Leaf morphology of 40 evergreen and deciduous broadleaved subtropical tree species and relationships to functional ecophysiological traits
Abstract
We explored potential of morphological and anatomical leaf traits for predicting ecophysiological key functions in subtropical trees. We asked whether the ecophysiological parameters stomatal conductance and xylem cavitation vulnerability could be predicted from microscopy leaf traits. We investigated 21 deciduous and 19 evergreen subtropical tree species, using individuals of the same age and from the same environment in the Biodiversity-Ecosystem Functioning experiment at Jiangxi (BEF-China). Information-theoretic linear model selection was used to identify the best combination of morphological and anatomical predictors for ecophysiological functions. Leaf anatomy and morphology strongly depended on leaf habit. Evergreen species tended to have thicker leaves, thicker spongy and palisade mesophyll, more palisade mesophyll layers and a thicker subepidermis. Over 50% of all evergreen species had leaves with multi-layered palisade parenchyma, while only one deciduous species (Koelreuteria bipinnata) had this. Interactions with leaf habit were also included in best multi-predictor models for stomatal conductance (gs) and xylem cavitation vulnerability. In addition, maximum gs was positively related to log ratio of palisade to spongy mesophyll thickness. Vapour pressure deficit (vpd) for maximum gs increased with the log ratio of palisade to spongy mesophyll thickness in species having leaves with papillae. In contrast, maximum specific hydraulic conductivity and xylem pressure at which 50% loss of maximum specific xylem hydraulic conductivity occurred (Ψ50) were best predicted by leaf habit and density of spongy parenchyma. Evergreen species had lower Ψ50 values and lower maximum xylem hydraulic conductivities. As hydraulic leaf and wood characteristics were reflected in structural leaf traits, there is high potential for identifying further linkages between morphological and anatomical leaf traits and ecophysiological responses.
Introduction
Plant functional traits have been successfully used to link biological diversity to ecosystem functioning (EF) of communities (Garnier et al. 2004; Díaz et al. 2007). However, the assessment of physiologically relevant traits often requires high expenditure of time and lab resources. For example, determination of xylem cavitation vulnerability as a measure of drought stress requires several hours per sample (Sperry et al. 1988; Kröber et al. 2014). Hence, there are only a few multi-species studies that connect leaf traits to more than a single ecophysiological response. However, establishing relationships between leaf traits and ecophysiological mechanisms across large species sets would certainly improve our understanding of ecosystem functioning (Sack & Holbrook 2006). For many leaf traits, the functional relevance is well known. For example, specific leaf area (SLA) was found to be closely linked to mass-based photosynthesis and respiration rates (Wright et al. 2004). In addition, leaves with low SLA are physically more robust, less prone to herbivory and tend to have a longer life span (Sterck et al. 2006). It should be considered that functional relationships of ecophysiological responses to leaf traits might not be universal, but are only valid in a specific context. For example, low SLA was found to be only associated with higher shade tolerance in evergreen species, but only weakly in deciduous trees (Lusk & Warton 2007). For many other leaf traits, links to ecophysiological functions still have to be established. In particular, our knowledge is limited on how morphological and anatomical leaf traits are related to physiologically relevant wood traits (Zanne et al. 2010; Böhnke et al. 2011).
Many structural leaf traits vary with the evergreen or deciduous leaf habit. In particular, evergreen leaves are often sclerophyllous and associated with a lower SLA, lower leaf nitrogen content and lower mass-based photosynthetic capacity (Reich et al. 1997; Shipley et al. 2006; Curtis & Ackerly 2008), which results in increased durability and leaf life span (Medina 1984). In contrast, deciduous leaves are often mesophyllous or hydrophyllous. Because of higher elasticity of leaf cells, deciduous species with mesophyllous leaves display more variation in cell volume but only tolerate small variation in leaf water potential (Ψ) as compared to evergreen species with sclerophyllous leaves (Sobrado 1986). Since Orians & Solbrig (1977) published their seminal paper on the trade-off between photosynthetic capacity and leaf longevity, many studies at the global (Kikuzawa 1991; Reich et al. 1997; Cornelissen et al. 2005) or regional scale (Mulkey et al. 1995; Diemer 1998; Pringle et al. 2011) have supported this view. Meanwhile, the concept of the leaf economics spectrum (LES; Wright et al. 2004) that reflects this trade-off has become widely accepted in trait-based ecology. The LES describes a universal spectrum of leaf economics consisting of key chemical, structural and physiological properties. The spectrum runs from rapid to slow return on investment in nutrients and dry mass of leaves, and operates largely independently of growth form, plant functional type or biome (Wright et al. 2004). However, much less attention has been paid to other ecophysiological functions that are not reflected in the LES or morphological traits other than leaf longevity or leaf toughness. One example of such a key ecophysiological function is xylem vulnerability. Comparing four species, each of deciduous and evergreen leaf habit from Costa Rican dry tropical forests, Brodribb et al. (2003) did not find a close link between xylem vulnerability and leaf traits, and in particular, no link to leaf habit. In contrast, Kröber et al. (2014) encountered significantly lower values of maximum specific hydraulic conductivity and lower Ψ50 values (xylem pressure at which 50% loss of maximum specific hydraulic conductivity occurs) in evergreen than deciduous species. Accordingly, Ψ50 decreased with leaf nitrogen content and log leaf area and increased with leaf carbon-to-nitrogen ratio. However, structural leaf traits such as thickness of the epidermis or wax layers have not yet been tested for relationships to xylem cavitation resistance.
In another study, Kröber & Bruelheide (2014) established links between parameters of stomatal control and traits of the LES. There was a positive relationship of stomatal conductance (gs) to leaf nitrogen content and a negative one to the leaf carbon-to-nitrogen ratio. In contrast, stomatal control parameters, which were derived from the conductance–vapour pressure deficit (vpd) curve, were not related to the LES but to stomatal traits. The stomatal traits, in turn, were unrelated to the LES. The vpd at the point of inflection of the conductance–vpd curve was the higher, the lower the stomata density and the higher the leaf carbon content. Furthermore, maximum conductance was positively associated with leaf carbon content and vein length. However, Kröber & Bruelheide (2014) only considered leaf traits related to stomata and veins and did not explore relationships to other anatomical and morphological traits.
The expectation of connections between leaf morphology and leaf functionality is based on the fundamental idea that leaf structure reflects functional coordination (Sisó et al. 2001; Sack et al. 2003). For example, a less densely packed leaf has more extensive intercellular spaces, which should facilitate CO2 and H2O diffusion, and thus be connected to stomatal or xylem conductance. However, Aasamaa et al. (2001) did not find the volume of intercellular space to be related to specific hydraulic conductivity. The leaf anatomy, in particular the regular arrangement of palisade mesophyll cells, affects light perception of deeper cell layers, and thus has consequences for photosynthetic efficiency (Smith & Hughes 2009). The regular arrangement of palisade mesophyll cells is thought to efficiently absorb incident radiation of high intensity, whereas spongy mesophyll results in higher scattering of light, which increases absorption at low light intensities (Beck 2010). The fact that thicker mesophyll requires increased gas exchange per leaf unit area explains why Sack & Frole (2006) found palisade mesophyll thickness to be negatively correlated with xylem hydraulic conductance in a set of ten tropical rain forest tree species. Furthermore, leaf hydraulic conductance was positively related to the ratio of palisade to spongy mesophyll thickness. This ratio of thickness of the two types of photosynthetically active tissues has been highlighted as being tightly correlated to leaf venation more than 50 yrs ago (Wylie 1946). The underlying mechanistic explanation is that with increasing ratio of palisade to spongy mesophyll thickness, the density of chloroplasts per unit leaf volume increases. This translates into higher photosynthesis rates per unit leaf area. This is only true up to a certain threshold of the palisade to spongy mesophyll thickness ratio, since some spongy mesophyll is required for intercellular gas diffusion. Popma et al. (1992) reported that lowland rain forest species in Mexico having leaves of high photosynthetic capacity had thicker palisade parenchyma and higher palisade to spongy parenchyma ratios. For five dipterocarp rain forest tree species, Kenzo et al. (2004) found leaf thickness and palisade layer thickness to be strongly correlated to photosynthetic capacity. Leaf hydraulic conductance was also linked positively to leaf thickness in temperate woody species (Sack et al. 2003). Finally, light absorption not only depends on the thickness and structure of the green mesophyll but also on epidermal traits. For example, Vogelmann et al. (1996a) reported that leaf epidermal cells can enhance photosynthesis through focusing light onto the underlying mesophyll.
The morphological and anatomical leaf structure is also expected to affect transpiration and drought resistance. In general, thin-leaved plants with high SLA are considered more vulnerable to drought stress than those with thick and robust leaves (Mediavilla et al. 2001; Li et al. 2009). As low SLA leaves transpire less water, they would be expected to have higher water use efficiency (WUE). However, in a study with six poplar species, Cao et al. (2012) found intrinsic WUE not to be related to SLA but only to increase with abaxial stomatal density and decrease with vessel lumen area. In contrast to SLA and mesophyll traits, the structure of the epidermis and the cuticle has received surprisingly little attention in comparative studies. As both a thick outer epidermal wall and a cuticle reinforced with wax layers should decrease transpiration during stomata closure, they would be expected to occur predominantly in leaves with high WUE. Accordingly, water permeability of epicuticular waxes has been reported to be positively correlated with plant sensitivity to vpd (Kerstiens 1997). However, linking water permeation across the cuticle directly to its structure or composition has turned out to be difficult (Riederer & Schreiber 2001) because the cuticle and epicuticular waxes also have additional functions, such as protecting the leaf from radiation, insect herbivory and microbial infection (Kerstiens 2006; Pruem et al. 2012).
Similarly, trichomes are thought to play an important role in leaf hydraulic properties. Similar to the cuticle, trichomes also have multiple functions: temperature insulation, reflection of radiation, increase in boundary layer thickness, and thus increase in the gas diffusion pathway, reduced ion leaching and trapping of moisture on the leaf surface (Gutschick 1999; Press 1999; Gates 2003).
In summary, relationships between ecophysiology and leaf morphology and anatomy are only partly established, although the need to compare these relationships across different life forms and habitats has been pointed out (Sack & Holbrook 2006). We set out to analyse these structure–function relationships using four ecophysiological key traits with high relevance for species performance and survival as functional responses. In addition, we investigated interrelationships among leaf microscopy traits, with particular emphasis on the species' evergreen or deciduous leaf habit. Thus, we asked which microscopy leaf traits were connected to: (i) stomatal regulation, expressed as the absolute maximum fitted stomatal conductance and to vpd at the maximum modelled stomatal conductance–vpd relationship, and (ii) maximum xylem specific hydraulic conductivity and xylem hydraulics, expressed as xylem pressure at which 50% loss of maximum specific hydraulic conductivity occurs (Ψ50). To our knowledge, our study is the first that explores such functional–morphological relationships systematically across a large set of plant species.
Material and Methods
Plant material
We studied the leaf anatomy of 40 indigenous tree species (Table S4), which are all typical of the subtropical monsoon forest in southeast China, Jiangxi Province (http://www.bef-china.de). Warm and humid summers and dry winters with occasional frost events characterise the local subtropical climate. Mean annual precipitation at the study site is 1821 mm and mean annual temperature is 16.7 °C, measured from 1971 to 2000 (Yang et al. 2013). The sampled plants were all planted in the Biodiversity-Ecosystem Functioning experiment (BEF-China) as 1-year-old or 2-year-old saplings in 2008 and 2009. Total tree number in the experiment is 219,000, arranged in 566 plots with 400 tree individuals per plot on an area of 38 ha (Bruelheide et al. 2014). The studied 40 tree species represent 19 families (Table S4). According to their representation in the natural forests in a nearby nature reserve (Bruelheide et al. 2011), some families were planted with numerous species, e.g. Fagaceae (12 species), Lauraceae (five), Sapindaceae (three) and Elaeocarpaceae (three). In total, 19 and 21 species were evergreen and deciduous, respectively (Table S4).
Leaf sampling
Five leaves were sampled each from five randomly chosen individuals per species (in total 25 leaves) for leaf trait analyses (SLA and microscopy leaf traits), taking the five individuals from different plots. Only fully exposed healthy sun leaves with no visible damage from e.g. herbivory were sampled, with a focus on sampling at comparable plant heights. SLA was measured following Cornelissen et al. (2003). The microscopy traits were measured in accordance to Gerlach (1984), with at least three replicates per nominal trait and species and at least 30 replicates per numerical trait and species. A full list of the morphological traits studied is provided in Table S8.
The four functional leaf traits related to stomatal conductance and hydraulic xylem properties (CONMAXFITA, absolute maximum of fitted stomatal conductance; VPDMAXFIT, vpd at maximum of modelled gs–vpd relationship; HYDCOND, maximum specific xylem hydraulic conductivity; PSI50, xylem pressure Ψ50, at which 50% loss of maximum specific hydraulic conductivity occurs) were assessed in two preceding studies (Kröber & Bruelheide 2014; Kröber et al. 2014). Stomatal conductance was measured with a SC1 porometer (Decagon, Pullman, WA, USA), based on steady-state technology. Daily courses were measured on at least three individuals per species, always on the same leaf, in the high-diversity plots of the experiment. Then gs–vpd relationships were aggregated by species. To derive parameters of stomatal control, we modelled these species-specific gs–vpd relationships by regressing the logits of gs/gsmax to vpd and the quadratic term of vpd using a generalised linear model with binomial error distribution. From these relationships, we extracted the absolute maximum fitted conductance as the maximum value of the fitted model and the vpd, thus the corresponding x-value for the maximum of the modelled gs–vpd relationship for every species. By making use of logits we ensured that modelled maximum values (CONMAXFIT) did not exceed gsmax, and that gs approached zero at high vpd. For further details see Kröber & Bruelheide (2014).
A second study focused on xylem vulnerability. Using three samples per species, maximum specific xylem hydraulic conductivity and Ψ50 were investigated. Freshly sampled twigs were placed in a cavitation chamber (PMS 1505D-EXP; PMS Instrument Co., Albany, OR, USA) connected to a Scholander pressure chamber (PMS M1000), and air was flushed out from previous embolism events following established protocols (Sperry et al. 1988; Perez-Harguindeguy et al. 2013). To ensure that all air was evacuated from the xylem, the twig segments were flushed for 1 h with 10 mm citric acid perfusion solution, using filtered and demineralised water at 0.15 MPa. Maximum specific xylem hydraulic conductivity was measured as the mass of water through-flow per time, related to the length of the twig and to the cross-sectional area of the twig. Then, with increasing air pressure, we simulated decreasingly negative water potentials and determined the xylem cavitation sensitivity by applying a sigmoid, three-parameter regression to the vulnerability data, where KS is the specific hydraulic conductivity of the xylem (kg·m−1·s−1·MPa−1; thus it is related to cross-sectional area). Ψ is the xylem pressure at which water flow was measured (MPa); Ψ50 is defined as the point of xylem pressure at which 50% loss of the original maximum specific hydraulic conductivity occurs. For further information see Kröber et al. (2014).
Anatomical preparations
We applied traditional botanical microtechniques as described in Gerlach (1984). Formalin–acetic acid–70% alcohol (FAA; 5 ml 40% formaldehyde, 5 ml glacial acetic acid, 90 ml 70% ethanol) was used as fixing agent to prepare microscope slides. Transverse sections of leaves, enclosed in natural cork, were made with a Reichert slide microtome at a thickness of 20–30 μm. Safranin was used to red-stain lignified tissue and Astra blue or alcian blue to blue-stain non-lignified cell walls. First, the sections were placed in Astra blue or alcian blue for 5 min, washed in distilled water, placed in Safranin (1% Safranin in 50% alcohol) for 2–5 min and transferred to 50% alcohol. After dehydration through an alcohol series (50%, 70%, 92% and 100% alcohol), the sections were placed in Histo-Clear® (distilled essential oils, food grade) or xylene and mounted in Euparal or Canada balsam. The embedded sections were dried in the laboratory oven at 60 °C overnight.
Light microscopy features
Slides were examined under a light microscope (Zeiss Axioskop 2; Zeiss, Jena, Germany) and digital photographs taken (Zeiss Axio Cam MRc). Measurements of leaf anatomy characteristics were made using the Axio Vision software (Zeiss, Rel. 4.8). Leaf transverse sections were prepared to measure thickness of the upper and lower epidermis, palisade and spongy parenchyma. In each leaf transverse section, ten measurements were made and averaged. In addition, we calculated the log10 ratio between thickness of palisade to spongy parenchyma (Table S8). The epidermis as a boundary tissue is build from one layer of cells; however, in several species, we found multilayer epidermal structures and defined the layer beneath the final layer as subepidermis. Density of the spongy parenchyma was assessed optically in three ordinal categories in relation to the frequency of intercellular spaces, and classified as: 0 = extensive intercellular space; 0.5 = some intercellular spaces; 1.0 = closely packed cells.
Scanning electron microscopy (SEM)
For sample preparation and investigation of the adaxial and abaxial leaf surfaces, we used the scanning electron microscope at the Jodrell Laboratory of the Royal Botanic Gardens, Kew (UK). Minute parts of leaves were fixed in 70% ethanol in small-sized caskets and dehydrated in an ethanol series (70%, 90% and 100%). To remove liquid in a controlled way, the dehydrated samples were subjected to supercritical drying in a Tousimus critical point dryer. This procedure avoids damage to delicate structures, such as breakage of cell walls through surface tension as the boundary of liquid–gas transition moves by. Supercritical drying employs high temperature and high pressure to avoid crossing phase boundaries. The fluid used was CO2, which has equal density to the liquid and vapour phase at the critical point. In the Tousimus critical point dryer liquid CO2 is heated to the critical point (31.04 °C), at which time pressure can be gradually released, allowing the gas to escape and leaving dried samples. Subsequently, the minute leaf samples were removed from the caskets and mounted on stubs using double-sided Sellotape. The stubs with the samples were put in the Quorum sputter coater Q150 T (Quorum Technologies, Lewes, UK) and coated with platinum under an argon gas atmosphere for 60 s. Subsequently, the platinum-coated samples on the stubs were subjected to Field Emissions Scanning Electron microscopy (Hitachi S-4700 II; Hitachi, Krefeld, Germany). The digital SEM images of the 40 species were used for description of leaf cuticle characters. The cuticle is basically a two-layered sheet of lipids, wax and cutin and hydroxyl fatty acids. In addition, the cuticle contains small amounts of other substances, such as phenolic compounds. We classified epicuticular waxes and cuticle characteristics following Ditsch & Barthlott (1997). Cuticle characteristics were recorded on both sides of the leaf, recording type of ornamentation of the outer surface of the cuticle (cuticular folding) and presence or absence of papillae, using the classification of Wilkinson (1979; see Table S8). Trichomes were classified as simple, stellate or bifurcate hairs.
Statistics
All numerical leaf traits were tested for differences between deciduous and evergreen leaf habit using anova. To reveal trait interrelationships, the 17 binary, numeric or ordinal leaf traits listed in Table S8 were subjected to a principal coordinate analysis (PCoA). We calculated Gower's distance to simultaneously handle traits of different scales using the ade4 package of R (Dray & Dufour 2007; Pavoine et al. 2009). The correlations between traits and PCoA axes were obtained by post-hoc correlation using the envfit function in the vegan package (Oksanen et al. 2013). Particularly relevant relationships were confirmed with bivariate linear regressions and Pearson correlations. Additional analyses were carried out for relationships to the four selected key ecophysiological traits: CONMAXFITA, VPDMAXFIT, HYDCOND and PSI50. Differences in these four ecophysiological traits between the four categorical cuticle characteristics (cuticular folding and presence or absence of papillae, both on upper and lower leaf surface) were tested using separate one-factorial anova for each ecophysiological variable. To test for relationships between the four key ecophysiological traits to morphological traits we used linear regression model selection based on information theory. In a first step, the best linear combination of main effects for the 14 non-nominal traits in Table S8 were identified using step-wise forward selection, according to the Akaike Information Criterion (AIC) in different models, each predicting one of the four ecophysiological traits. These analyses revealed that a maximum of three predictor variables was included in the best models. In a second step, we calculated all models with all possible combinations of these 13 traits, allowing models with up to three predictor terms. As we were particularly interested in the role of leaf habit, we also included all possible twofold interactions with leaf habit as possible model terms. The best model was identified using the dredge routine of the MuMIn package (Barton 2014). All statistical analyses and plots were conducted with the R software version 3.1.0 (R Core Team 2014).
Results
Morphology and anatomy of leaves
Leaf thickness ranged from 105 μm (Sapindus saponaria; Fig. S1E) to 367 μm (Manglietia yuyuanensis; Fig. S1D), with a mean of 180 μm (Table 1). While the deciduous species had the lower range in leaf thickness (105–218 μm), the evergreen trees ranged from 137 μm to 367 μm. All leaves studied were dorsiventral. Differences between evergreen and deciduous species were especially evident in the number of layers of palisade parenchyma. More than half of evergreen species had leaves with multi-layered palisade parenchyma (e.g. Daphniphyllum oldhamii; Fig. S1A; Castanopsis sclerophylla; Fig. S1C), while only one deciduous species (Koelreuteria bipinnata) had two- to three-layered palisade parenchyma. For further details of the mean values of the numerical leaf traits and their distribution, see Table 1. The complete raw data trait set for all study species can be found in Table S5. Most deciduous species (76%) had leaves with mono-layered palisade parenchyma, which was often very extensive, especially in Rhus chinensis (Fig. S1F). Among the evergreen species, only Phoebe bournei and Schima superba had mono-layered palisade parenchyma. Phoebe bournei (Lauraceae) was also the species with the lowest leaf thickness among all evergreen species. The deciduous species Meliosma flexuosa and Celtis biondii displayed the smallest log ratio of thickness of palisade to spongy parenchyma, with −0.2 and −0.21, respectively. However, the species with highest ratios, Ailanthus altissima (0.27) and Rhus chinensis (0.41), were also deciduous.
| trait [unit] | mean | max | min | SD | mean deciduous | mean evergreen | P |
|---|---|---|---|---|---|---|---|
| CONMAXFITA [mmol·m−2·s−1] | 904.30 | 1915.99 | 336.07 | 315.35 | 953.81 | 846.53 | 0.30 |
| VPDMAXFIT [hPa] | 22.97 | 73.81 | 6.17 | 10.35 | 22.34 | 23.70 | 0.68 |
| HYDCOND [kg·m−1·s−2·MPa−1] | 2.44 | 17.52 | 0.04 | 3.36 | 3.55 | 1.13 | 0.023 |
| PSI50 [MPa] | −3.78 | −1.09 | −6.60 | 1.51 | −3.05 | −4.64 | <0.001 |
| UPPEREPI [μm] | 13.41 | 24.83 | 5.50 | 4.40 | 13.83 | 12.94 | 0.53 |
| PALIS [μm] | 73.51 | 129.42 | 32.43 | 21.06 | 65.27 | 82.62 | 0.008 |
| SPONGY [μm] | 74.91 | 175.25 | 31.58 | 28.88 | 61.08 | 90.19 | <0.001 |
| LOG10RATIO [μm·μm−1] | 0.03 | 0.42 | −0.22 | 0.13 | 0.056 | 0.011 | 0.27 |
| LEAFTHICK [μm] | 180.97 | 366.57 | 105.23 | 51.50 | 155.77 | 208.82 | <0.001 |
| SLA [m2·kg−1] | 12.28 | 19.64 | 7.84 | 2.80 | 13.56 | 10.86 | 0.001 |
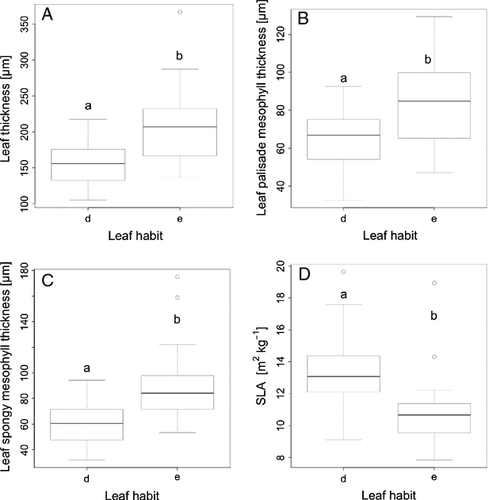
Overall, leaf habit was a good predictor of leaf anatomical characteristics; we found the following traits were significantly higher in evergreen than in deciduous leaves: leaf thickness (F = 14.15, P = 0.0006; Fig. 1A), palisade mesophyll thickness (F = 7.94, P = 0.0075; Fig. 1B) and spongy mesophyll thickness (F = 13.33, P = 0.0008; Fig. 1C). Furthermore, SLA was significantly higher in deciduous than in evergreen species (F = 11.82, P = 0.0014; Fig. 1D). The two leaf habits did not differ in the log ratio of thickness of palisade and spongy mesophyll and thickness of the upper epidermis (Table 1).
Ornamentation of the outer surface of the cuticle largely consisted of striae or ridges, which are folds of the cuticle. As an example for cuticular folding with striae of random orientation on the adaxial leaf side, see Ailanthus altissima (Fig. S2E). This cuticular folding represented an important character in deciduous leaves in the set of analysed species, but was much less pronounced in evergreen leaves. Epicuticular waxes were mostly present as smooth or fine-warty films, especially on the lower (abaxial) side of the leaf in most species studied (e.g. Fig. S2A). There were only three evergreen species with epicuticular wax plates on the abaxial side of the leaf (Manglietia yuyunanensis, Phoebe bournei and Quercus phillyraeoides; Fig. S2B), while deciduous species showed a large variation in epicuticular waxes on the abaxial leaf side (e.g. Acer davidii and Quercus serrata; Fig. S2A, F). On the upper (adaxial) leaf side, evergreen species showed a wider variety of epicuticular waxes than deciduous species, in the form of smooth or fine-warty films (Cinnamomum camphora; Figs S1B, S2C, D), heaps of grains or filaments, plates (Lithocarpus glaber, Quercus phillyraeoides; Fig. S2B) or scales (Cyclobalanopsis glauca).
Papillae as projections of the epidermis cell wall were encountered on the abaxial leaf side of three deciduous species in the form of striate papillae (Idesia polycarpa; Fig. S3A; Rhus chinensis; Fig. S3D; Triadica sebiferum) and on the abaxial side of three evergreen species in the form of simple papillae (Cyclobalanopsis myrsinifolia, Daphniphyllum oldhamii and Lithocarpus glaber; Fig. S3B, F). The deciduous species Rhus chinensis showed both striate papillae and simple trichomes on the abaxial side, and additionally, trichomes on the adaxial side (Fig. S3C, D). anovas on the effect of the presence of papillae on the four ecophysiological parameters did not reveal any significant relationship.
Trichomes occurred as simple, stellate or bifurcate hairs (Fig. S3E, F). In eight deciduous species, trichomes were present both on the adaxial and abaxial side, whereas trichomes did not occur in leaves of any of the evergreen species, In the studied evergreen species, adaxial trichomes were mainly a feature of Fagaceae and Lauraceae. Stellate hairs were mainly found in the Fagaceae but also in Alniphyllum fortunei (Styracaceae; Fig. S3E).
Interrelationships between morphological and anatomical leaf traits
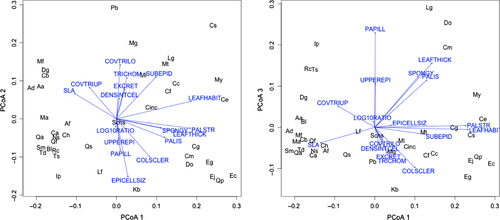
The results of the principal coordinate analysis (PCoA) of the 14 leaf traits characteristic of the leaf anatomy are shown in Fig. 2A and 2B for axis 1 versus 2 and 1 versus 3, respectively. For more detailed information see Table S6 with all correlations of the 14 traits with the three PCoA axes, and Table S7 for coordinates of all species. The strongest significant positive correlations with the first PCoA axis were observed for number of palisade layers, leaf habit, thickness of the palisade mesophyll layer, leaf thickness and thickness of the spongy mesophyll layer, in order of decreasing importance. SLA and cover of trichomes on the adaxial side of the leaf (COVTRIUP) were significantly negatively correlated with the first axis. The second PCoA axis was, to a large extent, characterised by a significant positive relationship to cover of trichomes on the abaxial side of the leaf (COVTRILO), type of trichomes (TRICHOM) and the presence of excretory glands (EXCRET), as well as by a significant negative relationship to epidermal cell size (EPICLLSIZ) and presence of a column of sclerenchyma cells through the leaf (COLSCLER).
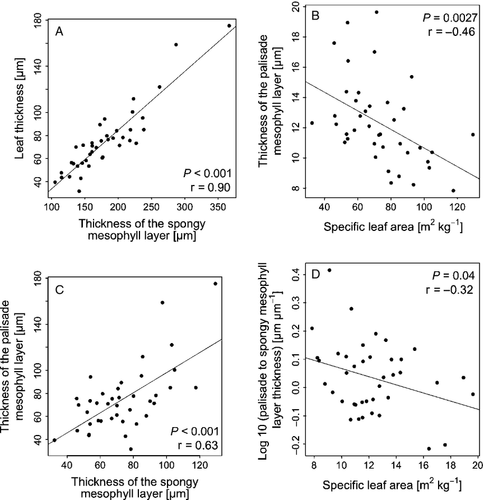
Leaf thickness was the variable with the most significant relationships to other anatomical and morphological leaf traits and increased with the number of layers of palisade mesophyll cells (r = 0.55, P = 0.0002), palisade parenchyma thickness (r = 0.80, P < 0.0001) and spongy parenchyma thickness (r = 0.90, P < 0.0001; Fig. 3A). Thickness of both mesophyll types was positively related to one another (Fig. 3C; r = 0.63, P < 0.0001). Both thickness of the palisade mesophyll layer and log ratio of palisade to spongy parenchyma thickness decreased with increasing SLA (Fig. 3B; r = −0.46, P = 0.0027; Fig. 3D; r = −0.32, P = 0.0427).
Relationships between morphological/anatomical and ecophysiological traits
Analysis of the relationships between the four ecophysiological key traits and cuticle characteristics did not reveal any significant relationships. However, anova analyses revealed marginally significant differences in fitted absolute maximum gs (CONMAXFITA) for different patterns of the upper epicuticular wax layer (EPIWAXUP; P = 0.0787).
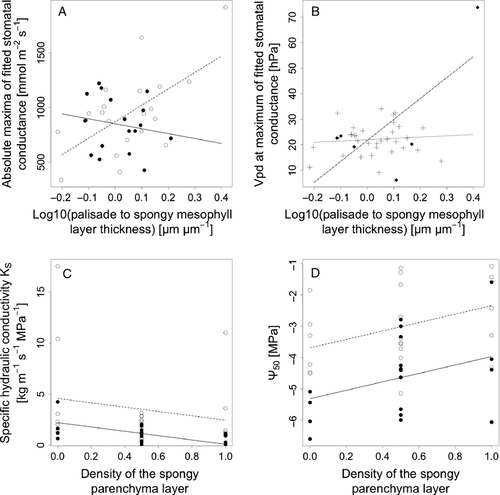
The best multi-predictor models with at most three terms were able to explain a proportion of the variation in the four ecophysiological parameters of gs and xylem cavitation vulnerability among species (r2 between 0.30 and 0.39), except for maximum specific xylem hydraulic conductivity (HYDCOND), for which the coefficient of determination was only 0.18 (Table 2). Three of the four ecophysiological characteristics contained leaf habit and interaction with leaf habit (Table 2, Fig. 4). Absolute maximum of fitted gs (CONMAXFITA) significantly increased with the log10 ratio of thickness of palisade to spongy parenchyma (LOG10RATIO; P = 0.006), with steeper slopes for deciduous than evergreen species (P < 0.001), but identical intercepts (P = 0.84; Fig. 4A). The vpd at the maximum of modelled gs–vpd relationship (VPDMAXFIT) showed an increase with LOG10RATIO only in the presence of papillae (PAPILL; P = 0.002), while neither LOG10RATIO (P = 0.71) nor PAPILL (P = 0.94) alone had a significant effect (Fig. 4B). Both parameters of stem xylem hydraulics depended on the same leaf traits, which were leaf habit (LEAFHABIT) and density of the spongy parenchyma (DENSINTCEL). Maximum specific xylem hydraulic conductivity (HYDCOND) was lower in evergreen than deciduous species (P = 0.023) and showed a tendency to decrease with increasing DENSINTCEL (P = 0.15). Xylem pressure at which 50% loss of maximum specific hydraulic conductivity occurred (PSI50) was higher in deciduous than evergreen species (P < 0.001), and increased with DENSINTCEL (P = 0.02; Fig. 4D).
| response | start AIC | final AIC | model of predictors | r2 | F-value | df | P |
|---|---|---|---|---|---|---|---|
| CONMAXFITA | 449.78 | 441.88 | CONMAXFITA ~ LOG10RATIO + LEAFHABIT + LOG10RATIO: LEAFHABIT | 0.30 | 4.991 | 3; 35 | 0.0055 |
| VPDMAXFIT | 183.31 | 170.49 | VPDMAXFIT ~ LOG10RATIO + PAPILL + LOG10RATIO: PAPILL | 0.3827 | 7.234 | 3; 35 | 0.00067 |
| HYDCOND | 95.47 | 91.60 | HYDCOND ~ LEAFHABIT + DENSINTCEL | 0.1827 | 4.024 | 2; 36 | 0.02647 |
| PSI50 | 32.91 | 17.90 | PSI50 ~ LEAFHABIT + DENSINTCEL | 0.3858 | 11.3 | 2; 36 | 0.00015 |
Discussion
Our study demonstrated that key ecophysiological traits of stomatal control and wood hydraulics were reflected in structural leaf traits. Interestingly, whether a leaf was evergreen or deciduous did explain a large proportion of the variation in morphological and anatomical leaf traits investigated. In addition, leaf habit was also among the best predictors for three of the four key hydrological traits. This justifies the use of leaf habit in ecophysiological models for woody species, as is the current practice in dynamic global vegetation models (DGVM; e.g. Woodward & Cramer 1996; Sitch et al. 2003). Just as leaf habit was related to leaf thickness, all leaf traits related to thickness of different layers also differed strongly between the two leaf habits. These interrelated traits also formed the first axis in the PCA analysis. Our findings confirm those in cross-species comparisons for different regions described in the literature. For example, in two studies with a comparable set of deciduous and evergreen study species, Roth et al. (1995) and Arambarri et al. (2006, 2008) described similar patterns in leaf structure for a montane forest in Venezuela and tree species from the lowest level of an Andine mountain forest of Tucumán (Argentina), respectively. For a set of 23 tree species native to seasonally dry tropical forest, Pringle et al. (2011) found differences in water availability were reflected in specific trait syndromes associated with evergreen and deciduous leaf habit.
Our first PCoA axis was related to the leaf economics spectrum and includes several morphological and anatomical traits. In a set of laboratory-grown seedlings of 52 European woody species Castro-Díez et al. (2000) showed that evergreen and deciduous species significantly differed in leaf thickness and thickness of mesophyll and spongy parenchyma, which we have now confirmed. Burrows (2001) examined leaves of 39 species on the east coast of sub-humid New South Wales, Australia (35° S); however, the relationships between, for example, mesophyll distribution and the ecological habitat of the species were weak.
Species with traits that implied an adaptation to dry habitats often co-occurred with mesophyllous species and vice versa (Medina et al. 1990; Gibson 1996). One reason for this might be that not all leaf traits related to ecophysiology are also related to the leaf economics spectrum (LES). This is reflected in our findings that not all variation in traits was captured by the first PCoA axis, as a further 25.1% of the variation was explained with axes two and three. In our study, we found cover and type of trichomes, presence of excretory glands, epidermal cell size and presence of a column of sclerenchyma cells through the leaf were unrelated to the LES. Similarly, stomatal size and density have been demonstrated to be independent of the LES, and thus of leaf thickness and leaf habit (Beerling & Kelly 1996; Kröber et al. 2012). Along this line, Brodribb & Holbrook (2005) concluded that leaf habit and the specific physiological solutions are not inevitably connected. Although leaf habit was a strong predictor in our analyses, as it sets certain boundaries for leaf physiology, within evergreen and deciduous leaves there is still a wide range of further differentiation (Kikuzawa 1991; Aerts 1995). One trait that was unrelated to the LES but was the best predictor for gs parameters was log10 ratio of palisade to spongy mesophyll layer thickness. This trait had already been suggested by Wylie (1946) as relevant for leaf hydraulics, and was confirmed to be also relevant to leaf hydraulic conductance (Sack & Frole 2006). The mechanistic explanation might be that with increasing log10 ratio, the amount of intercellular spaces decreases (Castro-Díez et al. 2000), which requires increased gas exchange, which in turn is reflected in higher maximum gs and stomata that remain open at higher vpd. Another function of a high log10 ratio might be related to resistance to air pollutants. Dineva (2004) suggested that wide intercellular spaces at low palisade/mesophyll ratios allow faster penetration and absorption of toxicants into photosynthetically active tissues.
Our study also revealed a relationship between leaf traits and xylem hydraulics. This contradicts the results of Baraloto et al. (2010), who found the main dimensions of leaf and stem functional traits were unrelated across a wide range of rain forest tree species. However, in contrast to Ψ50, maximum specific xylem hydraulic conductivity was only poorly explained by leaf traits and mainly depended on leaf habit, which confirms the results of Kröber et al. (2014). These authors found maximum hydraulic conductance and Ψ50 were mainly connected to leaf carbon to nitrogen ratio, which in turn was related to leaf habit. In their study, traits of xylem hydraulics were not related to traits of stomatal regulation, which is congruent with the findings of the present paper. In contrast, our observation that the Ψ50 increased with increasing density of the spongy parenchyma layer, was independent of leaf habit and thus of the LES, adds new aspects to the findings of Kröber et al. (2014). However, we do not have an explanation for why species with more drought-resistant xylem had less dense spongy parenchyma, as the opposite relationship of Ψ50 to density of the whole leaf would be expected (Niinemets 2001; Bacelar et al. 2004).
In contrast to expectations, leaf hydraulics were not significantly related to cuticle traits; however, with a larger species set and higher replication of the different types of cuticle structure, relationships might be detectable. Although cuticular waxes are generally considered as protection against water loss, Riederer & Schreiber (2001) stated that there is no simple link between the amount of wax and drought protection. Similarly, we also did not find a simple link between hydraulic traits and epicuticular wax structure. Thus, we must conclude that the ecological relevance of the different types of epicuticular waxes remains unsolved. Similarly, the presence of trichomes and especially papillae did not well reflect hydraulic functions, although their function to increase the leaf boundary layer, and thus the diffusion pathway for transpiration (Schuepp 1993), should confer higher drought resistance. The papillae found in Idesia polycarpa, Cyclobalanopsis myrsinifolia and Daphniphyllum oldhamii may have the same function as trichomes, as no trichomes were recorded for these species. An alternative explanation for papillae in contrast to trichomes might be that they reduce the adherence of water to the leaf surface, thus might facilitate gas exchange during precipitation events (Barthlott & Neinhuis 1997; Neinhuis & Barthlott 1997; Haworth & McElwain 2008).
For all results discussed, we should remember that all measurements were made on juvenile trees of a limited species set typical of subtropical forests in southeast China, which implies limitations. It is an open question whether the conclusions drawn are transferable to adult trees, as many such relationships vary with ontogeny (Lusk & Warton 2007). Furthermore, under different climate conditions, especially drought and other precipitation-related influences, the relationships between stomatal conductance, xylem hydraulics and morphological leaf traits may differ from the results obtained here. Finally, we should be aware that we could not provide mechanistic explanations for all observed relationships. In these cases, our analyses can only be regarded as explorative, reporting patterns that have not yet been described in the literature.
Conclusion
The aim of this study was to explore links between leaf anatomy and structure in relation to key ecophysiological traits. In addition to confirming the role of leaf habit in explaining leaf and xylem hydraulic traits, we identified further leaf traits that were unrelated to leaf habit and the leaf economics spectrum. In particular, the log ratio of palisade to spongy mesophyll layer thickness appeared to be a suitable predictor for stomatal regulation patterns and should be considered in future leaf trait analyses.
Acknowledgements
We thank Xuefei Yang, Sabine Both, Lin Chen and Kaitian Wang for coordinating the fieldwork when establishing the BEF-China experiment. We also highly appreciate support from the whole BEF-China research group. BEF-China is largely financed by the German Research Foundation (DFG FOR 891/1 and 2), and funding for this particular project was provided by the German Research Foundation to H.B. (DFG BR 1698/9-2). We are indebted to the Jodrell Laboratory of the Royal Botanic Gardens Kew for access to the scanning electron microscopy. Travel grants and summer schools were granted through the Sino-German Centre for Research Promotion in Beijing (GZ 524, 592, 698, 699 and 785).