The role of ABC genes in shaping perianth phenotype in the basal angiosperm Magnolia
Abstract
It is generally accepted that the genus Magnolia is characterised by an undifferentiated perianth, typically organised into three whorls of nearly identical tepals. In some species, however, we encountered interesting and significant perianth modifications. In Magnolia acuminata, M. liliiflora and M. stellata the perianth elements of the first whorl are visually different from the others. In M. stellata the additional, spirally arranged perianth elements are present above the first three whorls, which suggests that they have been formed within the domain of stamen primordia. In these three species, we analysed expression patterns of the key flower genes (AP1, AGL6, AP3, PI, AG) responsible for the identity of flower elements and correlated them with results of morphological and anatomical investigations. In all studied species the elements of the first whorl lacked the identity of petals (lack of AP3 and PI expression) but also that of leaves (presence of AGL6 expression), and this seems to prove their sepal character. The analysis of additional perianth elements of M. stellata, spirally arranged on the elongated floral axis, revealed overlapping and reduced activity of genes involved in specification of the identity of the perianth (AGL6) but also of generative parts (AG), even though no clear gradient of morphological changes could be observed. In conclusion, Magnolia genus is capable of forming, in some species, a perianth differentiated into a calyx (sepals) and corolla (petals). Spirally arranged, additional perianth elements of M. stellata, despite activity of AG falling basipetally, resemble petals.
Introduction
New developments in plant molecular genetics, which have elucidated some of the mechanisms governing floral ontogeny, have shed new light on the mysterious early evolution of the flower. This structure is both sufficiently conservative to be a major diagnostic feature in the taxonomy of angiosperms, yet sufficiently plastic to provide a tremendous diversity of floral form (Adams 2013; Amborella Genome Project 2013; Chamala et al. 2013; Rice et al. 2013). An important and new component of the ancestral flower is the perianth. The evolution of this structure appears to be complex. One theory states that its elements originated from bracts (bracteopetaly), as is true for most basal angiosperms, including Magnolia (Takhtajan 1991; Irish 2009). A second hypothesis, however, proposes that the perianth originated from stamens (andropetaly), and this appears to be valid for eudicots, monocots and certain basal angiosperms: e.g. the family Nymphaeaceae and the genus Saruma (Aristolochiaceae; Jaramillo & Kramer 2004). Indeed, both claims might be true, since the perianth in fact arose many times independently in unrelated lines of radiating angiosperms. Consequently, in various groups, the perianth may not be homologous (Soltis & Soltis 2004). The perianth may either be bipartite, and differentiated into both sepals and petals, or undifferentiated, in which case its components (perianth lobes) are more or less identical and referred to as tepals, although correct nomenclature is not always straightforward. The specialisation of early flowers, which initially possessed many floral parts, has progressed towards the differentiation of a perianth and a reduction in the number of its parts, together with a shift from a spiral to a whorled arrangement of organs (Ronse de Craene 2008). On that basis, most basal angiosperms should possess an undifferentiated perianth, spiral floral phyllotaxis and an elongate receptacle, as found in Amborella (Endress & Igersheim 2000) and Nymphaea (Luo et al. 2011). However, some species of basal angiosperms, which are considered to be more evolutionarily advanced, have a differentiated perianth e.g. Asimina and Annona (Soltis & Soltis 2004; Kim et al. 2005b; Soltis et al. 2009). Others, like Saururus chinensis, either have no perianth at all (Zhao et al. 2013) or, like Magnolia, have retained the ancestral, undifferentiated perianth (Kim et al. 2005a,b). It seems, therefore, that the differentiated perianth, like the perianth itself, is a product of convergent evolution (Soltis & Soltis 2004). The ancestral, undifferentiated perianth may still be observed in some early diverging lower eudicots, such as certain representatives of the family Ranunculaceae (Kramer et al. 2003), as well as in many monocots (Kanno et al. 2003). Besides this differentiation, other modifications of the perianth were possible during angiosperm evolution. These are exemplified by formation of organs of intermediate identity (sepaloidy, petaloidy) or the development of entirely new organs (Ronse de Craene 2007). The genetic mechanism for all these modifications is currently the subject of intense studies, with every indication that it will shortly be resolved.
The ABC model explains the identity of successive floral elements at the genetic level (Fig. 1A; Coen & Meyerowitz 1991). This model assumes that the characteristic floral phenotype depends on interaction of following three classes of homeotic genes: A class (APETALA1 – AP1, and APETALA2 – AP2), B class (APETALA3 – AP3, and PISTILLATA – PI) and C class (AGAMOUS – AG). Each class of genes is expressed in two adjacent whorls of floral organs. Furthermore, the A class genes are antagonistic to the C class genes. The expression of A class genes is responsible for sepal formation. Interaction of genes from the A and B classes and from the B and C classes results in the formation of a corolla and stamens, respectively. The carpels develop as a result of C class gene activity alone (Jack et al. 1994). Additional classes have been postulated to act in concert with the above three classes, namely the D class genes, required for ovule development, and E class genes, guaranteeing full functionality of other ABC genes (Li et al. 2003; Rijpkema et al. 2010). Some authors postulate, however, that the D class genes should not be considered as a separate class (Kramer et al. 2004).
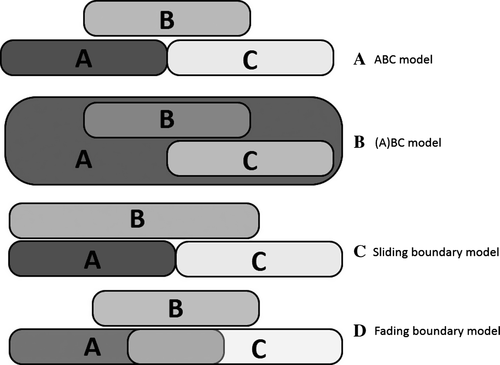
Molecular genetic studies conducted on a large number of plant species showed that modifications of this basic model are necessary to explain the enormous floral diversity present amongst the basal angiosperms. As a result an (A)BC model (Fig. 1B) and two variants of the classic ABC model have been proposed: the ‘sliding boundary’ model (Fig. 1C) and the ‘fading borders’ model (Fig. 1D). In the (A)BC model, only B and C class genes, both individually and together, play a role in establishing the identity of floral organs. The A class genes have no role in that process. Instead, they function in establishing the identity of the floral meristem and are involved in the regulation of transcription of B and C class genes (Causier et al. 2010). The ‘sliding boundary’ model (Kramer et al. 2003) assumes the basipetal extension of the domain of class B gene expression to the first whorl, resulting in the formation of an undifferentiated perianth (tepals), where the co-expression of functional class A and B genes occurs, as shown for Tulipa (monocots), Persea (magnoliids) and Illicium (basal angiosperms; Kim et al. 2005b). Even though they are mostly used to explain the presence of an undifferentiated perianth, other classes of genes may also shift the borders of their expression domains. This occurs for instance in Aquilegia, where an extended expression of C class genes results in the formation of a staminode (Kramer et al. 2007). According to the ‘fading borders’ model, floral identity genes are not expressed to the same degree throughout their domain, but have weaker expression at its outermost (lower) and innermost (upper) margins, which overlap with the expression of other floral organ identity genes, even those that are antagonistic. This leads to the gradual morphological transition of some organs, as in petaloidy (Luo et al. 2011). This latter variant of the ABC model was used to explain the gradual transition of spirally arranged petals and stamens (Nymphaea – water lily) and bracts and petals (Illicium and Amborella) in basal angiosperms (Buzgo et al. 2004a; Soltis et al. 2007). However, it is important to note that only the (A) BC and ‘sliding boundaries’ models have direct experimental support. The final proof for the dose-dependent function of ABC genes that would affect organ identity, and therefore unequivocally confirm the ‘fading borders’ model, has not yet been found.
In basal angiosperms, including magnoliids that diverged from the common tree prior to the splitting of core eudicots and monocots (Wikstrom et al. 2001; Soltis et al. 2002), floral diversity is immense, even though these plants represent <3% of all angiosperm species (Drinnan et al. 1994). Of these, the genus that deserves special attention is Magnolia, distinguished by its exceptional phyllotactic fingerprint, i.e. species- and genet-specific spectrum of floral phyllotaxis (Zagórska-Marek 1994, 2011). Until now, it was generally accepted that Magnolia flowers possess an undifferentiated perianth composed of three trimerous whorls of tepals and multiple spirally arranged generative elements borne on an elongate receptacle (Cronquist 1988; Soltis & Soltis 2004; Kim et al. 2005a,b). However, our observations, together with some published data (Figlar & Nooteboom 2004; Zagórska-Marek 2011; Vazquez-Garcia et al. 2015), clearly show that perianth organisation in Magnolia is not uniform and that there are species that display, at least at the morphological level, a differentiated perianth and/or petaloidy. Some authors (e.g. Duncan & Duncan 1988; Vazquez-Garcia et al. 2015), while describing Magnolia flowers, have already used the term ‘petal’ instead of ‘tepal’, but this is not substantiated either by morphological and anatomical analysis or by molecular data. So far, the only subject of experiments involving various ABC genes and models of their interactions has been M. grandiflora (Kim et al. 2005a,b), and recently also M. wufengensis, which both have an undifferentiated perianth and spirally arranged generative parts. Interestingly, in both species the B class genes (AP3 and PI) were expressed in all whorls of undifferentiated tepals (Kim et al. 2005a,b; Jing et al. 2014). This, and the fact that homologues of AP3 were proven as functional B class genes (Jing et al. 2014), strongly confirms the perianth identity. The AP1 homologue was detected in leaves, the perianth, stamens and carpels, indicating a lack of A class gene function (Kim et al. 2005a). It has, however, been also proposed (but not yet experimentally proved) that here the role of the A class gene might be assumed by AGL6 (Kim et al. 2005b). Its expression, as in the water lily and Persea americana (Lauraceae), has only been detected in the perianth (Chanderbali et al. 2006; Luo et al. 2011). Therefore, even though the ‘sliding boundary’ hypothesis, where the lower border of the domain of B class gene expression is shifted downwards, best explains undifferentiated perianth development in Magnolia, its other modifications still await interpretation. Testing the identity of perianth elements in the context of a modified ABC model in those species of Magnolia that have the perianth differently structured to the above two species is the main goal of this study.
Here we present the results of analysis of three selected species: M. acuminata (L.) L., M. liliiflora Desr. and M. stellata (Sieb. & Zucc.) Maxim. They all have a clearly differentiated perianth, which in the case of the last species has many additional parts, spirally arranged along the floral axis. We have discovered that the floral elements associated with the first whorl can be treated as sepals, as demonstrated both by genetic (lack of AP3/PI expression and presence of AGL6) and morphological/anatomical data. We also detected expression of AP3/PI and AGL6 in the additional perianth elements of M. stellata. Interestingly, a gradual reduction in the expression of AGL6 was detected there, paralleled by an increase in AG expression, with no gradual transition in morphology of additional perianth elements. Our results indicate that the ‘sliding boundary’ hypothesis explains quite well modifications of the magnolia perianth in its whorled region, whereas lack of gradual change in morphology of additional perianth elements in M. stellata is inconsistent with ‘fading borders’ model, suggesting that in this case a threshold value of specific gene products is required for a switch in floral organ identity.
Material and Methods
Plant material
For morphological and anatomical investigation, fully developed flowers were collected during the flowering period: early spring (March–April) for M. liliiflora and M. stellata and early summer (May–June) for M. acuminata. Fully developed foliage leaves were collected in June from all species. Material from plants whose identity had been confirmed was obtained from the Botanical Garden of the University of Wrocław and from Kórnik Arboretum, both in Poland.
For gene expression analyses, Magnolia flower buds (whose parts were all already formed) were collected twice: in October/November and later in February/March, in order to verify that the gene expression had not been affected by winter dormancy. Perianth elements (separately for each whorl), stamens, carpels, leaves from vegetative buds and perianth elements spirally arranged (in M. stellata) were isolated, immediately transferred to liquid nitrogen and stored at −80 °C.
Comparative morphology and anatomy of floral organs and leaves
The morphology of freshly collected, mature flower parts was studied using an Olympus SZX9 (Olympus Optical Co., Warsaw, Poland) stereoscopic microscope. The length and maximum width of all perianth elements were measured for 30 flowers of each Magnolia species. The shape of epidermal cells and stomatal frequency were determined for both the adaxial and abaxial surface of each organ type in a standardised manner: at the middle of the organ and not overlying veins for a sample of 20 leaves and for all perianth elements of 20 flowers. Semi-permanent slides were prepared. Plant material was fixed in FAA (formaldehyde acetic alcohol), bleached in 5% (w/v) aqueous sodium hypochlorite solution (NaClO) at room temperature for 3 h and stained with a 1% (w/v) alcoholic solution of nigrosin. Images of the epidermal surface were taken using an Olympus BX50 microscope, Olympus camera DP71 and Cell B software (Olympus). In order to compare the stomatal density on both surfaces of perianth elements and leaves, we conducted a Kruskal-Wallis statistical test using the Statistica 10 program (StatSoft, Melbourne, Vic., Australia).
Investigation of the internal anatomical structure of floral organs and leaves was conducted by preparing permanent slides using a standard paraffin tissue embedding procedure (Johansen 1940; Gerlach 1972). Transverse sections, 8-μm thick, were cut using a Leica rotary microtome (Leica Instruments, Germany), stained with Fast green and Safranin (Johansen 1940) and finally photographed as described above.
Genetic analyses
Total RNA was isolated using a standard protocol with Tri-Reagent (Molecular Research Center, Cincinnati, OH, USA). To remove any contaminating genomic DNA, RNA was treated with RNA-free DNA-ase (Roche Diagnostics, Mannheim, Germany). First strand cDNA was synthesised from 1500 ng RNA using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Carlsbad, CA, USA) according to manufacturer's protocol.
Primers were designed based on the only known Magnolia A, B and C class gene homologue sequences from the NCBI database and on primers reported in Kim et al. (2005b). All primers used in this study, their sequences and annealing temperatures can be found in Table S1. RT-PCR reaction was performed in 20 μl volume of a mixture containing equal amounts of cDNA, gene-specific primers, dNTPs, 1× PCR buffer, MgCl2 and Taq polymerase (Solis BioDyne, Tartu, Estonia) using a PTC-100TM programmable thermal controller (MJ Research, Waltham, MA, USA). The 18S rRNA gene was used as an indicator of cDNA template quality, and additionally, the reaction without reverse transcriptase was always performed as a negative control to check for DNA contamination. PCR products were separated on 1.5% agarose gel, stained with ethidium bromide and then photographed. In all three Magnolia species, all ABC gene expression studies were conducted on at least three replicates. For the sqRT-PCR performed to compare the expression level of ABC genes in the additional perianth elements of M. stellata, GelQuant.NET software from biochemlabsolutions.com was used to quantify the RT-PCR gel images. The expression level of analysed genes was normalised using the 18S gene.
Results
Morphology of the perianth
The descriptions of Magnolia traits available in the literature state, without going into depth on the problem, that although the perianth in this genus is predominantly uniformly structured, sometimes symptoms of differentiation can be observed (Takhtajan 2009; Ronse de Craene 2010; Vazquez-Garcia et al. 2015). Our survey confirmed that, indeed, in some Magnolia species the morphology of perianth parts constituting the first whorl is distinct. These parts have visually different colour and shape from the other parts (Zagórska-Marek 2011). To investigate these differences more closely, we selected three magnolia species where this feature was especially pronounced: M. acuminata, M. liliiflora, M. stellata. In the last species the additional perianth elements were present.
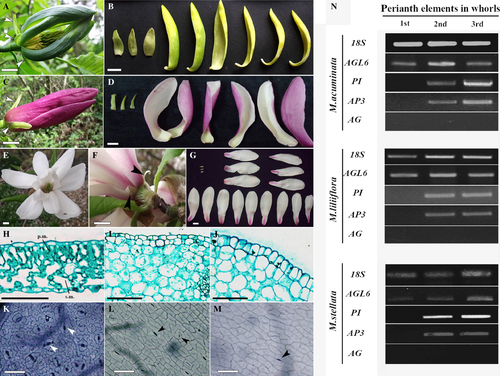
In the first step we carefully examined morphology of the perianth. In all M. acuminata flowers, without exception, the perianth elements of the first whorl were small, uniformly green and triangular in shape (Fig. 2A), and thus obviously different from those belonging to the two subsequent whorls. There, the perianth elements were more showy, longer, with a clearly different shape, incurved margins and characteristic for this species, gradation of colour from blue-green on the dorsal surface in a second whorl to yellow on their ventral surface in the third whorl (Fig. 2B). In M. liliiflora, the first whorl elements were small, narrow and green with pointed tips (Fig. 2C). These highly contrasted with the other perianth elements, which were large, fleshy, dark pink abaxially and pale pink adaxially, becoming wider at the end of their rounded tips (Fig. 2D). In M. stellata, the first whorl elements of the perianth were very small and thin, translucent white or greenish and strongly reflexed (Fig. 2E, F). The remaining perianth parts were fleshy and matte white, tinged pale pink at the base (Fig. 2G). In M. stellata, the additional, spirally arranged perianth elements were present in the area where stamens are typically produced (Zagórska-Marek 2011; Wiss & Zagórska-Marek 2012); their number varied from 8 to 12. They resembled those perianth elements that were arranged in whorls (with the exception of the first whorl) in terms of colour and shape (Fig. 2G). After analysing 30 flowers, we found the condition of the initial three trimerous whorls exclusively present in the perianth of M. stellata.
Anatomy of perianth elements
In order to check the degree of difference of the perianth elements from foliage leaves, we performed comparative analysis of these organs with regard to the shape of epidermal cells and mesophyll anatomy. We found that the epidermis on adaxial and abaxial sides of the leaf was always flat and composed of typical ‘jigsaw’ cells (Fig. 2K); the mesophyll was invariably differentiated into palisade and spongy parenchyma (Fig. 2H). The epidermal cells of the perianth organs, irrespective of the side or the whorl number, were always rectangular, with straight cell walls (Fig. 2L, M), and the mesophyll was undifferentiated (Fig. 2I, J).
Stomata distribution differed in the leaves and perianth organs and varied to a similar extent in all three Magnolia species. In the leaf, stomata were present on the abaxial side, but absent on the adaxial side (Table 1, Fig. 2K). In the perianth elements, stomata occurred on both sides, but their frequency gradually decreased upwards. In comparison to leaves, there was a 10-fold reduction in frequency of stomata in the second and third whorl of the perianth, but only a fivefold reduction in the first whorl (Table 1, Fig. 2L, M), and these differences were statistically significant. Another difference between whorls was the profile of the epidermis seen in transverse sections. The epidermis of the first whorl elements was always flat (Fig. 2I), as opposed to conical epidermal cells (Fig. 2J) characteristic of the second and third whorl elements. In short, the elements of the first perianth whorl, like the foliage leaves, have flat epidermal cells and a reduced, albeit still relatively high, stomata frequency. At the same time, they share with the remaining perianth elements such distinct features as: (i) a rectangular contour of the epidermal cells proper; (ii) presence of stomata on both sides; and (iii) undifferentiated mesophyll.
| Species | Mean stomata number 1 mm−2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Leaf | PE of whorls | Additional PE | ||||||
| 1st | 2nd | 3rd | 1st | 3rd | 6th | 9th | ||
| Magnolia acuminata | ||||||||
| ab | 125 ± 9 | 30 ± 5 | 10 ± 4 | 8 ± 2 | N/A | N/A | N/A | N/A |
| ad | – | 28 ± 3 | 9 ± 3 | 7 ± 2 | N/A | N/A | N/A | N/A |
| Magnolia liliiflora | ||||||||
| ab | 158 ± 20 | 24 ± 6 | 8 ± 3 | 8 ± 2 | N/A | N/A | N/A | N/A |
| ad | – | 25 ± 4 | 7 ± 2 | 7 ± 1 | N/A | N/A | N/A | N/A |
| Magnolia stellata | ||||||||
| ab | 371 ± 24 | 41 ± 7 | 9 ± 3 | 8 ± 3 | 8 ± 1 | 8 ± 2 | 8 ± 2 | 8 ± 1 |
| ad | – | 38 ± 5 | 8 ± 2 | 8 ± 2 | 7 ± 2 | 8 ± 1 | 8 ± 1 | 7 ± 2 |
- N/A = not applicable.
- Stomata in the leaves appear only on the abaxial side (ab). Statistically significant differences exist between the PE of the first whorl and leaves, the PE of the first whorl and the remaining PE, regardless of their arrangement: either whorled or spiral (M. stellata). There is no difference between the PE from the second and third whorl and between the whorled and spiral (additional) PE.
Both morphological and anatomical data clearly show that the elements of the first perianth whorl differed from those of the remaining two whorls and also from the leaves. It is evident that the characteristic features of these two kinds of organ are admixed in the elements of the first whorl.
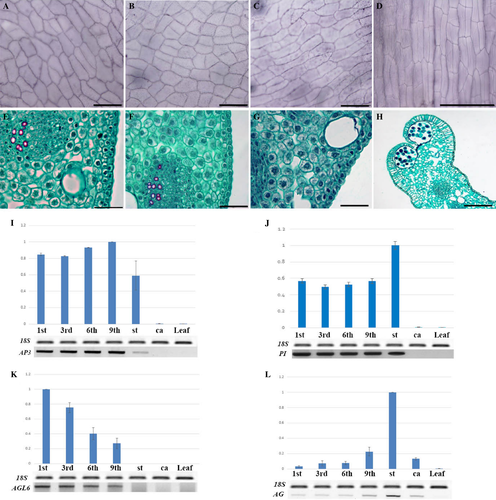
Similar analysis of additional, spirally arranged perianth elements of M. stellata showed that, as in whorls (Fig. 3A, E), they have rectangular epidermal cells (Fig. 3B, C), undifferentiated mesophyll (Fig. 3F, G) and stomata on both sides (Table 1). They also clearly differ in their anatomy from stamens (Fig. 3D, H). Statistically, there was no difference in the frequency of stomata between the additional perianth elements and those arranged in whorls (Table 1). Despite developing within a domain where stamens are usually formed, each of these elements is supplied with three vascular bundles, a feature typical of the leaves and bracteopetal perianth organs, not by a single bundle characteristic of the stamens. These results strongly indicate that these structures are in fact perianth elements, and only their distribution pattern is characteristic for stamens.
Genetic identity of the perianth organs
Our morphological and anatomical studies revealed that in all investigated Magnolia species the perianth elements of the first whorl are distinct. They are neither modified leaves nor the same as the elements of the second and third perianth whorl. Does this provide sufficient evidence that these elements are sepals? To address this question we conducted an analysis of the ABC genes and, for all three Magnolia species, obtained the same pattern of gene expression (Fig. 2N). This pattern was not affected by the time of the harvest; it was identical in floral buds collected before and after winter. Not once was expression of the AG gene detected in the perianth elements (Fig. 2N), but AP1 gene expression was present in all floral organs, as well as in the foliage leaves (Fig. S1), as previously reported by Kim et al. (2005a). Therefore, we analysed the expression pattern of the predicted A class gene, AGL6. This gene was expressed in all whorls of the perianth, both in the first and in the remaining two whorls (Fig. 2N). Interestingly, expression of AP3 and PI, the B class genes, was restricted to the second and third whorl (Fig. 2N).
Genetic identity of additional perianth organs in M. stellata
In terms of morphology and anatomy, especially vasculature, the additional elements found on the border between the whorled perianth proper and the androecium, despite their spiral arrangement, resembled those arranged in whorls. Because of their evidently mixed features: tepal-like outlook versus stamen-like distribution, the term ‘tepaloid stamens’ was used in our earlier work, where juvenile genets were also studied (Wiss & Zagórska-Marek 2012). The additional elements were in such cases less numerous and smaller, but their number and size increased with age of the genet (B. Zagórska-Marek, unpublished data). What then is their final identity? In order to clarify this, the genetic identity of additional perianth elements was examined in flowers with a well-developed ‘stellata’ phenotype. sqRT-PCR analyses of ABC gene expression on selected, additional perianth elements (1st, 3rd, 6th, 9th), as well as on generative organs and fully developed foliage leaves, were performed. Expression of the AP1 gene was detected throughout the flower, including additional perianth organs, but also in leaves (Fig. S1). The B class genes AP3 and PI were transcribed in additional, spirally arranged perianth elements, but no signal was detected in carpels (Fig. 3I, J). The pattern of expression of B class genes was therefore consistent with the classic ABC model. Next, we analysed the expression pattern of the AGL6 gene. Significantly, its expression was detected in the additional, spirally arranged perianth elements, with no signal found in generative parts (Fig. 3K). There was a small gradation in AGL6 expression level between the first and ninth additional perianth element. The signal fell acropetally along the floral axis (Fig. 3K). Expression of AG, the proven C class gene, was observed in generative parts, both stamens and carpels (as in the ABC model), but also faintly in the additional perianth elements (Fig. 3L). The degree of expression of AG in these additional elements was complementary to that of AGL6, decreasing basipetally (Fig. 3L). More importantly, no expression of AG was ever detected in the perianth elements arranged in whorls (Fig. 2N). No signal from AGL6, AP3, PI or AG was observed in foliage leaves (Fig. 3I–L).
Discussion
The diversity of flower architecture in basal angiosperms is enormous, and the mechanisms responsible for this phenomenon are still the subject of investigation. Some of these variations can be explained in terms of already well-understood modifications to the classical ABC model, but further work is necessary. For example, duplication events occurring during the evolution of basal angiosperms may also form the basis for the different ABC gene expression patterns and function, and may be treated as a potential source for the appearance of novel floral patterns (Kramer et al. 2003; de Bodt et al. 2005; Viaene et al. 2010; Amborella Genome Project 2013).
This investigation has focused on the mechanisms responsible for perianth development in the basal angiosperm genus Magnolia. The expression pattern of ABC genes in this genus is known mainly from studies on M. grandiflora (Kim et al. 2005a,b), a species that has an undifferentiated perianth composed of three whorls of elements, each having the same genetic identity. To explain this, the ‘sliding boundary’ hypothesis (Kramer et al. 2003) was proposed, and it was suggested that the AGL6 gene is an A class gene (Kim et al. 2005a,b). Our research has focused on three other species of Magnolia having a differently organised, clearly differentiated perianth: M. acuminata, M. liliiflora and M. stellata.
The most important discovery was that the above morphological differentiation was also expressed on the anatomical level, for example in terms of mesophyll structure, epidermal cell shape and stomata frequency, and confirmed unequivocally by the genetic data. The expression pattern of genes from A, B and C classes showed that AP3/PI expression was detected in the second and third whorl of the perianth, but not in the first whorl, which was visually different from the other two. The AGL6 gene was expressed in all perianth elements, but not in the generative zone, which supports the suggestion of its role as a A class gene, unlike AP1, which was expressed in all floral parts of the magnolias studied. Expression of AGL6 only in the first whorl, in the absence of AP3/PI expression, resulted in the formation of perianth elements that differed from those of other whorls. In our opinion, the floral organs of the first whorl of all three Magnolia species have an entirely new identity and can justifiably be referred to as sepals. This means that in Magnolia, the perianth may in some cases be composed of two types of organ, each having a distinct genetic identity. We are fully aware of the limitations of the RT-PCR technique, for which the gene expression analysis could not be performed at the most crucial primordial stage of floral organ development. Nevertheless, the fact that the identity (based on morphological/anatomical and genetic data) of these highly specific organs did not fade during differentiation, further growth and aging, is in some way proof of its stability. However, it is worth mentioning that the structure of the organs is not always fully dependent on the ABC genes expression pattern. It is probably associated with modifications of the genes within a particular evolutionary lineage (neo-functionalisation and sub-functionalisation) and/or with the existence of other genetic factors, obscure so far, and probably unrelated to the ABC genes, but involved in determining the flower organ identity, as postulated for example for Aristolochia (Jaramillo & Kramer 2004; Litt & Kramer 2010; for additional information on genome to phenotype correlations see e.g. Weiss 2005). Importantly, in our and other authors’ opinion, even uncorrelated morphological–anatomical and genetic data, in terms of organ homology, provide important information and allow correct labelling of the organs and therefore cannot be neglected (Buzgo et al. 2004b; Soltis et al. 2005). Despite these discrepancies in the case of M. acuminata, M. liliiflora and M. stellata, it is advisable to revise most common terminology currently in use and, in referring to the perianth elements of these species, always consider them in terms of sepals and petals, rather than simply tepals.
The relatively showy perianth of M. stellata, which should thus be treated as a case of petaloidy, represents yet another interesting phenomenon associated with the perianth structure of magnolias. Our analyses confirmed that at the morphological and anatomical level, petals both in whorls and spirally arranged are identical. The diminishing upward expression of AGL6 (a postulated A class gene), accompanied by increasing expression of AG discovered in consecutive additional petals suggests that petaloidy in this species may best be explained through the ‘fading boundary’ hypothesis. This case, however, differs from that shown for the water lily, another species for which this hypothesis was also proposed (Luo et al. 2011). In the water lily, there is an obvious, gradual morphological transition between tepals and stamens, whereas in M. stellata all perianth elements (except for the sepals in the first whorl, confirmed in this study) are identical. The latter could thus be the outcome of an insufficient AG protein level needed to trigger morphological transition, and consequently normal petals develop from the spirally arranged primordia.
Magnolia stellata is not the only member of the genus to have additional petals. In some other species, such as M. obovata and M. virginiana, the additional perianth elements, which are also arranged spirally (Zagórska-Marek 2011), might have a similar genetic background. Furthermore, it is claimed in ‘Notes on Magnoliaceae’ (Nooteboom 1985) that M. wilsoni and M campbelli also have an elevated number of perianth elements. Unfortunately, it is not yet clear how they are organised in these species; they may be spirally ordered, as in M. stellata, or the number of perianth whorls may be multiplied, as in M. wufengensis (Wu et al. 2012). Enlargement of the perianth increases its attractiveness to pollinators. Together with perianth differentiation, this is an extremely interesting developmental process involving genetic mechanisms capable of producing different phenotypes, and thus, responsible for the immense plasticity found in the genus Magnolia. It promises to be a most interesting field for future evolutionary research. The presence of petaloidy in M. stellata is also indicative of an unknown mechanism responsible for the spacing of floral primordia, which acts earlier than and independently of the mechanism that defines their final identity. Wiss & Zagórska-Marek (2012) studied the additional perianth elements but concentrated on a quality of their phyllotactic patterns without closer examination of their identity. Interestingly, our above hypothesis on the independence of the two developmental processes is also supported with an ABC-triple mutant of Arabidopsis thaliana, where leaf-like organs are arranged not spirally but in whorls (Bowman et al. 1991). The compact character of the floral shoot in this mutant implies the presence of yet another, possibly independent, mechanism that shortens the internodes of the floral shoot and is also involved in shaping floral architecture.
Contrary to the model species M. grandiflora, all three investigated species (M. acuminata, M. liliiflora and M. stellata) have a differentiated perianth, which is considered a derived character. The species M. acuminata and M. liliiflora are sister clades and, based on fossil evidence and nuclear and chloroplast data, are also closely related to M. stellata (Nie et al. 2008). They are, however, evolutionarily distant from M. grandiflora. Interestingly, Magnolia probably contains at least one other species with a differentiated perianth. This is M. obovata (Zagórska-Marek 2011), a taxon also well separated on the molecular phylogenetic tree from M. acuminata, M. liliiflora and M. stellata. If this trait can be confirmed with similar data to our genetic studies, combined with classic methods of phenotyping, it would demonstrate that the differentiated perianth arose independently on at least two occasions. That the genus Magnolia contains a mixture of both ancestral and progressive traits is already known. Our results relating to perianth architecture show that this highly ‘experimental’ clade, distinguished by its plasticity in terms of development and evolution, is valuable both from the perspective of evolutionary research and in the context of basal angiosperms studies. We have brought together the expression patterns of AGL6, AP3/PI and AG genes with distinct, visually accessible phenotypic identities of floral organs present in three Magnolia species with unusual perianth architecture, and demonstrated that in all cases the genetic and morphological/anatomical identity of various organs is well correlated. Nevertheless, because the function of AGL6 homologues in plants is still ambiguous and appears to be highly variable, being involved in determining the identity of the floral meristem (Oryza; Li et al. 2010), in development of the ovary (Hyacinthus; Fan et al. 2007; Rijpkema et al. 2009), in functioning as an A class gene (Oncidium; Hsu et al. 2003) and in the transition of juvenile to adult plant, or as a regulator of floral transition (Nicotiana; Tzeng et al. 2002) we plan to continue this research in order to prove or invalidate the role of AGL6 in Magnolia as an A class gene during flower development.
Acknowledgments
This work was supported by the University of Wroc?aw (grant no. 1068/S/IBE/14). We thank members of Department of Plant Developmental Biology for helpful comments on the manuscripts and Dr Kevin L. Davies for linguistic correction.




