hummingbirds leads to differences in pollen flow dynamics and creates selection pressure on plant breeding features (Linhart et al. 1987; Temeles et al. 2013; Betts et al. 2015).
At the community level, ornithophilous plants are more common in rainforest areas than in Neotropical savannas such as the Brazilian Cerrado (Gottsberger & Silberbauer-Gottsberger 2006). Yet, many plants belonging to different families are dependent on hummingbirds for their reproduction in these open savanna habitats (Maruyama et al. 2013). In this naturally patchy environment, flowers in open savanna habitats are commonly associated with more generalist, non-hermit hummingbirds (e.g. Bittencourt & Semir 2004; Consolaro et al. 2009; Machado et al. 2010; Stahl et al. 2012; Maruyama et al. 2014), whereas in forest habitats, specialised hummingbird flowers are frequently associated with the Planalto hermit – Phaethornis pretrei (e.g. Coelho & Barbosa 2004; Consolaro et al. 2005; Araújo & Oliveira 2007; Araújo et al. 2011; Melazzo & Oliveira 2012; Maruyama et al. 2014).
Here, we studied the pollination biology and reproductive system of three sympatric hummingbird-pollinated species in the Cerrado, belonging to three different plant families. They are very common and represent one third of the hummingbird-pollinated plants in the open savanna areas of the region (Machado & Oliveira 2015). In the first part of the study, we characterise the floral adaptations for hummingbird pollination in terms of the floral traits, and evaluate how floral display affected the diversity and visiting behaviour of pollinators. After describing these floral traits in the context of the general features described for ornithophily, we combined the studied species breeding features to data on reproductive biology of other hummingbird-pollinated plants in the Cerrado region. In light of this comprehensive information, we further discuss the reproductive pattern of hummingbird-pollinated Cerrado plants with reference to the aforementioned general survey (Wolowski et al. 2013). Although the majority of hummingbird-pollinated plants were found to be self-compatible in the Neotropics, most studies were conducted in the rainforest areas, and breeding systems seem to be phylogenetically conserved and related to the plant habit (Wolowski et al. 2013). For instance, as woody habit seems to be associated with self-incompatibility in hummingbird-pollinated plants (Wolowski et al. 2013), we might expect hummingbird plants in the Cerrado to be predominantly self-incompatible, because frequent fires select for woodiness (Simon & Pennington 2012). In this sense, we expanded the data available on the floral adaptation of hummingbird-pollinated plants and contrasted breeding system results with the previous survey comprising mainly rainforest plants.
Material and Methods
Study site and studied species
Fieldwork was carried out in the natural reserve that belongs to Clube de Caça e Pesca Itororó de Uberlândia (hereafter CCPIU: 18°59′21″ S, 48°18′06″ W) in the state of Minas Gerais, Brazil, South America. The study site has approximately 400 ha of natural vegetation, with typical savanna formation of the Cerrado as the dominant vegetation type (ca. 60%), besides areas of palm swamps and gallery forests. Climate in the region is highly seasonal, with a warm rainy season from October to March and a cooler dry season from April to September; the mean monthly temperature in the region is 22.8 °C and mean annual precipitation is 1482 mm (Cardoso et al. 2009).
Three plant species associated with hummingbirds were studied. They are the only species exhibiting specialised hummingbird-pollinated flowers in this open savanna area, together with previously studied Palicourea rigida (Machado et al. 2010; Machado & Oliveira 2015): (i) Bionia coriacea (Nees & Mart.) Benth. (Fabaceae: Faboideae) is a shrub up to 1-m high, common in open savanna vegetation in the Cerrado. Until recently recognised as Camptosema coriaceum Benth., and a matter of taxonomic confusion mostly due to the independent evolution of hummingbird pollination within the tribe Diocleae, the genus Bionia has recently been re-established (Queiroz et al. 2015); (ii) Esterhazya splendida Mikan (Orobanchaceae) is a shrub or treelet up to 2.5-m high, also common in coastal Restinga vegetation (Ormond et al. 1998); and (iii) Ananas ananasssoides (Baker.), L. B. Sm. (Bromeliaceae) is a terrestrial bromeliad widespread in the Cerrado region. Vouchers from the studied populations can be found in the Herbarium Uberlandense (HUFU), Uberlândia, MG, under numbers HUFU 41985 (B. coriacea = C. coriaceum), 39242 (E. splendida) and 6814 (A. ananassoides).
Floral biology and conformation of the studied species to ornithophily
For each species, we recorded aspects of the floral biology, including the time of flower opening, floral longevity, pollen release and nectar production, which are commonly used to define hummingbird pollination (Faegri & van der Pijl 1979; Cronk & Ojeda 2008). For morphological measurements we collected at least 30 flowers per species, from at least six individuals. We measured the minimum corolla diameter, internal corolla length and pistil and stamen height from the corolla base, using a digital calliper (error 0.01 mm). In B. coriacea, which is andromonoecious, we recorded pistil size in hermaphrodite flowers only.
Nectar production was estimated with graded micro-syringes (Hamilton, USA) and a hand-held refractometer (Bellingham and Stanley, Tunbridge Wells, UK) in flowers kept isolated from visitors with nylon mesh bags. Accumulated nectar production was estimated at distinct time intervals after flower opening: at 07:00–08:00 h, 12:00–13:00 h and 17:00–18:00 h in 20 flowers per interval, collected from ten distinct individuals for each species. To obtain accurate measures of nectar production at each sampling, flowers were thoroughly inspected and destroyed in the process. We compared the nectar volume between the sampling intervals (i.e. 07:00–08:00, 12:00–13:00 and 17:00–18:00 h) using non-parametric Kruskal–Wallis rank sum test (Zar 1999). After detecting significant difference among intervals, we performed post-hoc Dunn tests to evaluate the differences among each pair of time intervals (Zar 1999). To estimate nectar standing crop, nectar volume was measured in flowers that were accessible to floral visitors at the same intervals.
Sugar composition in the nectar was analysed using gas-liquid chromatography (GLC) in a Konik KNK 3000-HRGS gas chromatograph (Konik, Miami, FL, USA) equipped with a Spectra-Physics SP 4290 data integrator (Spectra-Physics, Santa Clara, CA, USA), a flame ionisation detector, and a SE 30 capillary column (Galetto & Bernardello 2005). Nitrogen was the carrier gas (2 ml min−1). One sample, each from a different individual, was collected from bagged flowers, and stored in Whatman No. 1 chromatography paper. We calculated the sucrose:hexose ratio (sucrose/(glucose + fructose)), which has been related to the pollination system in other studies (Galetto & Bernardello 2005; Amorim et al. 2012).
Flower availability and floral visitors
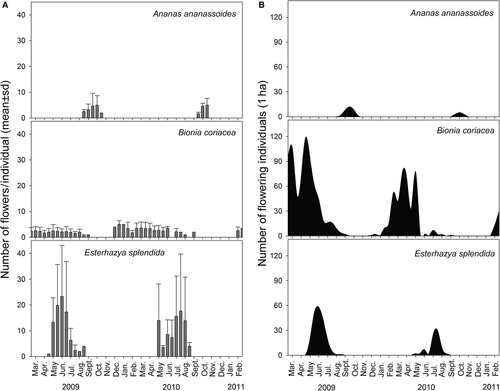
Flowering phenology was recorded fortnightly in a fixed plot of 1 ha, from March 2009 to February 2011, counting the number of flowering individuals and the number of flowers for each individual. Field observations were conducted to record hummingbirds and other potential pollinators. We observed visitor's behaviour on the flowers to see if they effectively pollinated flowers, their frequency of visits and if they engaged in aggressive or territorial behaviour. Focal sessions, 1 h each, were held from 06:00–18:00 h, totalling 40 h of sampling for each plant species.
Breeding system
We performed controlled pollination experiments to characterise the breeding system using four treatments: cross-pollination (between different individuals), self-pollination (within the same individual), spontaneous self-pollination (bagged non-manipulated flowers) and agamospermy (flowers emasculated before pollen release). All flowers in the four treatments were kept isolated from flower visitors with nylon mesh bags before and after the treatments. We used at least 30 flowers per treatment and tried to keep a similar number between treatments within each species, sometimes hindered by flower availability. In B. coriacea, the stigma was lightly brushed with a small paintbrush before pollinations in order to remove or puncture the protective pellicle on the stigma, commonly found in the flowers of this family (e.g. Tandon et al. 2003). Preliminary results had shown that without this procedure no fruit was set, regardless of the treatment. As a control, to estimate natural pollination success, we also marked and monitored flowers with no treatments. All treatments, including controls, were distributed in at least eight individuals for each species. To evaluate the results, we recorded fruit set and seed set for each treatment and control. We calculated the index of self-incompatibility (ISI; Zapata & Arroyo 1978) as the ratio between manual self-pollination and cross-pollination fruit set, and species with ISI below 0.25 were classified as self-incompatible (as in Oliveira & Gibbs 2000). The number of seeds per fruit was compared among treatments for each species with Kruskal–Wallis and post-hoc Dunn test (Zar 1999). In the case of E. splendida, in which enough fruits were formed, the fruit weight was also compared among treatments with the same statistics.
In order to assess the possible prevalence of self-incompatibility in Cerrado plants, we conducted a literature search for studies on hummingbird-pollinated plants in this region, both from open savanna habitats and forests. The survey included the online databases Institute for Scientific Information Web of Science and Google Scholar. As search terms, ‘pollination’, ‘hummingbird’ and ‘Cerrado’ were used, encompassing references up to January 2015. All studies were then sorted for data on pollination experiments as well as for information on the pollinators. From these data, we calculated the ISI as described previously. For dimorphic species, the mean ISI value was calculated from the values of each morph. We also included the life form of each species sensu Sarmiento & Monasterio (1983).
Results
Floral biology and conformation of the studied species to ornithophily
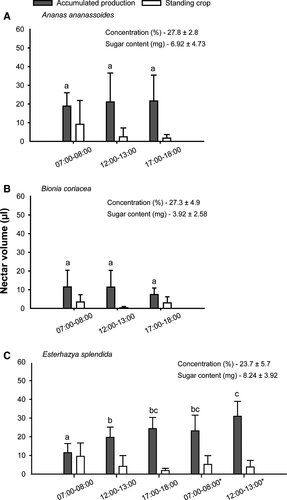
Flowers of the three studied species are markedly different in structure but share a reddish hue and all lack perceptible scent, traits which are consistent with the hummingbird pollination syndrome. Bionia coriacea has bright red flowers (Fig. 1b), while in Esterhazya splendida flowers can vary from red to orange with a spotted pattern near the flower opening (Fig. 1c). In Ananas ananassoides flowers are cream-white, with lilac in the tip of the corolla (Fig. 1a) and have pale reddish bracteoles. In these species, flower opening occurred between 06:00 and 07:00 h. Flowers of A. ananassoides and B. coriacea lasted 1 day, whereas those of E. splendida lasted almost 2 days. E. splendida offered almost three times more nectar in volume and two times more in sugar content than B. coriacea – with intermediate values in A. ananassoides (Fig. 2). Sugar concentration was slightly higher for A. ananassoides and B. coriacea than in E. splendida, which in turn was the only species with increasing nectar availability throughout flower span, suggesting subsequent nectar production after flower opening (Fig. 2). In all species, there was considerable consumption of nectar by floral visitors, which intensified after the first hours in the morning. Sucrose, fructose and glucose were found in the nectar of all species (Table 1). The predominant sugar was sucrose in all species, although this predominance over the other two kinds of sugar was more marked in E. splendida and less marked in A. ananassoides (Table 1).

| Flower features | Ananas ananassoides | Bionia coriacea | Esterhazya splendida |
|---|---|---|---|
| Morphometry (mm) | |||
| Corolla length | 18.5 ± 1.9 | 29.8 ± 3.5a | 28.2 ± 2.5 |
| Corolla diameter | 3.3 ± 0.4 | 3.7 ± 0.5 | 4.5 ± 0.5 (Min.) |
| Anther height | 13.0 ± 1.3 | 22.7 ± 2.8 | 31.9 ± 2.4 |
| Stigma height | 16.3 ± 1.7 | 26.8 ± 3.1 | 34.4 ± 3.7 |
| Sugar content (%) | |||
| Sucrose | 49.85 ± 5.97 | 70.44 ± 5.56 | 88.27 ± 0.84 |
| Fructose | 25.54 ± 2.79 | 14.85 ± 2.19 | 8.23 ± 0.71 |
| Glucose | 24.61 ± 3.28 | 14.72 ± 3.37 | 3.49 ± 0.13 |
| Sucrose/hexose ratio | 0.99 | 2.38 | 7.53 |
- a The actual floral restriction to visitors is ca. 33% of the corolla length.
Flower availability and floral visitors
Flowering time and length varied considerably among species (Fig. 3a). Esterhazya splendida showed the largest floral display (23.3 ± 19.7 flowers individual−1, mean ± SD, at the flowering peak), followed by A. ananassoides (4.7 ± 4.8) and B. coriacea (1.9 ± 1.4; Fig. 2). Considering floral resource, each flower of B. coriacea was far less rewarding than those of the other species (Fig. 2). At individual and population scale, E. splendida was the most rewarding of the three species, with more rewarding flowers than B. coriacea and higher abundance than A. ananassoides, which presented similar nectar reward per flower (Figs 2 and 3).
Overall, the same hummingbird species visited all three plant species (Table 2). Visits were more frequent to E. splendida and less frequent to A. ananassoides and B. coriacea (Table 2). Amazilia fimbriata was the most frequent pollinator for these species, but at least in E. splendida, the hummingbirds Eupetomena macroura and Heliomaster squamosus, both exhibiting territorial behaviour, were also commonly recorded. Pollinators other than hummingbirds were recorded only in A. ananassoides, and these included butterflies of the families Pieridade (e.g. Phoebis sennae) and Hesperiidae (e.g. Urbanus sp.), as well as bees (e.g. Bombus sp.); all were occasionally recorded making legitimate visits, although less frequently than hummingbirds.
| Hummingbird | Bill length (mm) | Ananas ananassoides | Bionia coriacea | Esterhazya splendida |
|---|---|---|---|---|
| Amazilia fimbriata (Gmelin, 1788) | 19 | 37 (5.65 ± 3.73) | 18 (4.22 ± 1.55) | 52 (13.15 ± 14.39) |
| Chlorostilbon lucidus (Shaw, 1812) | 18 | – | 5 (3.40 ± 2.79) | 9 (9.67 ± 7.79) |
| Colibri serrirostris (Vieillot, 1816) | 22 | – | 3 (5.33 ± 1.00) | – |
| Eupetomena macroura (Gmelin, 1788) | 19 | 2 (4.00 ± 2.83) | 1 (3) | 27 (8.31 ± 11.26) |
| Heliomaster squamosus (Temminck, 1823) | 31 | 2 (2.50 ± 2.12) | 3 (3.33 ± 0.58) | 18 (10.78 ± 8.42) |
| Phaethornis pretrei (Lesson & Delattre, 1839) | 35 | 3 (4.67 ± 1.53) | – | – |
| Total | 44 (5.37 ± 3.57) | 30 (4.07 ± 1.70) | 106 (11.97 ± 11.63) | |
| Visits h−1 | 1.10 ± 1.17 | 0.77 ± 1.07 | 2.70 ± 2.66 |
Breeding system
Although all three species bore some fruits after manual self-pollination, only E. splendida developed fruits by spontaneous self-pollination (Table 3). Moreover, fruit set, and seed set, from selfing was considerably smaller than that from cross-pollination, and effectively, all species had ISI values below 0.25 (B. coriacea – 0.15, E. splendida – 0.12, A. ananassoides – 0.21). Consequently, all three species can be characterised as self-incompatible. Natural pollination had a fruit set similar to cross-pollination in all three species, but seed set was lower (Table 3). Fruit weight in E. splendida also followed this pattern, with heavier fruits from cross-pollination than from open natural pollination, which in turn were heavier than selfed fruits (Table 3). In all species, self-pollination produced less seeds than natural pollination (Table 3).
| Pollination treatment | Ananas ananassoides | Bionia coriacea | Esterhazya splendida | ||||
|---|---|---|---|---|---|---|---|
| % fruit (n) | Seeds/fruit | % fruit (n) | Seeds/fruit | % fruit (n) | Seeds/fruit | Fruit weight (g) | |
| Natural pollination | 80.0 (40) | 4.9 ± 4.0a | 7.7 (114) | 8.3 ± 3.3b | 71.4 (70) | 86.8 ± 95.5b | 0.088 ± 0.044b |
| Cross-pollination | 89.7 (39) | 6.0 ± 4.1a | 6.7 (91) | 12.3 ± 3.3a | 71.4 (70) | 166.7 ± 144.3a | 0.122 ± 0.054a |
| Self-pollination | 18.9 (37) | 0.2 ± 0.5b | 1.0 (97) | 5a | 8.6 (70) | 7.7 ± 7.1c | 0.045 ± 0.025bc |
| Spontaneous self | 0.0 (30) | – | 0.0 (65) | – | 13.3 (60) | 13.9 ± 9.5c | 0.033 ± 0.021c |
| Agamospermy | 0.0 (30) | – | 0.0 (54) | – | 0.0 (50) | – | – |
- a Only one fruit was formed after this treatment.
In the literature survey, we found 20 case studies for 17 hummingbird-pollinated plant species from the Cerrado region (Table 4). From these plants, ten species were not previously included in the Wolowski et al. (2013) study. The reason we found more studies might be related to the fact that we conducted a search focused in one biome (i.e. Cerrado), which possibly allowed a more thorough survey, the inclusion of another database, Google Scholar, and that at least two studies in our survey were more recent than the previous survey (Table 4). For the three species with more than one study reporting the breeding system, values of ISI were similar (Table 4). Including the species studied here, we obtained a complete dataset of 20 species, ten of them from open savanna areas and other ten that occurred mostly in forests of the Cerrado region. Together they belonged to 11 different plant families, and 12 of the 20 (60.0%) species were self-incompatible. Splitting the results by the habitat, eight out of the ten savanna species were self-incompatible (80%), while only four out of the ten forest species in Cerrado region were so (40%). Most species were either subshrubs or shrubs of woody groups (75%), while five species were herbs or herbaceous lianas (25%). Among the savanna species, three were herbs (30%) and the remainder were woody subshrubs or shrubs (70%). The dominance of woody species was even higher among the forest species of the Cerrado region (80%).
| Plant species | Author | Family | Pollinators | ISI (BS) | Habitat | Habit | Source |
|---|---|---|---|---|---|---|---|
| Geissomeria pubescens | Nees | Acanthaceae | Thfu | 2.22 (SC) | Forest | ss | Matias & Consolaro (2014) |
| Zeyheria montana | Mart. | Bignoniaceae | Cose | 0.00 (SI) | Savanna | s | Bittencourt & Semir (2004) |
| Amfi, Euma, Phpr | 0.00 (SI) | Barbosa (1997) | |||||
| Ananas ananassoides | (Baker) L.B.Sm. | Bromeliaceae | Amfi | 0.21 (SI) | Savanna | h | This study |
| Costus spiralis | (Jacq.) Roscoe | Costaceae | Phpr | 0.86 (SC) | Forest | h | Araújo & Oliveira (2007) |
| Gaylussacia brasiliensis | (Spreng.) Meisn. | Ericaceae | Chlu, Amfi | 0.98 (SC) | Forest | s | Araújo et al. (2011) |
| Bionia coriacea | (Nees & Mart.) Benth. | Fabaceae | Amfi | 0.15 (SI) | Savanna | ss | This study |
| Cuphea melvilla | Lindl. | Lythraceae | Phpr, Amfi | 0.61 (SC) | Forest | s | Melazzo & Oliveira (2012) |
| Helicteres sacarolha | A.Juss. | Malvaceae | Amfi, Phpr | 0.00 (SI) | Savanna | ss | Franceschinelli (1989) |
| Amfi, Phpr | 0.00 (SI) | Barbosa (1997) | |||||
| Esterhazya splendida | J.C.Mikan | Orobanchaceae | Amfi, Euma | 0.12 (SI) | Savanna | ss | This study |
| Ferdinandusa speciosa | (Pohl) Pohl | Rubiaceae | Phpr, Chlu | 0.61 (SC) | Forest | s | Castro & Oliveira (2001) |
| Manettia cordifolia | Mart. | Rubiaceae | Phpr | 0.17 (SI) | Forest | l | Consolaro et al. (2005) |
| Palicourea coriacea | (Cham.) K.Schum. | Rubiaceae | Heco, bees | 0.32 (SC) | Savanna | ss | Consolaro et al. (2009) |
| Palicourea croceoides | Ham. | Rubiaceae | Thfu, Euma | 0.09 (SI) | Forest | s | Coelho (2013) |
| Palicourea macrobotrys | (Ruiz & Pav.) Schult. | Rubiaceae | Thfu | 0.97 (SC) | Forest | s | Coelho & Barbosa (2003) |
| Thfu | 0.88 (SC) | Consolaro et al. (2009) | |||||
| Palicourea marcgravii | A.St.-Hil. | Rubiaceae | Amfi | 0.06 (SI) | Forest | s | Consolaro et al. (2009) |
| Palicourea officinalis | Mart. | Rubiaceae | Amfi, Chlu | 0.10 (SI) | Savanna | ss | Consolaro et al. (2009) |
| Palicourea rigida | Kunth | Rubiaceae | Cose, Euma | 0.04 (SI) | Savanna | s | Machado et al. (2010) |
| Psychotria poeppigiana | Müll.Arg. | Rubiaceae | Thfu | 0.12 (SI) | Forest | s | Coelho & Barbosa (2004) |
| Amasonia hirta | Benth. | Verbenaceae | Not identified | 0.00 (SI) | Savanna | h | Barbosa (1997) |
| Stachytarpheta gesnerioides | Cham. | Verbenaceae | Amfi, Caam | 0.83(SC) | Savanna | h | Barbosa (1997) |
- Cose = Colibri serrirostris; Amfi = Amazilia fimbriata; Euma = Eupetomena macroura; Phpr = Phaethornis pretrei; Chlu = Chlorostilbon lucidus; Heco = Heliactin cornuta; Thfu = Thalurania furcata; Caam = Caliphlox amethystina. We also list the habitat where the plants can be found within the Cerrado biome (forest or savanna) and the plant habit (woody or herb). SI = Self-incompatible and SC = Self-compatible. Habit terms are used sensu Sarmiento & Monasterio (1983): h = herbs, ss = subshrub, s = shrub, herbaceous liana = l.
Discussion
Although limited in number, the species studied here present convergent flower features and conformation to hummingbird pollination. The data, both for the studied species and the general survey, suggest that plant breeding system may be involved in the adjustment to hummingbird pollination in open savanna areas.
Conformation of the studied species to ornithophily
The three plants studied span a continuum from generalised to specialised ornithophilous flowers, both in terms of floral traits and floral visitor composition. Adaptations to bird pollination include contrasting colour display, abundant nectar as reward and lack of scent as attractants. According to floral features commonly associated with ornithophily (Faegri & van der Pijl 1979; Cronk & Ojeda 2008), B. coriacea and E. splendida show most of the traits associated with hummingbird pollination. These floral traits can be found to a certain extent also in A. ananassoides, which also received occasional visits from insect pollinators. Morphologically, the shorter corolla tube in A. ananassoides might favour more generalist pollination, and this kind of complementary role of insect pollinators seems usual in short-corolla bromeliads, as reported for another population of A. ananassoides (Stahl et al. 2012) and for other species in the family (Canela & Sazima 2005; Wendt et al. 2008; Schmid et al. 2011). In contrast, other floral traits, such as the exerted reproductive structures in E. splendida and the pendant flowers in B. coriacea, have been interpreted as specialised adaptations favouring hummingbirds instead of bees for pollination (Castellanos et al. 2004). Flower morphology of the species studied here, as well as of other hummingbird plants in the Cerrado region, show that most have tubular specialised flowers (literature in Table 4). These flowers contrast with other flowers visited opportunistically by hummingbirds, but mostly pollinated by other visitors in the region (Maruyama et al. 2013; Machado & Oliveira 2015).
Nectar concentration in the three species was similar to the usual 20–26% found in ornithophilous flowers (Stiles & Freeman 1993; Perret et al. 2001; Schmidt-Lebuhn et al. 2007; Cronk & Ojeda 2008; Krömer et al. 2008). Hummingbirds show preference for sucrose (Martinez del Rio 1990), and hummingbird-pollinated plants, in correspondence, usually produce sucrose-rich nectar as the species studied here (Stiles & Freeman 1993; Perret et al. 2001; Krömer et al. 2008; see also Amorim et al. 2012 for a very specialised nectar adaptation). Nevertheless, insect pollinators can also be associated with sucrose-rich nectars (Perret et al. 2001; Schmidt-Lebuhn et al. 2007; Krömer et al. 2008) and other floral traits have to ensure specific association with hummingbird pollinators (e.g. Perret et al. 2001).
Flower availability and floral visitors
Floral display size and nectar offer varied greatly among these species, and were reflected in the main pollinators' activity. Hummingbirds have high energy demands, and are very sensitive to nectar availability, which affects their behaviour and flower choice (Cotton 1998; Justino et al. 2012). In accordance, E. splendida, with the largest floral display, received many more visits than the other two species. But despite differences in floral display, nectar availability and visitation rate, the same short-billed hummingbirds visited all three species studied here, with Amazilia fimbriata being most frequent in all plants. This species is also the main pollinator of several other hummingbird-pollinated plants in the Cerrado (e.g. Justino et al. 2012; Melazzo & Oliveira 2012 and species in Table 4), emphasising the importance of this bird in this ecosystem. Larger species of hummingbird, such as Eupetomena macroura and Heliomaster squamosus, are usually associated with highly rewarding plants, and these birds were more common only in E. splendida. Moreover, hummingbirds only established territories in E. splendida, a foraging strategy that is adopted in rich patches where the resource availability is worth the energetic cost of defence against intruders (Cotton 1998; Justino et al. 2012). An expected consequence of territoriality is limited pollen flow and higher level of self-pollination (Linhart et al. 1987; Devaux et al. 2014). When considering fruit set, natural pollination was roughly equal to cross-pollination, indicating no pollen limitation. Nevertheless, seed set was lower in control open-pollination than in cross-pollinated flowers, especially in E. splendida, in which hummingbirds presented territoriality.
Breeding system
In a recent survey of 73 hummingbird-pollinated plants, Wolowski et al. (2013) showed that 77% of species were self-compatible. This is an unusually high incidence of self-compatibility when compared to the broader survey conducted by Raduski et al. (2012), which compiled data on ISI for 1238 species in 144 plant families and showed that only 30% of plants were strongly self-compatible (44% of plants were classified as strongly self-incompatible in the same study). It is important to note that self-incompatibility is rarely well defined and most plants present some variation in breeding system, as shown in other reproductive features (Gibbs 2014). Nevertheless, the ISI has been largely used to establish usual limits to what are considered self-incompatible and obligate outcrossing species (Raduski et al. 2012).
The higher proportion of self-incompatible species in our Cerrado survey likely reflects the somewhat distinct composition of hummingbird-pollinated plants in this ecosystem in relation to the rain forests. While species from the predominantly herbaceous and/or self-compatible plant families Acanthaceae, Bromeliaceae and Gesneriaceae dominate in rain forests (Buzato et al. 2000; Matallana et al. 2010; Wolowski et al. 2013), many species of Rubiaceae and other woody self-incompatible groups are important hummingbird plants in the Cerrado region. Since woody habit shows an association with self-incompatibility in hummingbird-pollinated species (Wolowski et al. 2013), and fire resistant woody species dominate open savanna vegetation of the Cerrado (Simon & Pennington 2012), a higher proportion of self-incompatible hummingbird-pollinated species would be expected in this habitat. In contrast, the high proportion of self-compatible plants reported in Wolowski et al. (2013) at least partially stems from the dominance of rain forest understorey species in their database, historically better studied for plant–hummingbird interactions (Maruyama et al. 2013).
As closely related hummingbird-pollinated plants tend to have similar values of ISI (Wolowski et al. 2013), the difference among Cerrado and rain forest areas may thus be a result of phylogenetic bias, reflecting the major plant clades more frequent in each system. At the same time, considering the results for all Cerrado plants, as well as the differences between open and forest habitats in the Cerrado, it is tempting to speculate that in open savanna areas, the territorial and more generalist behaviour of non-hermit hummingbirds limit pollen flow (Justino et al. 2012) and may be a pressure selecting for self-incompatibility (as suggested in Linhart et al. 1987). As an example, hummingbird pollination is the predominant system in Bromeliaceae (Kessler & Krömer 2000; Wendt et al. 2008) and they seem to be predominantly self-compatible (Matallana et al. 2010). However, the bromeliad representative from the open savanna studied here, A. ananassoides, showed self-incompatibility.
Concluding remarks
Interaction among plants and hummingbirds in the Cerrado is asymmetrical, with hummingbirds depending less strictly on ornithophilous flowers (Maruyama et al. 2013). On the other hand, many plant species show specialised adaptations and rely on hummingbirds as primary pollinators, as those studied here. These open savanna species were associated with more generalist, and often territorial, non-hermit hummingbirds, which might limit pollen flow due to their lower mobility, and select for outbreeding mechanisms (e.g. Linhart et al. 1987; Garcia-Meneses & Ramsay 2012; Betts et al. 2015). However, the difference of Cerrado plant mating systems to the general pattern in rainforest species may be more associated with the plant assemblage considered rather than the pollination system itself. Since breeding systems have been recently associated with range sizes of plant species (Milla dos Santos et al. 2012; Grossenbacher et al. 2015), the predominance of self-incompatibility found, especially for open savanna plants, may have even larger implications. For instance, widespread plants in temperate regions have been shown to be predominantly self-compatible (Grossenbacher et al. 2015), while for the Neotropical Melastomataceae an opposite trend has been found (Milla dos Santos et al. 2012). Disentangling some confounding factors such as hummingbird's behaviour and plant habit (e.g. woodiness), which seem to relate to the breeding system (Wolowski et al. 2013) and its relation to plant distribution (Milla dos Santos et al. 2012; Grossenbacher et al. 2015), would help in further investigations of the relationship between plant reproductive strategy and other life traits. In this sense, within lineage comparisons contrasting related taxa associated with different pollinators might be an interesting approach for future studies.
Acknowledgements
We thank Peter Gibbs for thorough reading and suggestions on an earlier version of the manuscript. Comments provided by Marlies Sazima, Pedro J. Bergamo, Felipe W. Amorim and Clarisse Palma-Silva, as well as two anonymous reviewers, improved the quality of the manuscript. We also thank Alexandra Bachtöld for identifying the lepidopteran species and Felipe Amorim and Leonardo Galleto for nectar sugar analysis. FAPEMIG (Fundação de Amparo a Pesquisa do Estado de Minas Gerais) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) provided financial support for this study. We dedicate this study to the CCPIU administration, which has preserved the small but valuable area of native vegetation where hummingbirds and plants interact peacefully.




