Physiological integration enhanced the tolerance of Cynodon dactylon to flooding
Abstract
Many flooding-tolerant species are clonal plants; however, the effects of physiological integration on plant responses to flooding have received limited attention. We hypothesise that flooding can trigger changes in metabolism of carbohydrates and ROS (reactive oxygen species) in clonal plants, and that physiological integration can ameliorate the adverse effects of stress, subsequently restoring the growth of flooded ramets. In the present study, we conducted a factorial experiment combining flooding to apical ramets and stolon severing (preventing physiological integration) between apical and basal ramets of Cynodon dactylon, which is a stoloniferous perennial grass with considerable flooding tolerance. Flooding-induced responses including decreased root biomass, accumulation of soluble sugar and starch, as well as increased activity of superoxide dismutase (SOD) and ascorbate peroxidase (APX) in apical ramets. Physiological integration relieved growth inhibition, carbohydrate accumulation and induction of antioxidant enzyme activity in stressed ramets, as expected, without any observable cost in unstressed ramets. We speculate that relief of flooding stress in clonal plants may rely on oxidising power and electron acceptors transferred between ramets through physiological integration.
Introduction
Flooding is detrimental to most terrestrial plants, exerting significant effects on species composition, productivity and stability in numerous plant communities worldwide. Although much progress has been made in elucidating mechanisms of hypoxia/anoxia tolerance in plant tissues (Jackson et al. 2009; Perata et al. 2011; Pucciariello et al. 2014), how physiological integration (a unique physiological phenomenon in clonal plants) affects the performances of flooded clonal plants has received limited attention (Wang et al. 2009; Xiao et al. 2010).
Clonal growth is widespread in the plant kingdom (Klimes et al. 1997), and defined as ‘vegetative production of modules (ramets) which may achieve independence’ (Mogie & Hutchings 1990). The genetically identical offspring can remain connected to the parent for a considerable period through stolons, rhizomes or roots (Xu et al. 2012). Physiological integration allows substance transport between interconnected ramets in habitats with different resource availability, and therefore can support the survival and growth of stressed ramets (de Kroon & van Groenendael 1997; Yu et al. 2004; Xu et al. 2010; Roiloa & Hutchings 2013). Numerous studies have shown the importance of physiological integration on increasing individual fitness under abiotic stresses, such as shade (Xu et al. 2010), salinity (Ikegami et al. 2008), nutrient deficiency (Marshall & Anderson-Taylor 1992), defoliation (Jonsdottir & Callaghan 1989; Xu et al. 2012) and soil contamination with heavy metals (Salemaa et al. 1999). Moreover, many flood-tolerant species such as Phragmites australis (Barclay & Crawford 1982), Glyceria maxima (Monk et al. 1987), Typha domingensis (Pezeshki et al. 1996), Acorus calamus (Arpagaus & Braendle 2000), Alternanthera philoxeroides and Hemarthria altissima (Luo et al. 2011) are clonal plants. Therefore, in-depth studies of the relationship between physiological integration and flooding tolerance will extend the scientific interpretation of plant tolerance to flooding.
In the last decade, physiological integration between interconnected ramets has proved beneficial for growth of flooded A. philoxeroides (Wang et al. 2009) and Spartina alterniflora (Xiao et al. 2010), of which young ramets physically connecting to old ones grow better compared to those without connections. However, the metabolic alteration induced by physiological integration and its contribution to the benefits remains to be elucidated.
Upon flooding, plants suffer growth inhibition attributed to O2 shortage. Limited O2 availability in plants restricts aerobic respiration, decreases adenylate energy charge, which may lead to carbohydrate starvation (Bailey-Serres & Voesenek 2008; Bailey-Serres et al. 2012). On the other hand, hypoxia/anoxia within plant tissue creates an over-reduced environment that favours the generation of reactive oxygen species (ROS), which are toxic in excess amounts (Bailey-Serres & Voesenek 2008; Sairam et al. 2008). These metabolic changes slow down plant growth and are sometimes lethal. Since flooded ramets can overcome the growth inhibition through connections to older ramets (Wang et al. 2009; Xiao et al. 2010), here we expect to reveal the effects of physiological integration on changes in carbohydrate utilisation and ROS detoxification.
With Cynodon dactylon, a stoloniferous perennial grass capable of inhabitation in mesic riparian environments where frequent flooding and drainage can occur (Stromberg et al. 2005; Ye et al. 2013), we specifically address the following hypotheses: (i) flooded ramets may receive support with resources and signal compounds from non-flooded ramets to alleviate carbohydrate starvation and ROS toxic effects; and (ii) upon resource export, the non-flooded ramets might grow more slowly than those not connected to flooded ramets.
Material and Methods
Experiment design
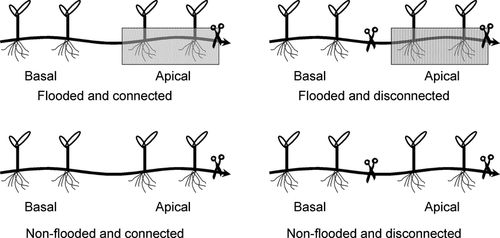
Stolons of C. dactylon (L.) Pers. were collected from the riparian vegetation of Yangtze River near Yichang, China (30°41′19.01″ N, 111°17′18.29″ E) and were reproduced for 2 months through clonal growth in river sand in a glasshouse of the China Three Gorges University. Plants were watered with half-strength Hoagland solution (Hoagland & Arnon 1950) once a week. After reproduction, 40 pinched stolons of equal size, each with four ramets, among which the two youngest were tagged as apical and the others as basal, were transplanted in plastic containers (32 × 13 × 20 cm, length ×width × height) filled with river sand. In addition, any terminal buds of the stolons, if present, were cut off to equalise the size of clonal fragments. After recovering growth for 1 month, plants were randomly assigned to four treatments of a factorial design, in which apical ramets are either not flooded or flooded and either disconnected or connected to basal ramets growing in well-drained conditions watered with tap water daily (Fig. 1). The flooding was carried out with tapwater, the surface of which remained 5 cm above the soil and was replaced as necessary. The treatments lasted for 1 month, with average air temperature of 24 °C and air humidity of 80%.
Growth measurements
At harvest, plants were divided into two equal groups, one for determinations of biomass (dried at 65 °C for 48 h before measurement) and non-structural carbohydrates (soluble sugars and starch). Biomass of above- (including stolons) and belowground apical/basal parts was measured. The other group was for analysis of ROS metabolism.
Enzyme analysis
Leaves and roots were sampled and frozen immediately in liquid nitrogen for enzyme analysis. Enzymes were extracted and activity measured according to a modification of Jiang & Zhang (2002). Samples (~0.2 g) were crushed into fine powder in liquid nitrogen with pre-cooled mortars and pestles. Soluble proteins were extracted in 5 ml of 50 mm potassium phosphate buffer (pH = 7.0) containing 1 mm EDTA and 1% polyvinylpyrrolidone. The solution was centrifuged at 15000 × g for 20 min at 4 °C, and the supernatant retained for enzyme determination.
Superoxide dismutase (SOD; EC 1.15.1.1) activity was measured by inhibition of photochemical reduction of nitro-blue tetrazolium (NBT) in the presence of riboflavin. An aliquot of 100 μl was added to a 3-ml reaction system at 25 °C, containing 50 mm potassium phosphate buffer (pH = 7.8), 13 mm methionine, 75 μm NBT, 2 μm riboflavin and 0.1 mm EDTA. The mixture was illuminated for 10 min with 200 μmol·m−2·s−1 light. One unit of SOD activity was defined as the amount of protein inhibiting 50% of NBT reduction, which was monitored as absorbance change at 560 nm. Correction was made with a non-illuminated mixture as blank and with enzyme replaced by distilled water as control.
Ascorbate peroxidase (APX; EC 1.11.1.11) was measured as ascorbate (absorption coefficient 2.8 mm−1 cm−1) consumption rate in H2O2. The 3-ml reaction mixture contained 50 mm potassium phosphate buffer (pH = 7.0), 0.1 mm EDTA, 0.03 mm ascorbate, 0.06 mm H2O2 and 100 μl enzyme extract. The reaction was started at 20 °C by adding H2O2, and changes in absorption at 290 nm were monitored every 30 s for 2 min. One unit of APX activity was defined as protein required to cause loss of 1 μmol ascorbate s−1. Distilled water was used as blank and replaced enzyme as control, and the linearity of the reaction rate was confirmed for 5 min from initiating reaction.
Catalase (CAT; EC 1.11.1.6) was measured as the decrease in substrate H2O2 (absorption coefficient 39.4 mm−1 cm−1). An aliquot of 200 μl enzyme extract was added to form a 3-ml system containing 50 mm potassium phosphate buffer (pH = 7.0) and 10 mm H2O2 at 20 °C. Absorbance change at 240 nm was read every 30 s for 2 min and one unit CAT activity was defined as a decrease of 1 μmol·H2O2·s−1. Correction and linearity confirmation were made as in APX determination.
Determination of H2O2 concentration
Roots and leaves (~0.1 g) were sampled, immersed in 25 mm H2SO4 dissolved in acetone and frozen in liquid nitrogen until analysis. The H2O2 was extracted with acidified acetone (w/v = 20 mg·ml−1) and determined according to Wolff (1994) and Cheeseman (2009). An aliquot of 50 μl H2O2 extract was mixed with 950 μl FOX1 solution and incubated at 25 °C for 45 min. Floccules were removed by centrifugation. The H2O2 concentration was calculated based on the difference in absorbance at 550 nm and 850 nm, with a standard curve ranging between 0–100 μm measured using the same protocol.
Determination of non-structural carbohydrates
Soluble sugar and starch were measured with a modified method based on sugar determination (Gravatt & Kirby 1998; Chow & Landhäusser 2004). A total of 50 mg dried tissue was ground to powder for each sample. Soluble sugars were extracted three times with 5 ml 80% ethanol in a 95 °C water bath for 10 min. Supernatants were combined and diluted to 25 ml with 80% ethanol for sugar analysis, after centrifugation at 500 g for 5 min. The remaining residue was dried at 50 °C for starch digestion. The residue was added to 2 ml 0.1 m NaOH and kept at 50 °C for 30 min in a shaker. After neutralised with 2.5 ml 0.1 m acetate, the mixture was digested at 55 °C for 48 h with amyloglucosidase in 100 mm acetate buffer, pH 4.5. The supernatant was diluted with 80% ethanol to 50 ml for starch determination after centrifugation at 500 g for 10 min.
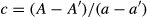 (1)
(1)Statistical analysis
Two-way anova was used to evaluate the effects among treatments on apical and on basal parts of C. dactylon. Duncan's multiple range test was run to evaluate differences within significant factorial interactions. Necessary square root or log transformations were applied in order to meet the assumptions of normality. All analyses were performed with SPSS 16.0 (SPSS, Chicago, IL, USA), and differences were considered significant at P < 0.05.
Results
Growth
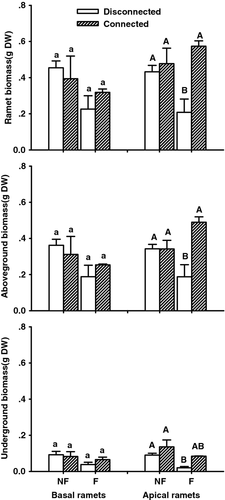
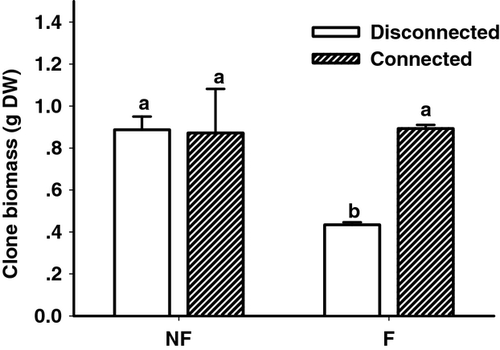
Both flooding and connection treatments significantly affected growth of C. dactylon in apical ramets. Flooding reduced root growth by 54% (P = 0.017), but did not affect aboveground or total biomass (Fig. 2, Table 1). Connections to basal ramets (allowing physiological integration) remarkably enhanced both root and shoot growth of apical ramets 100% (P = 0.026) and 57% (P = 0.011), respectively (Fig. 2). At the clone level, the flooded and connected group had clone biomass equal to the non-flooded group, and double that of the disconnected group (P = 0.011, Fig. 3). Generally, connection to basal ramets prevented growth inhibition in flooded apical ramets. Regarding basal ramets, we observed no significant changes in growth (Fig. 2, Table 2); neither flooding nor connection affected the above- or belowground biomass of basal ramets.
| traits | flooding (F) | connection (C) | FxC | |||
|---|---|---|---|---|---|---|
| F | P | F | P | F | P | |
| ramet biomass | 1.105 | 0.324 | 11.412 | 0.010↑ | 6.954 | 0.030 |
| aboveground biomass | 0.005 | 0.944 | 10.996 | 0.011↑ | 11.079 | 0.010 |
| underground biomass | 9.028 | 0.017↓ | 7.412 | 0.026↑ | 0.213 | 0.656 |
| root sugars | 14.060 | 0.004↑ | 7.968 | 0.018↓ | 8.029 | 0.018 |
| root starch | 19.531 | 0.001↑ | 1.813 | 0.203 | 5.714 | 0.034 |
| stem sugars | 5.319 | 0.044↑ | 6.239 | 0.032↓ | 1.065 | 0.326 |
| stem starch | 2.513 | 0.141 | 0.137 | 0.718 | 1.421 | 0.258 |
| leaf sugars | 3.202 | 0.104 | 0.649 | 0.439 | 1.237 | 0.292 |
| leaf starch | 0.471 | 0.508 | 0.166 | 0.692 | 0.762 | 0.403 |
| root SOD | 6.345 | 0.030↑ | 0.812 | 0.389 | 6.893 | 0.025 |
| root APX | 7.038 | 0.022↑ | 0.065 | 0.804 | 0.180 | 0.680 |
| root CAT | 3.638 | 0.079 | 0.167 | 0.690 | 3.818 | 0.073 |
| root H2O2 | 2.372 | 0.149 | 0.068 | 0.798 | 0.044 | 0.838 |
| leaf SOD | 0.010 | 0.924 | 0.432 | 0.530 | 2.635 | 0.143 |
| leaf APX | 1.409 | 0.255 | 0.040 | 0.845 | 1.438 | 0.250 |
| leaf CAT | 1.276 | 0.276 | 0.158 | 0.696 | 0.889 | 0.361 |
| leaf H2O2 | 0.000 | 0.991 | 0.446 | 0.514 | 0.715 | 0.411 |
- The effects of flooding, connection and flooding × connection (F × C) on biomass, concentration of soluble sugars, starch and H2O2, and activities of antioxidant enzymes from different parts/organs of apical ramets of C. dactylon. Significant differences (P <0.05) are indicated in bold, and arrows indicate significant increase (↑) or decrease (↓) of the variables with flooding treatments or allowing stolon connections.
| traits | flooding (F) | connection (C) | FxC | |||
|---|---|---|---|---|---|---|
| F | P | F | P | F | P | |
| ramet biomass | 4.032 | 0.080 | 0.045 | 0.837 | 1.017 | 0.343 |
| aboveground biomass | 3.558 | 0.096 | 0.014 | 0.908 | 0.888 | 0.373 |
| belowground biomass | 3.698 | 0.091 | 0.223 | 0.649 | 0.964 | 0.355 |
| root sugars | 0.654 | 0.438 | 0.305 | 0.593 | 0.123 | 0.734 |
| root starch | 14.708 | 0.003↑ | 2.233 | 0.163 | 0.029 | 0.869 |
| stem sugars | 10.385 | 0.007↑ | 0.073 | 0.791 | 3.362 | 0.092 |
| stem starch | 2.910 | 0.116 | 0.105 | 0.752 | 0.102 | 0.755 |
| leaf sugars | 0.298 | 0.600 | 0.039 | 0.849 | 0.489 | 0.504 |
| leaf starch | 0.488 | 0.502 | 1.197 | 0.302 | 0.086 | 0.776 |
| root SOD | 0.356 | 0.560 | 2.619 | 0.128 | 0.453 | 0.512 |
| root APX | 0.647 | 0.438 | 0.831 | 0.382 | 2.710 | 0.128 |
| root CAT | 4.226 | 0.059 | 0.180 | 0.677 | 2.282 | 0.153 |
| root H2O2 | 1.984 | 0.181 | 0.478 | 0.501 | 0.080 | 0.782 |
| leaf SOD | 0.147 | 0.707 | 2.887 | 0.113 | 0.452 | 0.513 |
| leaf APX | 0.129 | 0.725 | 2.901 | 0.112 | 0.005 | 0.947 |
| leaf CAT | 0.858 | 0.371 | 0.154 | 0.701 | 0.000 | 0.990 |
| leaf H2O2 | 4.254 | 0.064 | 0.275 | 0.611 | 1.222 | 0.293 |
- See the footnote of Table 1 for meanings of symbols.
Carbohydrate concentrations
Flooding and connection affected carbohydrate concentrations of C. dactylon in different ways, and most of the effects were observed in apical ramets (Fig. 4, Table 1). Two-way anova showed that flooding generally increased carbohydrate concentrations in apical ramets of C. dactylon. In roots, flooding increased both soluble sugar and starch concentrations by 220% (P = 0.004) and 183% (P = 0.001), respectively. Flooding also stimulated accumulation of soluble sugars in stems of apical ramets. However, flooding did not affect starch concentration in stems, nor either carbohydrate in leaves (Fig. 4, Table 1).
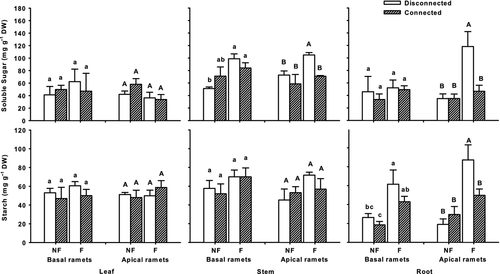
In contrast to flooding, connection between ramets tended to reduce concentrations of carbohydrates in apical C. dactylon ramets (Fig. 4, Table 1). Stolon connection reduced soluble sugars both in roots (P = 0.018) and in stems (P = 0.032), although starch was not significantly affected (P = 0.203 in roots, P = 0.0.718 in stems). Moreover, there were no remarkable changes in carbohydrate concentrations of leaves in apical ramets (Fig. 4, Table 1).
In the combined flooding and connection treatment, only apical ramets of C. dactylon accumulated considerable amounts of soluble sugars (P = 0.018) and starch (P = 0.034) in roots in the flooded and disconnected treatment, while carbohydrates remained at relatively low levels in other treatments (Fig. 4, Table 1). However, we did not observe similar result in stems and leaves of apical C. dactylon (Fig. 4, Table 1). It appeared that physical connections with basal ramets prevented carbohydrate accumulation as a stress response in apical ramets.
For basal ramets, flooding and connection had limited effects on carbohydrate status. Basal ramets contained higher concentrations of soluble sugar in stems and starch in roots in flooded treatments, and connection of stolons to apical ramets had no effect on basal ramets (Fig. 4, Table 2).
Anti-oxidant metabolism
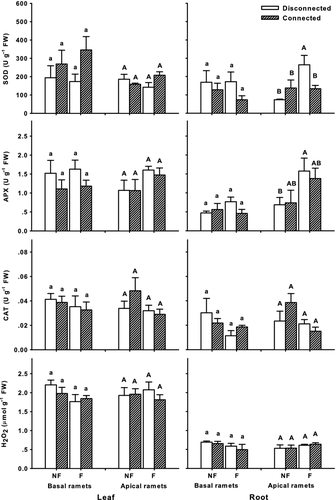
Only on SOD and APX activities among responses of antioxidant enzymes and H2O2 changed significantly, and were confined to roots of apical C. dactylon (Fig. 5, Table 1). Flooding nearly doubled SOD activity (P = 0.030), and APX activity was 2.5-fold (P = 0.013) higher in comparison to non-flooded treatments, while concentrations of H2O2 and CAT did not show remarkable differences between flooding and non-flooding treatments (Fig. 5, Table 1). Physiological integration alone did not affect the activities of the three investigated enzymes, nor did the H2O2 concentration. In regard to combined factors, SOD activity in the disconnected and flooded treatment was 92–258% higher than that in other treatments (P = 0.025; Fig. 5). Moreover, neither leaves of apical ramets nor basal ramets responded to flooding and/or connection in terms of H2O2 concentration and enzyme activities (Fig. 5, Tables 1 and 2).
Discussion
Physiological integration counteracted C. dactylon's responses to flooding
As predicted, physiological integration reduced most of the stress responses in flooded C. dactylon; namely, growth inhibition (Figs 2 and 3), carbohydrate accumulation (Fig. 4) and induction of SOD activity (Fig. 5).
Coupling of growth inhibition and carbohydrate accumulation is common in flooding events. Growth of flooded ramets slows (Figs 2 and 3), with metabolism decreasing because of O2 limitation and/or active down-regulation (Blokhina et al. 2003; van Dongen et al. 2009). This hampered growth probably induces carbohydrate accumulation in roots of flooded ramets (Fig. 4) due to reduced consumption rather than enhanced supply from shoots (Gharbi et al. 2009). In addition, flooding may impair loading and unloading processes in the phloem (Ferner et al. 2012), as evidenced in the higher concentration of soluble sugars in stems than in leaves when flooded (Fig. 4).
Physiological integration relieved C. dactylon growth inhibition and carbohydrate accumulation (Figs 2-4). The potential mechanism of these changes might involve transferring compounds that neutralise the effects of flooding on plant metabolism. Oxidising power and electron acceptors (e.g. NAD(P)+; van Dongen et al. 2003) are expected to be among the translocated chemicals. These can facilitate metabolic functioning in flooded ramets by re-oxidising the electron transport chain when it is over-reduced (Sairam et al. 2008). In this case, flooded ramets may be able to utilise carbohydrates in a more efficient way than in anaerobic respiration, allowing recovering root/shoot growth (Fig. 2) and decreasing carbohydrate accumulation (Fig. 4) during flooding.
Activities of SOD and APX were up-regulated in flooded treatments, suggesting oxidative stress (Blokhina et al. 2003; Møller et al. 2007), although H2O2 concentrations remained at the control level in roots of apical ramets (Fig. 5). The antioxidant activities in flooded ramets of C. dactylon with connections are also consistent with the suggestion of transferred electron acceptors. The over-reduced environment in flooded tissues would become oxidised to some extent, with less ROS produced if compounds such as NAD(P)+ are transferred to flooded ramets under physiological integration. Under these circumstances, absence of responses to flooding in antioxidant enzyme activities seems appropriate in apical ramets of the flooded and connected treatment (Fig. 5).
In general, physiological integration between flooded and non-flooded ramets counteracts the flooding responses of C. dactylon in terms of carbohydrate accumulation and induced antioxidant enzymes activity. We hypothesise that translocation of electron acceptors through physiological integration may be involved in the underlying mechanisms, and that carbohydrate supply is less important in reciprocal resource translocation among the interconnected C. dactylon during flooding.
Physiological integration did not cost the supporting basal ramets
In the basal ramets of C. dactylon, we observed no influence on biomass, carbohydrate concentrations and ROS metabolism induced by physical connections (Figs 2-5), suggesting no cost to basal ramets. This is consistent with some clonal plant species subjected to other environmental stresses (van Kleunen & Stuefer 1999; Yu et al. 2002; Janeček et al. 2008) and with our deduction that carbohydrates are not a major component in physiological integration. In addition, no costs were observed within our study possibly because of: (i) prevention of apical dominance by pinching out the apex before the treatments; and (ii) a compensatory effect of photosynthetic capacity of unstressed basal ramets (Roiloa & Retuerto 2006). However, physiological integration does result in costs in some other studies, and is attributed to excess export of carbohydrates and/or presence of apical dominance (Evans 1991; Charpentier et al. 1998; Wang et al. 2009).
Acknowledgements
ZL and DF contributed equally to this paper. ZL carried out the experiment, ZL and DF performed the statistical analyses and drafted the manuscript, FC assisted in the experiment, and FC, QY, WSC and ZX commented on the manuscript. All authors read and approved the manuscript. This work was financially supported by the Chinese Academy of Sciences (KZCX2-XB2-07), the Australian Research Council (DP1093827) and the National Natural Science Foundation of China (31070356).