The type of competition modulates the ecophysiological response of grassland species to elevated CO2 and drought
Abstract
The effects of elevated CO2 and drought on ecophysiological parameters in grassland species have been examined, but few studies have investigated the effect of competition on those parameters under climate change conditions. The objective of this study was to determine the effect of elevated CO2 and drought on the response of plant water relations, gas exchange, chlorophyll a fluorescence and aboveground biomass in four grassland species, as well as to assess whether the type of competition modulates that response. Elevated CO2 in well-watered conditions increased aboveground biomass by augmenting CO2 assimilation. Drought reduced biomass by reducing CO2 assimilation rate via stomatal limitation and, when drought was more severe, also non-stomatal limitation. When plants were grown under the combined conditions of elevated CO2 and drought, drought limitation observed under ambient CO2 was reduced, permitting higher CO2 assimilation and consequently reducing the observed decrease in aboveground biomass. The response to climate change was species-specific and dependent on the type of competition. Thus, the response to elevated CO2 in well-watered grasses was higher in monoculture than in mixture, while it was higher in mixture compared to monoculture for forbs. On the other hand, forbs were more affected than grasses by drought in monoculture, while in mixture the negative effect of drought was higher in grasses than in forbs, due to a lower capacity to acquire water and mineral nutrients. These differences in species-level growth responses to CO2 and drought may lead to changes in the composition and biodiversity of the grassland plant community in future climate conditions.
Introduction
It is anticipated that the concentration of CO2 in the atmosphere will double by the end of this century compared to current values (i.e. reaching at least 700 μmol·mol−1; IPCC 2007). This rise, together with the predicted increase in the duration and intensity of drought periods this century, associated with irregular precipitation and higher temperatures (IPCC 2007), will have different consequences for vegetation, from short-term physiological responses to long-term changes in structure and function of ecosystems (Mooney et al. 1991; Curtis et al. 1995). Ultimately, these changes will affect the productivity of plant communities (Naudts et al. 2014).
Drought conditions change the aeration and hydraulic conductance of soils; soil water potential becomes more negative (Rozema 1993), and plant uptake of water and mineral nutrients from soil are affected (Robredo et al. 2007). To reduce water loss, plants close their stomata, reducing the availability of CO2 in the chloroplast and leading to a reduction in photosynthesis (Chaves et al. 2003; Raven et al. 2004; Robredo et al. 2007). Stomatal closure is a major limiting factor for photosynthesis under drought conditions; however, when drought becomes more severe, plant biochemical reactions and use of light energy can also be effected (Flexas et al. 2002; Lawlor & Cornic 2002; Peña-Rojas et al. 2004) leading to a decrease in productivity. In contrast, elevated CO2 concentrations in well-watered conditions increase photosynthetic assimilation, especially in C3 plants (Lawlor & Keys 1993; Drake et al. 1997). Moreover, the increase in photosynthesis associated with the elevated CO2 concentration increases the availability of carbon skeletons to augment biomass (Kimball 1983; Manning & von Tiedemann 1995; Champigny & Mousseau 1999; Schapendonk et al. 2000).
When plants are subjected to drought under elevated CO2, they lose water more slowly due to stomatal closure and consequently reduce transpiration compared to plants growing under ambient CO2 (Bunce & Ziska 1998; Robredo et al. 2007; Vu & Allen 2009; Wall et al. 2011). It has also been suggested that elevated CO2 could increase plant tolerance to drought, permitting better osmotic adjustment (Wullschleger et al. 2002). The higher rates of photosynthesis under elevated CO2 might alleviate reductions in biomass production caused by environmental stress (Pérez-López et al. 2013b).
Several studies have examined plant responses of water relations and gas exchange to the interactive effects of elevated CO2 and water stress when grown singly or in monoculture. However, competition between plants regulates ecophysiological responses to abiotic stresses, thus increasing or decreasing plant growth (Thomas & Bazzaz 1993; Körner 1995; Wayne & Bazzaz 1995; Lüscher et al. 1996; Warwick et al. 1998; Poorter & Navas 2003; Verlinden et al. 2013). Nevertheless, few studies have examined the effect of elevated CO2 in combination with water stress in plants grown in a mixture (Dijkstra et al. 2010). To our knowledge, no study has addressed the concerted effects of elevated CO2 and drought on water relations, gas exchange and chlorophyll a fluorescence in plant monocultures and mixtures.
In natural ecosystems plant mixtures coexist with the same or different species. This coexistence implies competition for available resources, such as water, mineral nutrients and light (Van der Werf et al. 1993; Warwick et al. 1998). In real environmental conditions, growth of each species depends on resource availability. If environmental conditions change, the growth of the species may also change, altering the distribution of species within the ecosystem. Grasslands are a major ecosystem, covering approximately 25% of the Earth's surface (FAO 2007). Despite the importance of this ecosystem, few studies have focused on the influence of future climate conditions on the ecophysiology of grassland species when plants are grown in monoculture or mixture and how the grassland will adapt to climate change. This void in the field prompted us to: (i) study the effects of water stress and elevated CO2 on plant water relations, gas exchange, chlorophyll a fluorescence and aboveground biomass of four grassland species; (ii) elucidate any differences between these species and functional groups in such responses; (iii) analyse whether the type of competition alters the response; and (iv) investigate if future climate conditions will alter competition between species and functional groups.
Material and Methods
Plant material and experimental design
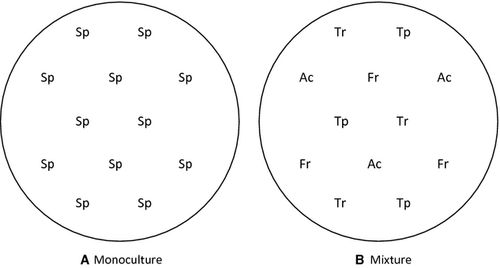
Four grassland species were used in this study, two grasses (Agrostis capillaris L. and Festuca rubra L.) and two forbs (Trifolium pratense L. and Trifolium repens L.). Seedlings were grown in a 1:1 mixture of peat/vermiculite in 3-l pots. Twelve seeds of the same or different species were sown per pot, reaching a final density of 520 plants m−2. The plants were grown in two conditions of competition: monoculture (intraspecific competition) and mixture (interspecific competition). In monoculture, all the plants were of the same species, whereas in mixture, three plants per species were equally distributed (Fig. 1). From sowing, plants were grown under ambient (370 μmol·mol−1) or elevated (740 μmol·mol−1) CO2 in a Conviron E15 controlled environment growth chamber (Conviron, Manitoba, Canada). These CO2 conditions were maintained for 24 h·day−1. The photoperiod was 14-h light/10-h dark, with day/night temperature of 24/20 °C and relative humidity of 70/80%. During the light period, the photosynthetic photon flux density (PPFD) was 400 μmol·m−2·s−1, provided by a combination of incandescent bulbs and warm-white fluorescent lamps (Sylvania F48T12SHO/VHO; Sylvania, USA).
Plants were watered with Hoagland's solution (Arnon & Hoagland 1940) twice per week and with deionised water between each application of Hoagland's solution until the beginning of the drought experiment, 28 days after sowing (DAS). Drought was imposed for 10 days by limiting watering to 20% of daily evapotranspiration, while well-watered plants received 100% of their daily evapotranspiration. During the drought period, watering was alternated daily between Hoagland's solution and deionised water. All parameters (see below) were measured at the end of the experiment (38 DAS).
Measurement of plant water relations
Leaf water potential (Ψ) was measured using the Scholander pressure-equilibration technique (Scholander et al. 1965). Hydraulic conductance (HC) was estimated for individual plants by dividing the instantaneous transpiration rate (E) by the difference between measured predawn leaf water potential (Ψpd) minus midday leaf water potential (Ψmd): [HC = E/(Ψpd - Ψmd)] (Pérez-López et al. 2009).
Gas exchange and chlorophyll a fluorescence
Leaf gas exchange and chlorophyll a fluorescence were determined using an open system, Li-6400 (Li-Cor Inc., Lincoln, NE, USA) with an integrated fluorescence chamber head (Li-6400-40; Li-Cor). Leaf gas exchange was measured as described in Pérez-López et al. (2013b). Measurements were performed 3 h after dawn at a stable temperature of 24 °C and relative humidity of 60%. The PPFD was provided by a red/blue LED source (model Li 6400-02B; Li-Cor) at 400 μmol·m−2·s−1 to match the PPFD of growing plants. The CO2 concentration used was the same as in growth conditions. Instantaneous transpiration efficiency (ITE) was calculated by dividing CO2 assimilation rate (A) by instantaneous transpiration rate (E). Li-Cor software was used to calculate stomatal conductance (gs) and the intercellular CO2 concentration (Ci) from A and E, according to the method of von Caemmerer & Farquhar (1981).
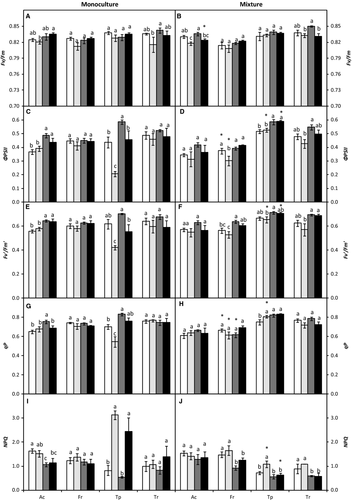
Chlorophyll a fluorescence was measured as described in Pérez-López et al. (2012). Plants were dark-adapted for 30 min at room temperature to relax all energy-dependent fluorescence quenching coefficients. The background fluorescence signal (Fo) was determined with a 660 nm output of 0.25 μmol·m−2·s−1 set at 500 Hz. The maximum fluorescence (Fm) was measured with a saturating flash of 7800 μmol·m−2·s−1 for 0.8 s. Maximum efficiency of PSII (Fv/Fm) was calculated as Fv/Fm = (Fm − Fo)/Fm. Actinic illumination was provided at 400 μmol·m−2·s−1. During light exposure, we induced a transient closure of PSII reaction centres by applying saturating pulses every 15 s until steady state variable fluorescence (Fs) was achieved. At that point, the light was switched off to allow maximum oxidation of the PSII, and a far-red pulse (735 nm) of 5.88 mW·s−1 was applied for 10 s. The following parameters were calculated (Pérez-López et al. 2012) for actual quantum yield of PSII (ΦPSII): 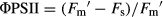 , where
, where 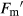 is maximum light-adapted fluorescence. The photochemical efficiency of PSII open centres (
is maximum light-adapted fluorescence. The photochemical efficiency of PSII open centres (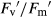 ) was calculated using the following equation:
) was calculated using the following equation: 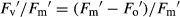 , where
, where 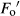 was the minimum light-adapted fluorescence. The coefficients of photochemical (qP) and non-photochemical (NPQ) quenching were calculated using the following equations, respectively:
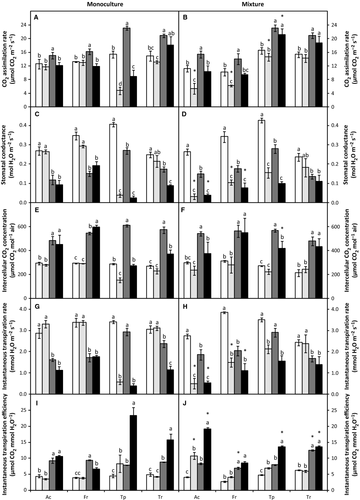
was the minimum light-adapted fluorescence. The coefficients of photochemical (qP) and non-photochemical (NPQ) quenching were calculated using the following equations, respectively: 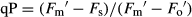 and
and 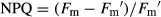 .
.
Growth parameters

where ABmono is total aboveground biomass per plant of a species grown in monoculture, and ABmix is total aboveground biomass per plant of the same species grown in mixture. An RCI of 0 indicates there is no effect, a positive value indicates competition, and a negative value indicates facilitation.
Statistical analysis
Results are reported as mean ± SE of three independent experiments (three ambient CO2 and three elevated CO2). In each experiment, we measured at least three replicate plants. At each CO2 concentration, each measured physiological parameter remained statistically similar in the three independent experiments; therefore, we pooled measurements from the three experiments in each condition. The SE was directly calculated from raw data. Data analyses were performed using SPSS 19.0 (SPSS Inc., Chicago, IL, USA). Three-way anova was used to evaluate the main effects of drought, CO2 concentration, competition and their interactions on all dependent variables. Means were compared using Duncan's multiple range test; P ≤ 0.05 was considered statistically significant.
Results
Water relations
After 10 days of drought under ambient CO2, midday leaf water potential (Ψmd) of A. capillaris was −1.07 MPa in monoculture (Fig. 2A), while in mixture it reached −1.42 MPa (Fig. 2B). Under elevated CO2, both singly and in mixture, the decreases in Ψmd after drought were similar to those under ambient CO2. The hydraulic conductance (HC) was 30% higher in mixture than in monoculture in well-watered conditions and ambient CO2. Elevated CO2 reduced HC by 23% in monoculture and 47% in mixture in well-watered conditions (Fig. 2C and D). Drought decreased HC by 70% and 90%, regardless of CO2 concentration, in monoculture and mixture, respectively.
For F. rubra deprived of water, grown under ambient CO2 and in monoculture, Ψmd was −1.47 MPa (Fig. 2A), whereas in mixture the reduction was to −1.68 MPa (Fig. 2B). Elevated CO2 mitigated drought in monoculture, but not significantly in mixture. Elevated CO2 decreased HC 61% in monoculture and 27% in mixture in well-watered conditions (Fig. 2C and D). Under ambient CO2, drought decreased HC 64% and 85% in monoculture and mixture, respectively. However, under elevated CO2 in monoculture, drought did not decrease HC, whereas in mixture the fall was less marked than in ambient CO2.
For T. pratense in monoculture at ambient CO2, drought decreased Ψmd to −2.84 MPa (Fig. 2A), while in mixture the decrease was less, to −2.25 MPa (Fig. 2B). Under combined drought and elevated CO2, the decreases were less marked in both types of competition, at −2.13 MPa in monoculture and −1.32 MPa in mixture. In well-watered conditions and ambient CO2, HC was 20% lower in mixture than in monoculture (Fig. 2C and D). Elevated CO2 reduced HC 40% in monoculture but not in mixture under well-watered conditions. Drought reduced HC ca. 90%, regardless of the type of competition or CO2 level.
Drought reduced Ψmd of T. repens under ambient CO2 to ca. −1.6 MPa regardless of the type of competition (Fig. 2A and B). In elevated CO2, the decrease in Ψmd under drought was less marked and similar in both types of competition, at ca. −1.35 MPa. HC was 57% lower in mixture than in monoculture under ambient CO2 and well-watered conditions (Fig. 2C and D). Under elevated CO2, HC was ca. 30% lower regardless of competition in well-watered conditions. Drought decreased HC by ca. 80% regardless of the type of competition or CO2 level.
Gas exchange parameters
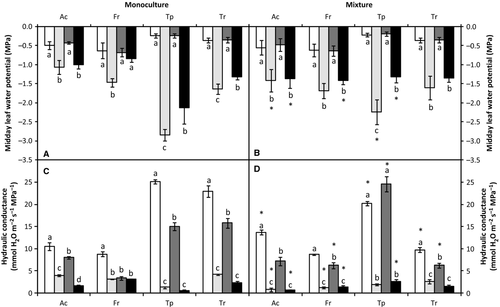
In well-watered conditions, elevated CO2 increased CO2 assimilation rate (A) of A. capillaris by 26% in monoculture and 37% in mixture (Fig. 3A and B). In monoculture, drought did not affect A under ambient or elevated CO2, whereas in mixture, A decreased 51% under ambient CO2 and 14% under elevated CO2. In well-watered conditions, elevated CO2 reduced stomatal conductance (gs) 55% and transpiration rate (E) 43% in monoculture, and in mixture by 43% and 32%, respectively (Fig. 3C,D,G and H). Drought decreased gs and E to a similar extent only in mixture, ca. 80% under ambient and 70% under elevated CO2. As expected, intercellular CO2 concentration (Ci) of A. capillaris increased 70% in response to elevated CO2 in well-watered conditions in both types of competition (Fig. 3E and F). Drought only significantly affected Ci at elevated CO2 in mixture. The instantaneous transpiration efficiency (ITE) in well-watered conditions was 123% and 102% higher under elevated CO2 compared to ambient CO2 in monoculture and mixture, respectively (Fig. 3I and J). Drought did not affect ITE in monoculture at either CO2 concentration, while in mixture it increased ITE 163% under ambient and 130% under elevated CO2.
In well-watered conditions, F. rubra increased A 22% in monoculture and 37% in mixture under elevated CO2 (Fig. 3A and B). Drought did not lead to significant differences in monoculture, but reduced A 39% under ambient CO2 in mixture. Under combined elevated CO2 and drought, the decrease was less noticeable. Elevated CO2 decreased gs ca. 50% in well-watered and 30% in droughted plants regardless of the type of competition (Fig. 3C and D). Drought decreased gs 69% in mixture under ambient CO2 and 57% under elevated CO2. In well-watered conditions, Ci increased 80% under elevated CO2 regardless of the type of competition (Fig. 3E and F). Drought did not affect Ci regardless of CO2 or competition condition. Elevated CO2 reduced E 45% compared to ambient CO2 regardless of the type of competition in well-watered conditions (Fig. 3G and H). Water stress only decreased E in mixture, and the decrease was only significant under ambient CO2. Elevated CO2 increased ITE 139% in monoculture and 159% in mixture in well-watered conditions (Fig. 3I and J). Drought did not affect ITE in either CO2 concentration or type of competition, except under elevated CO2 and drought in monoculture.
In T. pratense, elevated CO2 in well-watered conditions increased A 35%, regardless of the type of competition (Fig. 3A and B). Drought decreased A in monoculture 68% under ambient CO2 and 56% under elevated CO2. In mixture, drought did not cause any significant differences regardless of CO2 concentration. In well-watered conditions, elevated CO2 reduced gs nearly 30% regardless of the type of competition (Fig. 3C and D). Drought decreased gs 90% in monoculture under both ambient and elevated CO2. In mixture, the decreases in response to drought were less marked, ca. 60%, regardless of CO2 concentration. In well-watered conditions, elevated CO2 increased Ci ca. 110% regardless of type of competition (Fig. 3E and F). In monoculture, drought reduced Ci ca. 50% regardless of CO2 concentration. In mixture, drought only decreased Ci under elevated CO2. Elevated CO2 affected E in well-watered conditions in both types of competition, but this effect was not significant (Fig. 3G and H). Drought reduced E ca. 85% in monoculture under both CO2 concentrations. In mixture, the decreases after drought were less noticeable, ca. 40% regardless of the CO2 concentration. Elevated CO2 increased ITE in well-watered conditions by 74% and 68% in monoculture and mixture, respectively (Fig. 3I and J). Drought increased ITE more in monoculture compared to mixture under both ambient and elevated CO2.
In T. repens, elevated CO2 increased A ca. 40% regardless of type of competition in well-watered conditions (Fig. 3A and B). Drought did not significantly decrease A in either type of competition or CO2 concentration. In well-watered conditions, elevated CO2 reduced gs ca. 40% regardless of the type of competition (Fig. 3C and D). The effect of drought on gs was only significant in monoculture under elevated CO2. At elevated CO2, Ci increased ca. 120% regardless of type of competition in well-watered conditions (Fig. 3E and F). Drought only affected Ci in monoculture under elevated CO2. E was 20% higher in monoculture than in mixture (Fig. 3G and H). Elevated CO2 reduced E ca. 30% in well-watered conditions regardless of type of competition; E fell 60% in monoculture and 40% in mixture under drought conditions. Drought only affected E in monoculture under elevated CO2. Elevated CO2 increased ITE ca. 90% in either type of competition (Fig. 3I and J). Drought did not affect ITE under ambient CO2; however, under elevated CO2, water stress increased the effect of elevated CO2 in monoculture but not in mixture.
Chlorophyll a fluorescence
There were no significant differences in A. capillaris between treatments for maximum efficiency of PSII photochemistry (Fv/Fm) in monoculture (Fig. 4A). In mixture, drought reduced Fv/Fm, regardless of CO2 concentration (Fig. 4B). In monoculture and well-watered conditions, comparing elevated to ambient CO2, actual quantum yield of PSII (ΦPSII), the photochemical efficiency of PSII open centres (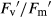 ) and photochemical quenching (qP) increased by 32%, 15% and 16%, respectively (Fig. 4C,E and G), whereas non-photochemical quenching (NPQ) was 35% lower (Fig. 4I). However, in mixture under actinic illumination, elevated CO2 did not affect any chlorophyll fluorescence parameter (ΦPSII,
) and photochemical quenching (qP) increased by 32%, 15% and 16%, respectively (Fig. 4C,E and G), whereas non-photochemical quenching (NPQ) was 35% lower (Fig. 4I). However, in mixture under actinic illumination, elevated CO2 did not affect any chlorophyll fluorescence parameter (ΦPSII, 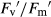 , qP, NPQ; Fig. 4D,F,H and J). Nor were these parameters affected by drought in any type of competition or CO2 level, except for qP, which decreased 10% under elevated CO2 in monoculture.
, qP, NPQ; Fig. 4D,F,H and J). Nor were these parameters affected by drought in any type of competition or CO2 level, except for qP, which decreased 10% under elevated CO2 in monoculture.
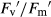 ) in monoculture (E) and mixture (F), photochemical quenching (qP) in monoculture (G) and mixture (H), and non-photochemical quenching (NPQ) in monoculture (I) and mixture (J). Growth conditions and statistical analysis are depicted in Fig. 2.
) in monoculture (E) and mixture (F), photochemical quenching (qP) in monoculture (G) and mixture (H), and non-photochemical quenching (NPQ) in monoculture (I) and mixture (J). Growth conditions and statistical analysis are depicted in Fig. 2.In F. rubra, there were no significant differences between treatments in any of the fluorescence parameters analysed in monoculture (Fig. 4A,C,E,G and I), although ΦPSII and qP increased 16% and 10%, respectively, compared to mixture. In mixture, Fv/Fm remained unchanged between treatments (Fig. 4B). Elevated CO2 did not affect ΦPSII and qP (Fig. 4D,H). 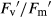 was 13% higher under elevated compared to ambient CO2 in both well-watered and drought conditions (Fig. 4F). NPQ fell 35% under elevated CO2 in well-watered conditions (Fig. 4J). Drought decreased ΦPSII 20% in mixture only under ambient CO2, but
was 13% higher under elevated compared to ambient CO2 in both well-watered and drought conditions (Fig. 4F). NPQ fell 35% under elevated CO2 in well-watered conditions (Fig. 4J). Drought decreased ΦPSII 20% in mixture only under ambient CO2, but 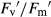 , qP and NPQ were not affected by drought, regardless of CO2 level.
, qP and NPQ were not affected by drought, regardless of CO2 level.
For T. pratense, there were no significant differences in Fv/Fm between treatments (Fig. 4A and B). In well-watered plants in monoculture, elevated CO2 increased ΦPSII and qP 33% and 18%, respectively, while in mixture they increased 13% and 10%, respectively; 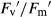 did not change under elevated CO2 in any type of competition (Fig. 4C–H). Under ambient CO2, drought decreased ΦPSII,
did not change under elevated CO2 in any type of competition (Fig. 4C–H). Under ambient CO2, drought decreased ΦPSII, 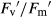 and qP in monoculture by 52%, 31%, and 22%, respectively, but these parameters were not altered in mixture. In monoculture, under combined elevated CO2 and drought, elevated CO2 mitigated the drought-induced reduction. Elevated CO2 did not affect NPQ in well-watered conditions (Fig. 4I and J). Under ambient CO2, drought increased NPQ 280% in monoculture and 50% in mixture. Elevated CO2 mitigated the drought-induced increases by 31% and 42% in monoculture and mixture, respectively.
and qP in monoculture by 52%, 31%, and 22%, respectively, but these parameters were not altered in mixture. In monoculture, under combined elevated CO2 and drought, elevated CO2 mitigated the drought-induced reduction. Elevated CO2 did not affect NPQ in well-watered conditions (Fig. 4I and J). Under ambient CO2, drought increased NPQ 280% in monoculture and 50% in mixture. Elevated CO2 mitigated the drought-induced increases by 31% and 42% in monoculture and mixture, respectively.
There were no significant differences in T. repens between treatments in any of the fluorescence parameters analysed (Fig. 4), except NPQ, where elevated CO2 decreased NPQ ca. 40% in mixture, regardless of water treatment (Fig. 4J).
Aboveground biomass
The forbs produced more aboveground biomass than the grasses in all treatments, especially when grown in mixture (Fig. 5).
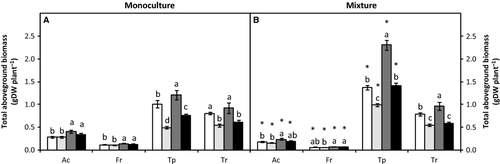
The aboveground biomass of A. capillaris under ambient CO2 and well-watered conditions was 0.27 g DW·plant−1 in monoculture (Fig. 5A) and 0.18 g DW·plant−1 in mixture (i.e. 34% less; Fig. 5B). Elevated CO2 increased aboveground biomass by 44% in monoculture and 28% in mixture in well-watered plants. Drought did not significantly affect the aboveground biomass in any type of competition under either CO2 concentration.
For F. rubra under ambient CO2 and well-watered conditions, the aboveground biomass was 0.11 g DW plant−1 in monoculture (Fig. 5A) and 46% lower in mixture (i.e. 0.06 g DW·plant−1; Fig. 5B). In well-watered conditions, elevated CO2 increased the aboveground biomass by 24% in monoculture and 15% in mixture. Drought did not affect the aboveground biomass, regardless of the type of competition or CO2 condition.
For T. pratense under ambient CO2 and well-watered conditions, the aboveground biomass was 1.00 g DW·plant−1 in monoculture (Fig. 5A) and 1.37 g DW·plant−1 in mixture (i.e. 37% higher; Fig. 5B). Elevated CO2 increased the aboveground biomass by 19% in monoculture and 67% in mixture in well-watered conditions. Under ambient CO2, drought decreased the aboveground biomass by 51% in monoculture and 27% in mixture. When T. pratense was subjected to combined conditions of elevated CO2 and drought, the decreases were less marked in monoculture.
Trifolium repens showed the same aboveground biomass under ambient CO2 and well-watered conditions in both types of competition, ca. 0.80 g DW·plant−1 (Fig. 5). In well-watered plants, elevated CO2 increased the aboveground biomass by 14% (not significant) and 22% in monoculture and mixture, respectively. Under ambient CO2, drought decreased the aboveground biomass by 32% in monoculture and 30% in mixture. Elevated CO2 did not reverse the effect of drought.
Productivity was lower in grasses than in forbs (Table 1), yielding 1.5 t·ha−1 in A. capillaris, 0.6 t·ha−1 in F. rubra, 5.3 t·ha−1 in A. capillaris and 4.2 t·ha−1 in T. repens in well-watered conditions at ambient CO2. In mixture, the productivity was 3.1 t·ha−1. The monoculture of T. pratense displayed higher productivity values, except when plants were subjected to drought under ambient CO2 conditions, where the monoculture of T. repens achieved higher productivity. Elevated CO2 increased the productivity of all treatments. Drought decreased the productivity of monocultures of T. pratense and T. repens and the mixture at all CO2 concentrations.
| CO2 levels | ambient CO2 | elevated CO2 | ||
|---|---|---|---|---|
| water regime | well-watered | drought | well-watered | drought |
| monoculture of A. capillaris | 1.46 ± 0.09b | 1.46 ± 0.08b | 2.10 ± 0.16a | 1.81 ± 0.13a |
| monoculture of F. rubra | 0.59 ± 0.03b | 0.54 ± 0.03b | 0.73 ± 0.04a | 0.65 ± 0.04ab |
| monoculture of T. pratense | 5.26 ± 0.45b | 2.56 ± 0.13d | 6.30 ± 0.54a | 3.96 ± 0.21c |
| monoculture of T. repens | 4.21 ± 0.16a | 2.83 ± 0.16b | 4.80 ± 0.59a | 3.19 ± 0.24b |
| mixture | 3.13 ± 0.13b | 2.31 ± 0.09c | 4.66 ± 0.28a | 2.83 ± 0.15b |
- Values represent means ± SE of at least three independent experiments, each performed in triplicate. Different letters in the same row indicate significant differences (P ≤ 0.05); the same letters indicate no significant differences.
Despite the dominance of T. pratense in mixed culture, the relative proportion of each species to the productivity changed depending on water regime and CO2 concentration (Fig. 6). Hence, the proportion of the contribution (%) of each species differed depending on environmental conditions. In well-watered plants, increased CO2 concentration enhanced the proportion of T. pratense and reduced the proportion of T. repens, A. capillaris and F. rubra (Fig. 6C). In response to water deficiency, elevated CO2 increased the contribution of T. pratense to productivity and reduced the contribution of T. repens, while the percentage contribution of A. capillaris and F. rubra did not change. In contrast, the effect of water availability on the contribution of each species to productivity in mixture was different, depending on the environmental CO2 concentration. At ambient CO2, the contribution percentage of A. capillaris and F. rubra increased in response to water shortage, whereas the contribution of T. pratense fell, and the contribution of T. repens did not change (Fig. 6B). Under elevated CO2, drought increased the contribution of A. capillaris and F. rubra and reduced the contribution of T. repens; the T. pratense contribution was maintained almost constant (Fig. 6D).
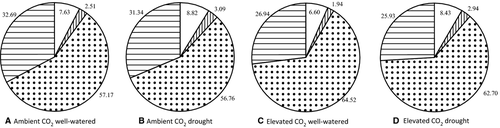
In the grasses, the relative competition intensity (RCI) was positive in all the treatments, indicating competition (i.e. grasses grew more in monoculture than in mixture; Fig. 7). For T. pratense, the RCI was negative for all the treatments, indicating facilitation (i.e. plants grew more in mixture than in monoculture). The RCI in T. repens was nearly zero for all treatments, indicating that the aboveground biomass in monoculture was similar to that in mixture.
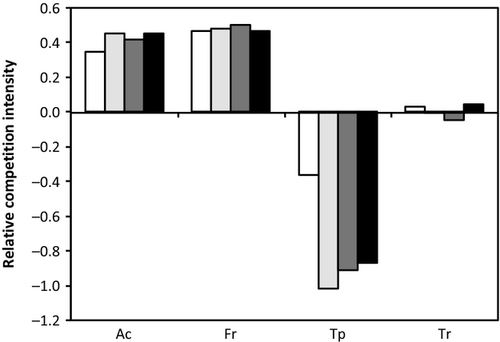
Elevated CO2 in well-watered conditions increased the RCI for grasses, while it produced more negative values for forbs (Fig. 7). Drought at ambient CO2 provoked the same pattern in RCI for grasses and forbs compared to the value in elevated CO2 (i.e. more positive values for grasses and more negative for forbs compared to well-watered conditions and ambient CO2). When plants were subjected to elevated CO2 and drought, RCI values of grasses did not change, but T. pratense presented less negative values and T. repens slightly positive values.
Discussion
Effects of elevated CO2 under non-stress conditions
Under non-stress conditions, CO2 levels did not affect plant water potential of any of the studied species, regardless of the type of competition (Fig. 2A and B), as previously found for barley (Pérez-López et al. 2010a). However, the response of Ψmd to elevated CO2 in well-watered plants is unclear; higher (Tognetti et al. 2000), unaffected (Ferris & Taylor 1994, 1995; Tognetti et al. 1998) or lower (Centritto et al. 1999; De Luis et al. 1999) water potentials have all been reported. Previous reports demonstrated that gs fell by an average of 22% under elevated CO2 concentrations (Ainsworth & Long 2005). However, in our study, the decrease in gs was higher: 30–40% in forbs and 50% on average in grasses, in both types of competition (Fig. 3C and D). Stomatal closure in all four species reduced E, as reported for numerous species (Robredo et al. 2007, 2010; Jiang et al. 2012; Pérez-López et al. 2012, 2013b). Despite stomatal closure, Ci was higher under elevated CO2 than ambient CO2 due to the higher external CO2 concentration, which increased A for all the species in this study (Fig. 3A and B); other authors have reported similar results for grassland species (Lee et al. 2011; Naudts et al. 2013). The higher A and lower E increased ITE, indicating that plants under elevated CO2 are more efficient in terms of water use. The increases in photosynthesis due to elevated CO2 increased availability of carbon skeletons to produce more biomass (Sæbø & Mortensen 1995; Nowak et al. 2004; Ainsworth & Long 2005; Pérez-López et al. 2013a). The aboveground biomass increased between 1.1- and 1.67-fold that obtained with ambient CO2 (Fig. 5); these results concur with those collected in Poorter & Navas (2003) for 350 species.
Moreover, the growth response of plants to elevated CO2 can change significantly (often reduced but also enhanced) when grown in competing populations with plants of different species compared to when growing with plants of the same species or in the absence of neighbours (Owensby et al. 1993; Wayne & Bazzaz 1995; Goverde et al. 2002). In our study, elevated CO2 enhanced the growth of grasses more in monoculture than in mixture, while forb growth was more enhanced when grown in mixture (Fig. 5). The different response of a species to increased CO2 (i.e. growth enhancement or reduction) when grown in interspecific competition depends on an adequate supply of resources, such as water, light and nutrients. In mixture, forbs displayed higher A, leading to higher carbohydrate production and higher HC, which allows these plants to acquire more water and nutrients from the soil than the grasses, and increases their productivity (Berntson et al. 1998; Maestre et al. 2005).
Drought effects at ambient CO2
Similar to reports from other authors, in monoculture drought had different implications depending on the species analysed (Dijkstra et al. 2010; Suriyagoda et al. 2011). In grasses, Ψmd reached −1.07 MPa for A. capillaris and −1.47 MPa for F. rubra. However, these decreases were not sufficient to alter plant growth, as gs, ΦPSII, A and aboveground biomass remained constant. Drought did not affect these parameters, despite the fact that it markedly reduced Ψmd. Thus, these grass species might require a threshold, more negative value of Ψmd to alter photosynthetic processes, and this threshold differs among species (Polley et al. 2012).
In T. repens, Ψmd was reduced to −1.6 MPa. Despite the fact that the drop-off of Ψmd was higher than in grasses, drought did not reduce A, but did reduce the aboveground biomass. This finding indicates a change in carbon allocation pattern, directing photoassimilate to roots and other processes. In general, plants respond to a relative shortage of any essential resource by increasing allocation to the structures and functions responsible for the acquisition of that limiting resource (Bloom et al. 1985; Coleman et al. 1989), in this case the roots for water and nutrient uptake. Although we did not detect changes in A, Jiang et al. (2010) observed that when T. repens was under more severe drought, where Ψmd reached values of ca. −2 MPa, gas exchange and photochemical parameters decreased. Thus, in our study, T. repens did not reach its threshold level to translate the effect of drought into alterations in photosynthetic processes.
The highest decrease in Ψmd of all the species was recorded in T. pratense, reaching −2.8 MPa. In parallel, gs decreased, which produced a smaller Ci, and consequently A was strongly reduced. According to Flexas et al. (2004), when gs decreases >60%, drought is considered severe, which is similar to our observation for T. pratense; in such conditions, other limitations to photosynthesis could appear. Fv/Fm did not differ between drought treatments and controls, indicating that the photosynthetic electron transport machinery was intact; other authors have reported similar findings (Robredo et al. 2010; Signarbieux & Feller 2011). However, ΦPSII was reduced by water stress. Water deficit produced the largest effect on 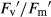 , reducing energy transduction from photosystem antennae to the reaction centres during illumination to prevent energy overload. At the same time, NPQ increased, which allowed the plant to dissipate the excess radiant energy not consumed in photosynthesis and to be wasted as heat in the PSII antennae complex (Robredo et al. 2010). Thus, the reduction in light energy channelled to the reaction centres implies a reduction in the quantity of energy transferred to photochemical reactions (qP). Therefore, as reported by others, under ambient CO2 and water deficit, there are stomatal and photochemical limitations to A, decreasing aboveground biomass, as found in grassland species under drought conditions (Naudts et al. 2011). However, other limitations, e.g. decreases in mesophyll conductance, could not be discounted. The decrease in E was higher than that of A; thus, ITE increased with drought (Robredo et al. 2007), indicating that T. pratense uses water more efficiently under drought conditions.
, reducing energy transduction from photosystem antennae to the reaction centres during illumination to prevent energy overload. At the same time, NPQ increased, which allowed the plant to dissipate the excess radiant energy not consumed in photosynthesis and to be wasted as heat in the PSII antennae complex (Robredo et al. 2010). Thus, the reduction in light energy channelled to the reaction centres implies a reduction in the quantity of energy transferred to photochemical reactions (qP). Therefore, as reported by others, under ambient CO2 and water deficit, there are stomatal and photochemical limitations to A, decreasing aboveground biomass, as found in grassland species under drought conditions (Naudts et al. 2011). However, other limitations, e.g. decreases in mesophyll conductance, could not be discounted. The decrease in E was higher than that of A; thus, ITE increased with drought (Robredo et al. 2007), indicating that T. pratense uses water more efficiently under drought conditions.
In mixture, the decrease in Ψmd was different to that in monoculture, changing the response of plants depending on the species analysed; Suriyagoda et al. (2011) reported similar results in forbs and grasses. This could be explained by the fact that in mixture, the HC of forbs was higher than that of grasses, allowing the former to acquire more water in mixture than the grasses. Smith & Roberts (2003) suggested that when the root systems of two or more species occupy the same soil volume, water is partitioned in proportion to the relative ability of each species to take up water.
In grasses, Ψmd was reduced more in mixture than in monoculture, −1.42 MPa in A. capillaris and −1.68 MPa in F. rubra. To reduce E and water loss, the grasses closed their stomata. However, this stomatal closure did not decrease Ci, possibly due to reduced Rubisco activity, less consumption of CO2 and, consequently, no CO2 was reduced, representing a possible biochemical limitation. For A. capillaris, no photochemical limitations were detected, as demonstrated in the constancy of the fluorescence parameters analysed. Nevertheless, in F. rubra, photochemical limitations were observed, as demonstrated by the decrease in ΦPSII. Therefore, the reduction in A was due to stomatal and possible biochemical limitations in A. capillaris, as well as stomatal, photochemical and possible biochemical limitations in F. rubra. However, reductions in A in response to a decrease in mesophyll conductance should not be discounted. Nevertheless, the decreases in A did not correlate with a significant reduction in aboveground biomass, possibly because the effects on biomass were in the belowground plant parts, which we did not measure. This finding could be caused by a shift in the direction of the photoassimilates to acquisition of the most limiting resource, in this case light for the grasses in the mixture. In short, plants would invest proportionally more in aboveground biomass than belowground biomass. Hardacre et al. (1986) also observed this response in grass species in competition with forbs in a mixture of Lolium perenne and T. repens.
The decrease in Ψmd of T. repens was similar in mixture and monoculture. The response of the gas exchange parameters was similar, and A did not decrease. However, the aboveground biomass fell, suggesting the same strategy as in monoculture (i.e. a change in carbon allocation pattern occurred, directing photoassimilates to structures and functions responsible for acquisition of the limiting resource, in this case, water availability).
The only species that showed a less negative value of Ψmd in mixture was T. pratense, reaching −2.2 MPa in mixture, and −2.8 MPa in monoculture. This improved water status resulted in less marked stomatal closure, which permitted the plant to maintain similar A values to those of well-watered plants. As mentioned for T. repens in monoculture and mixture, the aboveground biomass decreased despite a similar A, possibly due to a change in carbon allocation pattern, directing photoassimilates to structures and functions responsible for the acquisition of water, e.g. roots, changing the ratio of source and sink organs.
The different response of the diverse species to drought indicates that some species are more adaptable to water deficit than others (Signarbieux & Feller 2011). In our study, in spite of higher reduction in Ψmd, the forbs, especially T. pratense, maintained higher A values than the grasses, with higher aboveground biomass production.
Drought effects at elevated CO2
Under the combined effects of drought and elevated CO2, decreases in Ψmd were less marked; therefore, the negative impact of water deficit could be smaller. Other authors have also observed this result (Polley et al. 2002; Robredo et al. 2007). In monoculture, droughted grasses displayed decreased A compared to plants grown under elevated CO2 and well-watered conditions. Grasses did not show decreases in gs, and the fact that Ci was constant compared to well-watered plants at elevated CO2 may indicate that other factors are limiting A under drought. In A. capillaris, there was a decrease in qP, suggesting photochemical limitation of A. In F. rubra, qP did not decrease, indicating that photochemical processes were not a limiting factor for A. Nevertheless, it is possible that in both species, mesophyll conductance could be affected by drought (Flexas et al. 2004). Despite the slight decrease in A, aboveground biomass was not affected.
In T. repens, a similar response to drought detected under ambient CO2 was observed under elevated CO2. Photosynthesis did not decrease, despite stomatal closure and reduced Ci. This finding indicates that the light energy supply (400 μmol·m−2·s−1) at which A was measured (the same used at growth) was not sufficient to regenerate ribulose-1,5-bisphosphate and allow the plant to fix all the CO2 available (Rasineni et al. 2011). Therefore, no differences in A were recorded between well-watered and droughted plants. Despite the lack of a decrease in A, the higher stomatal closure in droughted than in well-watered conditions implied a reduction in water loss through transpiration; thus, the plants increased ITE, indicating that plants under elevated CO2 and drought are more efficient than under elevated CO2 and well-watered conditions. Despite a higher A under elevated CO2 compared to ambient CO2, the aboveground biomass did not increase. This could be due to the different use of the photoassimilates for non-growth processes, e.g. for nutrient uptake (Pérez-López et al. 2014), osmotic adjustment (Wullschleger et al. 2002) and antioxidant metabolism (Pérez-López et al. 2010b).
In the case of T. pratense, a similar reduction in gs was observed under elevated and ambient CO2; however, due to the higher external CO2 concentration, Ci increased. Higher CO2 availability with a lower decrease in the energy transferred to photochemical reactions (ΦPSII) permitted a superior A compared to that obtained at ambient CO2; Robredo et al. (2010) obtained similar results for barley. The constancy of transpiration and the abovementioned increase in A led to a much higher ITE, indicating that T. pratense under drought at elevated CO2 uses water more efficiently than under ambient CO2. The increase in photosynthesis under drought conditions because of elevated CO2 increased the availability of carbon skeletons to produce more biomass (Fig. 5).
When plants were grown under elevated CO2, the response to drought in mixture was different to that in monoculture; Dijkstra et al. (2010) reported similar findings in grasses and forbs. In the grasses, drought under elevated CO2 led to less stomatal closure than under ambient CO2. In spite of the lower decrease in gs, the absolute values were similar between CO2 concentrations with drought. Nevertheless, because of the higher external CO2 concentration, Ci was higher than that at ambient CO2, reducing the diffusional effect of drought on A. In addition, in A. capillaris, elevated CO2 could mitigate the negative effects of drought on a hypothetical biochemical limitation reported for drought at ambient CO2. In F. rubra, elevated CO2 reversed the effects of drought on photochemical limitation, but not on the likely biochemical limitation observed in drought at ambient CO2. This reduction in the limitation of photosynthesis allowed the plants to achieve a higher A and enhance aboveground biomass compared to the effect of drought at ambient CO2 (Fig. 5).
The behaviour of forbs under the combined conditions of elevated CO2 and drought was species-dependent; thus, whereas T. repens displayed no differences in gs or A between the competition types, both higher gs and A were observed in mixture for T. pratense. As mentioned above, the higher external CO2 concentration allows more CO2 to enter the chloroplast, yielding a higher A than under ambient CO2 in drought conditions. The increase in A, with the same value of E, translated into a better ITE, indicating that forbs in mixture use water more efficiently under elevated CO2 and drought conditions than under elevated CO2 and well-watered conditions. In the case of T. pratense, the higher A at elevated CO2 than at ambient CO2 produced a similar level of aboveground biomass compared to well-watered conditions at ambient CO2.
Effects on productivity and competitiveness
Overall, the total primary production of the mixed community was intermediate between the production of the grass and forb communities, regardless of the water regime and CO2 conditions. In all except ambient CO2 and drought conditions, the type of culture that displayed the highest productivity was the monoculture of T. pratense, followed by the monoculture of T. repens, the mixture, the monoculture of A. capillaris and the monoculture of F. rubra (Table 1).
Enrichment of CO2 usually alters the contribution of plant species to biomass production in grasslands (Teyssonneyre et al. 2002; Polley et al. 2003), favouring forbs over grasses (Niklaus et al. 2001; Teyssonneyre et al. 2002). On the other hand, CO2 enrichment usually increases the production of one or more species without affecting the biomass of other species (Morgan et al. 2004); however, occasionally, CO2 enrichment causes a strong opposite response among species or functional groups (Polley et al. 2003, 2012). In our study, under elevated CO2 concentrations and well-watered conditions, aboveground productivity increased; however, the response varied between species, as described in Berntson et al. (1998). Thus, all types of cultures, except the monoculture of T. repens, for which the increase was not significant, displayed more growth under elevated CO2 than under ambient CO2 in monoculture. In the mixture, the contribution of each species changed, increasing the contribution of T. pratense and decreasing the contribution of the other species; several authors have reported that increased CO2 alters the contribution of different species to a mixture (Hardacre et al. 1986; Overdieck 1986).
Drought decreased productivity at any CO2 concentration. In parallel, the contribution of grasses to the mixture productivity increased under drought conditions. Several authors have suggested that the presence of coexisting species may modify or amplify the environmental responses of the species (Dunnett & Grime 1999; Sala et al. 2000) due to differences in their physiological and morphological traits, leading to different abilities to maintain performance in competition (Greiner La Peyre et al. 2001). Moreover, when plants were subjected to drought and elevated CO2 simultaneously, the aforementioned higher A observed in those conditions permitted higher productivity than in drought under ambient CO2 (Centritto et al. 2002). Accordingly, our results show that interspecific competition is influenced by biological and physiological variables of the plants that are concurrent in a community and that the effect of multiple environmental changes may be synergistic or antagonistic.
Navas et al. (1999) suggested that the presence of a specific neighbour might be sufficient to increase or debilitate the competitiveness of a species or functional group. In well-watered conditions under ambient CO2, the RCI parameter of the grasses indicated that the mixture constituted competition (i.e. a decrease in their growth), while for T. pratense it was facilitation (i.e. an increase in its growth). In T. repens, RCI was near zero, as the aboveground biomass remained similar among the competition types in this species. Zhang et al. (2008) obtained similar results for the growth of F. rubra and T. pratense, observing competition for F. rubra and facilitation for T. pratense. In all cases, CO2 concentration affected the competitive interaction between species, as also found by Carter & Peterson (1983). Under elevated CO2 in well-watered conditions, RCI values under ambient CO2 were accentuated; thus, the grasses suffered higher competition, while the forbs experienced higher facilitation.
In drought conditions and ambient CO2, biomass of the forbs decreased in monoculture and in mixture, and more so in monoculture. Therefore, with drought, the forbs experienced higher facilitation than under well-watered conditions, while the grasses, due to their higher decrease in growth in mixture than in monoculture, suffered higher competition. Other authors have reported this species-specific response and shift in competitiveness along the environmental gradient (Reekie & Bazzaz 1989; Richardson et al. 2002) because species differ in their optimal growth environment and their tolerance to abiotic stress (Hartley & Mitchell 2005). Under the combined conditions of elevated CO2 and drought, aboveground biomass was higher than at ambient CO2 under drought conditions. In these growth conditions, T. pratense was the only species that was favoured, while the other species suffered from the competition. The competition intensity for the grasses increased with decreased productivity, whereas the opposite was true for T. pratense.
Thus, we conclude that elevated CO2 in well-watered conditions increased aboveground biomass by increasing photosynthesis (A). Drought reduced biomass and A, mainly due to stomatal limitation, and when drought was more severe, non-stomatal limitation also played a role. When plants were grown under combined conditions of elevated CO2 and drought, the limitations observed under ambient CO2 were reduced, permitting higher A and reducing the observed decrease in aboveground biomass. The response to climate change was species-specific and dependent on the type of competition. Thus, the response of grasses to elevated CO2 in well-watered conditions was higher in monoculture than in mixture, while it was higher in mixture than in monoculture for forbs. In contrast, the forbs were more affected by drought in monoculture than the grasses, while in mixture the negative effect of drought was higher in grasses than in forbs because of their lower capacity to acquire water and nutrients. These differences in species-level growth in response to CO2 and drought may lead to changes in the composition and biodiversity of the grassland plant community in response to future climate conditions.
Acknowledgements
This research was financially supported by the following grants: MICINN-BFU2010-16349/BFI co-funded by ERDF, K-EGOKITZEN IE10-277, UFI11/24 and GRUPO Gobierno Vasco-IT577-13. J. Miranda-Apodaca was the recipient of a grant from the Departamento de Educación, Universidades e Investigación del Gobierno Vasco (Spain).