Pericarp anatomy and hormone profiles of cypselas in dormant and non-dormant inbred sunflower lines
Abstract
The pericarp anatomy and the effects of storage after harvest, storage temperature and early cypsela imbibition on phytohormone profiles were studied in inbred sunflower lines B123 and B91. On day 0, germination of B123 cypselas was near 0%, indicating dormancy, whereas that of B91 cypselas was near 100%, indicating non-dormancy. The germination of B123 and B91 on day 33 at room temperature (25 °C) storage was similar. Cell wall thickness and sclerification of the pericarp were higher in B123 than B91, suggesting that structural characteristics may contribute to physical dormancy in B123. Jasmonates (JAs), salicylic acid (SA) and abscisic acid (ABA) were measured in dry and imbibed pericarps. SA content of dry pericarp was higher on day 33 than day 0. SA content during imbibition on day 33 was similar for room and low (−20 °C) storage temperatures. ABA content after 12 h imbibition was similar on days 0 and 33 at low temperature, but it increased on day 33 at room temperature for B123. 12-Oxo-phytodienoic acid (OPDA) was maximal on day 0 for B123, but peaked at day 33 at low temperature for B91. JA was higher on days 0 and 33 at room temperature as compared with low temperature. Our findings indicate that pericarp hormone profiles are affected in the two lines with different dormancy degree depending on storage conditions and imbibition processes.
Introduction
Seed dormancy, defined as the lack of capacity of a viable seed to germinate under favourable conditions, is an adaptive trait optimising germination to the time that is most suitable for the seed to complete its life cycle (Finch-Savage & Leubner-Metzger 2006). In regard to the fruit (technically speaking, cypsela) of sunflower (Helianthus annuus L., Asteraceae), dormancy causes difficulties in commercial production (Snow et al. 1998) and delays in sowing. Dormancy and delayed germination of sunflower cypselas may be functions of the embryo, seed cover layers (seed coat, pericarp) or both (Brunick 2007). Maiti et al. (2006) observed that embryo dormancy is often relatively short (4–8 weeks), whereas the effect of the seed coat and pericarp on seed dormancy may persist for longer periods of time (>32 weeks). In particular, dormancy caused by the pericarp affects seed germination and seedling establishment through accumulation of chemical inhibitors, prevention of water absorption, prevention of leakage of chemical inhibitors from the seed and inhibition of radicle protrusion (Sari et al. 2006). The pericarp thus has complex effects on seed germination and dormancy, involving both physical and chemical factors (Hu et al. 2009). Almost all physiological, molecular and genetic studies on seed dormancy to date have focused on complete seeds, without considering the roles of distinct tissues (Graeber et al. 2012).
The sunflower pericarp consists of an epidermis, hypodermis, phytomelanin layer, middle layer (composed of ten to 15 layers of axially oriented cells) and parenchyma (composed of thin-walled, loosely-packed cells adjacent to the inner epidermis). In mature fruit, sclerification of the middle layer decreases toward the centre and part of the inner parenchyma, and the vascular bundles and inner epidermis are collapsed (Lindström et al. 2007). In many species, freshly harvested mature seeds become non-dormant following prolonged dry storage (termed ‘dry after-ripening’), allowing germination under a wider range of environmental conditions (Iglesias-Fernández et al. 2011). Sunflower is an excellent model for studies of the dormancy process because the seeds are deeply dormant at harvest, and the degree of dormancy decreases gradually during dry storage (Corbineau et al. 1991).
Temperature is the other critical environmental factor affecting dormancy during dry storage. In most species, the degree of dormancy is reduced more quickly as after-ripening temperature increases. In sunflower cypselas, a complex relationship has been demonstrated between temperature and seed moisture content in controlling the mechanisms of dormancy release during dry storage. Stored mRNA is oxidised during seed after-ripening, and this process may be involved in regulation of dormancy (Bazin et al. 2011a). Further physiological and molecular studies are needed regarding the changes induced by after-ripening signals in dry viable seeds and the role of these changes during imbibition (water uptake by seeds) (Kucera et al. 2005; Finch-Savage & Leubner-Metzger 2006; Holdsworth et al. 2008).
Seed germination and dormancy are regulated by interactions of plant hormones in both synergistic and competitive fashions. Experimental treatments that promote dormancy release (e.g. after-ripening, which in sunflower occurs following dry storage conditions) are often correlated with changes in seed hormone content and/or sensitivity (Finch-Savage & Leubner-Metzger 2006). Studies during the past decade indicate that the hormones abscisic acid (ABA) and gibberellins (GAs) function synergistically in the regulation of seed germination and dormancy. There is considerable evidence that interactions occur among ABA, GAs and other hormones such as jasmonates (JAs) and salicylic acid (SA), and that the processes of seed dormancy and germination involve extensive cross-talk among their respective signalling pathways (Kucera et al. 2005).
Our group determined ABA distribution among different parts of sunflower dry seeds, showing that ABA accumulates in the pericarp and embryonic axis (Andrade et al. 2009). High ABA content in sugar beet pericarp was also reported (Hermann et al. 2007). Although ABA appears to stimulate dormancy (Nonogaki et al. 2010), there is often no clear relationship between degree of dormancy and ABA content of the mature dry seed or grain. ABA plays an important role in initiation of dormancy in developing seeds, but maintenance of dormancy (e.g. during late seed maturation and desiccation) does not require high ABA levels (Feurtado & Kermode 2007).
Moreover, a previous study from our group reported highest accumulation of JAs in the pericarp, followed by the embryonic axis and cotyledons of sunflower B71 and B59 dry seeds (Vigliocco et al. 2007). In addition, Miersch et al. (2008) quantified 12-oxo-phytodienoic acid (OPDA), JA, 12-hydroxyjasmonic acid (12-OH-JA), 11-hydroxyjasmonic acid (11-OH-JA), 12-hydroxyjasmonic acid sulphate (12-HSO4-JA) and 12-hydroxyjasmonoylglucoside (12-O-Glc-JA) in the pericarp of Cucumis sativa and Cucurbita pepo. JAs inhibit germination in some species, but stimulate germination of dormant seeds in Acer tataricum (Tatarian maple; Berestetzky et al. 1991) and Malus domestica (apple; Yildiz et al. 2007). Quantitative analyses of dry seeds of Arabidopsis mutants with impaired seed germination revealed a strong correlation between germination frequency and elevated levels of OPDA, JA and JA-Ile. OPDA, but not JA or JA-Ile, contributes to blocking of germination in these seeds. OPDA has also been suggested to promote dormancy under adverse environmental conditions, such as high temperature (Dave et al. 2011). These recent findings suggest that JAs are involved in the regulation of dormancy and germination, but the mechanism of their effect remains unclear.
The role of SA in seed germination is also controversial. A recent study suggests that this process is controlled by complex interactions among SA, ABA and GAs (Rivas-San Vicente & Plasencia 2011). In this study the determination of SA was approached because it remains unclear how endogenous concentrations are modulated in dry seed and during early imbibition of sunflower. We describe here (i) the pericarp anatomy of dry cypselas of the two inbred H. annuus lines (B123 and B91), (ii) effects of storage conditions (time after harvest, temperature) on cypsela germination in two inbred lines, (iii) hormone profiles in pericarps of dry versus imbibed cypselas of the two lines, and (iv) effects of exogenous JA and SA on germination and early growth of line B123.
Material and Methods
Plant material
Cypselas of inbred H. annuus lines B123 and B91, supplied by MSc Daniel Alvarez, were sown in an experimental field at EEA-INTA Manfredi (31°51′9.00′′ S, 63°44′55.91′′ W), Argentina.
Germination assays
Cypselas were harvested and stored at −20 ± 1 °C to maintain dormancy and at room temperature (25 ± 1 °C, relative humidity 50%) to release dormancy. Germination assays were performed on cypselas stored at 25 ± 1 °C for 11, 22, 33 or 44 days after harvest. A second germination test was conducted on dry cypselas at harvest (day 0) and 33 days after harvest at two storage temperatures (−20 ± 1 and 25 ± 1 °C). Four biological replicates (each 25 cypselas) were sown in 16 × 12-cm pots between filter papers moistened with 25 ml deionised water. Germination experiments were conducted in a growth chamber with alternating periods of 16-h light/28 °C/60% relative humidity and 8-h dark/20 °C/70% relative humidity. Relative germination percentages were scored at 10 days and pare resented as mean ± SE.
Assay of cypselas with or without pericarp
To evaluate the role of the pericarp in seed dormancy, cypselas were divided into two groups. In group 1 (cypselas without pericarp), pericarps were carefully peeled off by hand to avoid mechanical damage. In group 2 (cypselas with pericarp), cypselas were kept intact. For both groups, the germination assay was performed under the experimental conditions described above. Cypselas with pericarp were considered to have germinated when the radicle protruded through the covering layers (seed coat, pericarp). Germination in cypselas without a pericarp was defined as visible growth of the radicle through the seed coat. Germination status was recorded every day, and the experiment continued until no new germination was observed for five consecutive days. The assay employed four biological replicates (each 25 cypselas). The viability of ungerminated cypselas was determined using a standard tetrazolium test (Moore 1962).
Histological analysis of the pericarp
Cypselas were fixed in formalin/acetic acid/alcohol (FAA) solution. To obtain cross-sections of the middle portion and proximal end of the pericarp (Fig. 3), fixed samples were embedded in paraffin wax and processed using conventional techniques for cutting (10 μm) and staining (safranin–fast green). Additional free-hand cross-sections of cypselas were also obtained. All sections were mounted in glycerine/water (1:1). Photographic observations and recordings were made using a Nikon Labophot-2 microscope with attached Nikon Coolpix 4500 camera and ocular micrometer.
Imbibition assays
Mature dry cypselas stored for 33 days at −20 ± 1 °C and 25 ± 1 °C were subjected to imbibition assays under the experimental conditions described above. At imbibition times of 6, 12, 18 and 24 h, cypselas were collected and pericarps carefully removed by hand to avoid mechanical damage. The isolated pericarps were immediately frozen in liquid N2, lyophilised, and stored at −80 °C. The assay employed four biological replicates (each 25 cypselas).
Extraction and purification of endogenous hormones
The JAs, SA and ABA were extracted from 0.2 g dry weight (DW; g·plant−1) pericarps using the method of Durgbanshi et al. (2005) with some modifications. Plant material was homogenised in an Ultraturrax T25 basic (IKA, Staufen, Germany) with 5 ml deionised water. D5-OPDA, D6-JA, D5-12-OH-JA, D3-JA-Ile, D6-SA (Leibniz-Institute of Plant Biochemistry, Halle, Germany) and D6-ABA (NRC-Plant Biotechnology Institute, Saskatoon, Canada) were used as internal standards. A total of 50 ng of each was added to samples. Samples were centrifuged at 1,540 g for 15 min. The supernatant was adjusted to pH 2.8 with 15% (v/v) acetic acid and extracted twice with diethyl ether. The organic fraction was evaporated under vacuum. The dried extracts were dissolved in 1 ml methanol and filtered on a vacuum manifold at a flow rate <1 ml·min−1. The eluate was evaporated at 35 °C under vacuum in a SpeedVac SC110 (Savant Instruments, New York, NY, USA). The assay employed four biological replicates.
Hormone identification and quantification with liquid chromatography-electrospray ionisation tandem mass spectrometry (LC-ESI MS-MS)
The JAs (OPDA, JA, 12-OH-JA, JA-Ile), SA and ABA were separated from tissues using reverse-phase high-performance liquid chromatography (HPLC). An Alliance 2695 separation module (Waters, Milford, MA, USA) equipped with a Restek C18 column (100 × 2.1 mm, 3 μm) was used to maintain performance of the analytical column. Fractions were separated using a gradient of increasing methanol concentration, constant glacial acetic acid concentration (0.2% in water) and initial flow rate 0.2 ml·min−1. The gradient was increased linearly from 40% methanol/60% water–acetic acid at 25 min, to 80% methanol/20% water–acetic acid. After 1 min, the initial conditions were restored, and the system was allowed to equilibrate for 7 min. The identification and quantification of all hormones was performed with a quadruple tandem mass spectrometer (Quattro Ultima, Micromass, Manchester, UK) fitted with an electrospray ion (ESI) source, in multiple reactions monitoring mode (MRM) using precursor ions and their transitions (m/z) to OPDA (m/z 291/225), D5-OPDA (m/z 296/230), JA(m/z 209/59), D6-JA (m/z 215/59), 12-OH-JA (m/z 226/138), D3-12-OH-JA (m/z 229/141),JA-Ile (m/z 322/128), D3-JA-Ile (m/z 325/131), SA (m/z 137/93), D3-SA (m/z 141/97), ABA (m/z 263/153) and D6-ABA (m/z 269/159), with retention times of 11.35, 14.30, 7.20, 16.75, 4.35 and 8.25 min, respectively. The collision energies used were 20 eV for JA and 30 eV for OPDA, and the cone voltage was 35 V. The spectrometry software used was MassLynx version 4.1 (Micromass).
Exogenous hormone treatments
The effects of exogenous JA and SA (Sigma-Aldrich, Buenos Aires, Argentina) on germination and early growth of line B123 were evaluated. Twenty-five cypselas were placed in 16 × 12-cm pots between filter papers moistened with various hormone solutions. Based on previous studies of effects of exogenous application of SA and JA on germination (Dave et al. 2011; Rivas-San Vicente & Plasencia 2011) and seedling growth (Gutiérrez-Coronado et al. 1998; Corti Monzón et al. 2011), we used concentrations of 0.5 mm and 1.0 mm for SA, and 10−6 m, 10−8 m and 10−10 m for JA. SA was dissolved in 100 μl DMSO and diluted with distilled water (control: same volume of DMSO). JA was dissolved in 200 μl methanol and diluted with distilled water (control: same amount of distilled water + 200 μl methanol). The chamber conditions described under ‘Germination assays’ were used for exogenous hormone treatments. ‘First count’ and ‘final count’ germination were the percentages of germinated cypselas at days 4 and 10, respectively. Early growth characteristics (primary root length, hypocotyl length) were recorded at the end of the experiment (day 10). The assay employed three biological replicates.
Statistical analysis
The germination percentage data were arc-sine transformed to satisfy the assumptions of normality (Zar 1996). Differences in germination percentage and hormone content were analysed with one-way anova. Data were subjected to a Multiple Range Test a posteriori (Tukey HSD test). The statistical software used was Statgraphics Plus, version 3 (Manugistics 1997).
Results
Cypsela germination
The germination of mature and dry sunflower cypselas was monitored during dry storage at various intervals following harvest to determine germination capacity and degree of dormancy. The cypsela population of line B123 was essentially dormant on the day of harvest (day 0), with a germination percentage near 0%. The germination percentage reached 64% on day 11 and continued increasing until day 33, the time required to break dormancy. The cypsela population of line B91, in contrast to B123, had a germination percentage near 100% at all times after harvest, indicating that the B91 cypselas were non-dormant. B123 and B91 showed similar germination percentages on day 44. These findings indicate that dry storage at room temperature after harvest promoted breaking of dormancy under our experimental conditions (Fig. 1A).
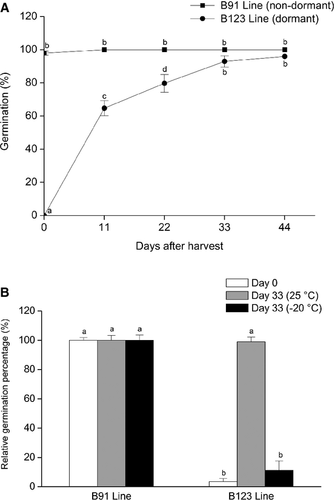
In view of the effect of after-harvest storage time on germination percentage, we evaluated the effect of two storage temperatures (25 ± 1 °C [room temperature] and −20 ± 1 °C) on germination of the two lines on day 33. B123 cypselas stored for 33 days at 25 ± 1 °C showed a high germination percentage, similar to that of non-dormant B91 cypselas. The B123 cypsela germination percentages were much lower after storage at −20 ± 1 °C than at room temperature. The B91 cypsela germination percentages were the same at the two storage temperatures (Fig. 1B). Thus, under our experimental conditions, storage at low temperature was important for maintaining dormancy of B123 cypselas.
Effect of pericarp removal on seed germination
The B123 cypselas with an intact pericarp were strongly dormant (germination of 0% on day 5), whereas cypselas with the pericarp removed had germination of 3% on day 5. In B91 cypselas, germination percentage on days 0–2 was not affected by the presence or absence of a pericarp. Starting on day 3, however, the percentage was higher in the intact pericarp group than in the pericarp-removed group, indicating that these cypselas were non-dormant (Table 1).
| time (days) | germination (%) | |||
|---|---|---|---|---|
| cypselas with pericarp | cypselas without pericarp | |||
| B91 | B123 | B91 | B123 | |
| 1 | 64a | 0a | 52a | 0a |
| 2 | 80a | 0a | 70a | 1a |
| 3 | 86a | 0a | 69b | 2a |
| 4 | 89a | 0a | 75b | 2a |
| 5 | 89a | 0a | 76b | 3b |
- Values with the same letter in column 2 versus 4 or column 3 versus 5 are not significantly different at P ≤ 0.05.
Pericarp anatomy
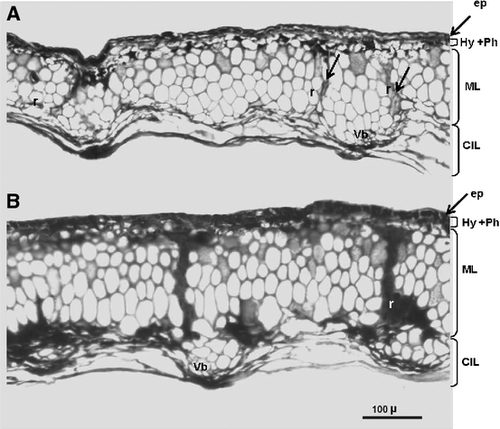
Cross-sections taken halfway along the length of the cypsela showed that thickness (183 versus 138 μm), number of cell strata (6–7 versus 4–5) and pericarp cell wall thickness and sclerification were higher in B123 dormant cypselas than in B91 non-dormant cypselas (Fig. 2A and B). The nuclei of parenchyma ray cells in B91 cypselas were clearly distinguishable in some cells (Fig. 2A). In B123 cypselas, pericarp rays and some cells of the inner side of the middle layer were impregnated with a substance similar to the phytomelanin found in the subepidermal layer (Fig. 2B). Cross-sections through the micropylar end of the cypsela showed that the middle layer at the binding of the two carpels (suture) was not sclerified in B91 (Fig. S1A) but was strongly sclerified and impregnated with phytomelanin in B123 (Fig. S1B). Differences between the lines in pericarp cell wall thickness were much more pronounced in this area of the fruit (Fig. S1A, B).
Hormone profiles in dry pericarps
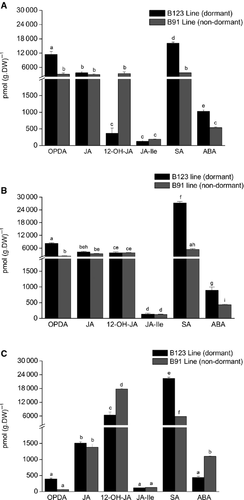
Endogenous content of JAs, SA and ABA was evaluated in dry pericarps of the two lines on day 0 (at harvest) and day 33 (after harvest) at storage temperatures of 25 ± 1 °C and −20 ± 1 °C. On day 0, OPDA and SA were the major compounds in B123. The content of these two compounds was, respectively, four-fold and 4.5-fold higher in B123 than in B91. 12-OH-JA content was higher in B91 than in B123. ABA content was twofold higher in B123 than in B91, but was relatively low for both lines. JA-Ile content was low in both lines. JA and JA-Ile content did not differ significantly between the two lines (Fig. 3A). On day 33, SA was the most abundant compound in pericarps of both B123 and B91 stored at 25 ± 1 °C. SA content was higher in B123 than in B91. Among the JAs, OPDA, JA and 12-OH-JA content was elevated in both lines. JA-Ile content was markedly lower (131.25 pmol·g−1 DW) than that of the other JAs. OPDA content was four-fold higher in B123 than in B91. The content of JA, 12-OH-JA and JA-Ile did not differ significantly between the two lines. ABA content was much higher (~50-fold) in B123 than in B91 (Fig. 3B). On day 33, SA content was approximately the same for the two storage temperatures in both lines (Fig. 3B andC). The low temperature caused a notable reduction in OPDA and JA content in both lines (Fig. 3C), whereas 12-OH-JA content was twofold higher at the low temperature (Fig. 3B and C). JA-Ile content was unaffected by temperature in both lines. ABA content was higher at the low temperature in B91, but higher at room temperature in B123.
Pericarp hormone content during imbibition time course
We analysed the hormone content of B123 and B91 cypselas at various imbibition times to evaluate the effect of imbibition processes at days 0 and 33 at 25 ± 1 °C and −20 ± 1 °C. In B123, imbibition on day 0 triggered a sharp decrease in SA content. In B91, SA content did not vary markedly during the imbibition time course and was always lower than in B123 (Fig. 4A). On day 33 under storage at room temperature, for B123 the SA content declined significantly at imbibition time 6 h and then remained relatively stable until 24 h. For B91, SA content decreased at imbibition time 6 h and then showed slight increases at 18 h and 24 h until reaching the control value (Fig. 4B). On day 33 under low temperature (−20 ± 1 °C), SA content at imbibition time 6 h decreased six-fold for B123 and three-fold for B91. Subsequently, SA content remained low and stable for B123, but peaked at 18 h with a significant decrease at 24 h for B91 (Fig. 4C). ABA content peaked at an imbibition time of 12 h for both lines, but the peak was three-fold higher in B123 than in B91. ABA content decreased significantly at 18 h (four-fold for B91, twofold for B123; Fig. 5A). On day 33 at room temperature, a peak in ABA content was observed at imbibition time 12 h in both lines; the peak was much higher in B123 than in B91. Decreases in ABA content (six-fold for B91; onefold for B123) were seen at 18 h (Fig. 5B). On day 33 under low temperature, ABA content increased at imbibition times 6 h and 12 h for both lines (particularly B123), and subsequently decreased (Fig. 5C). On day 0, OPDA content at imbibition time 6 h was onefold higher in B123 and twofold higher in B91 relative to time 0 h. In B123, OPDA content declined from 6 h to 18 h, and then remained fairly constant until 24 h. In B91, OPDA content was variable after 12 h (Fig. 6A). On day 33 at room temperature, OPDA content in B123 decreased notably from imbibition time 6 h to 18 h and then increased at 24 h. B91 showed a similar trend, but with an increase at 12 h (Fig. 6B). On day 33 at low temperature, OPDA content in B123 decreased at 6 h, increased subsequently, and remained constant thereafter. OPDA content in B91 peaked at 12 h (Fig. 6C). On day 0, JA content of B123 was high at imbibition time 6 h, and then declined gradually until 24 h. JA content of B91 decreased three-fold at 6 h, and remained constant thereafter (Fig. 7A). On day 33 at room temperature, JA content declined steadily throughout the imbibition time course in both lines, but was higher at each time point in B123 than in B91 (Fig. 7B). On day 33 at low temperature, JA content in B123 decreased at 6 and 12 h and peaked at 18 h, whereas that in B91 it peaked at 6 h and decreased subsequently (Fig. 7C).
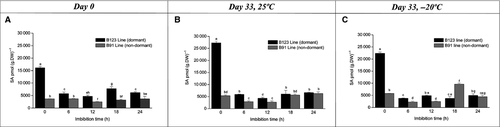
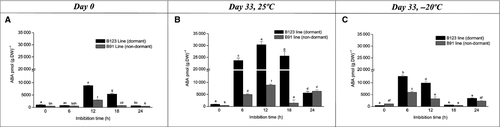
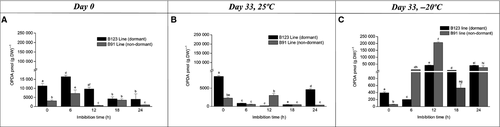
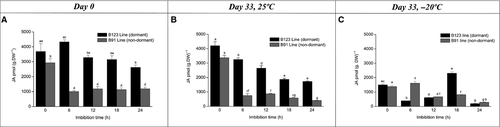
For dry pericarps (imbibition time 0 h), SA content at both temperatures was higher on day 33 (~25000 pmol·g−1 DW for B123, ~5000 pmol·g−1 DW for B91; Fig. 3B and C) than on day 0 (~16000 pmol·g−1 DW for B123, ~3000 pmol·g−1 DW for B91; Fig. 3A). On day 33, SA content was similar for the two temperatures (Fig. 4B and C). At the low temperature and imbibition time 12 h, ABA content was similar on days 0 and 33 (Fig. 5A–C). At room temperature, ABA content of B123 on day 33 (Fig. 5B) was higher than on day 0 (Fig. 5A). For both lines, the ABA peak was observed at imbibition time 6 h for the low temperature and 12 h for the room temperature (Fig. 5B and C). OPDA profiles differed between the two lines. The OPDA content was maximal at day 0 for B123 (Fig. 6A) and day 33 (under low temperature) for B91 (Fig. 6C). For both lines, JA content at both days 0 and 33 at room temperature (Fig. 7A and B) was higher than that on day 33 at low temperature (Fig. 7C).
Effects of exogenous SA and JA on cypsela germination and early growth
In view of the high SA content observed in B123 embryos on day 33 after harvest (unpublished data) and the reported action of JA on root architecture of sunflower seedlings (Corti Monzón et al. 2011), we examined the effects of exogenously applied SA and JA on germination and early growth of dormant B123 cypselas. The effects of SA and JA treatment are shown in Table 2. After 4 days of treatment, the first germination count was unchanged. In contrast, after 10 days of treatment the final germination count increased by 10−8 m JA. In regard to post-germination events, treatment with 1 mm SA resulted in significantly higher (1.44- and 1.61-fold, respectively) primary root and hypocotyl lengths on day 10. Treatment with 10−8 m JA resulted in a slight decrease in primary root length but had no effect on hypocotyl length.
| treatment | germination (%) | early growth | ||
|---|---|---|---|---|
| first count (Day 4) | final count (Day 10) | primary root length (mm) | hypocotyl length (mm) | |
| control | 0 ± 0 | 8 ± 0 | 1.15 ± 0.16a | 0.7 ± 0.14a |
| JA 10−10 m | 0 ± 0 | 0 ± 0 | 0.00 | 0.00 |
| JA 10−8 m | 0 ± 0 | 14 ± 2 | 0.83 ± 0.23a | 0.74 ± 0.16a |
| JA 10−6 m | 0 ± 0 | 0 ± 0 | 0.00 | 0.00 |
| SA 0.5 mm | 0 ± 0 | 8 ± 0 | 1.07 ± 0.39a | 0.72 ± 0.16a |
| SA 1 mm | 0 ± 0 | 8 ± 4 | 1.66 ± 0.14c | 1.13 ± 0.23c |
- Values with the same letter in column 4 versus 5 are not significantly different at P ≤ 0.05.
Discussion
Seeds that are not imbibed may undergo a variety of biochemical processes during storage, including changes in metabolites (Bailly 2004), proteome (Chibani et al. 2006) and gene expression (Leubner-Metzger 2005). Such changes provide possible mechanisms for the after-ripening process. Changes in transcription and protein metabolism occur even during the dry quiescent state, reflecting alteration of dormancy status, enhancement of germination vigour and/or effects on post-germination functions related to seedling growth (Holdsworth et al. 2007).
We can distinguish the two sunflower lines with regard to the degree of seed dormancy based on germination capacity. The high germination percentage of B123 on day 33 after harvest indicates low seed dormancy. In contrast, B91 seeds displayed high germination at all times after harvest and were therefore characterised as non-dormant. Subrahmanyam et al. (2002) similarly demonstrated high variability in the degree of seed dormancy among sunflower genotypes. Maiti et al. (2006) examined 59 sunflower genotypes and found that some were highly dormant and others essentially non-dormant. Non-dormant lines such as B91 may be useful in breeding programmes to reduce dormancy in hybrid sunflower. In our conditions, the findings suggest that the pericarp is partly responsible for B123 seed dormancy, because germination increased only 3% after pericarp removal. In agreement, germination of Rosa multibracteata was not improved after breakdown of the pericarp with H2SO4, mechanical scarification, or complete removal of the pericarp, indicating that other parts of the fruit are involved in dormancy regulation (Zhou et al. 2008). The seed coat was not removed in the present study; it is therefore possible that the seed coat plays a role in regulation of B123 seed dormancy, perhaps through the presence of germination inhibitor(s) and/or physical obstruction.
Based on our anatomical analysis, the establishment of physical dormancy may be related to the increased cell wall thickness, sclerification of the middle layer and the absence of parenchyma in the micropylar area of B123 dormant cypselas, in comparison with B91 non-dormant cypselas. However, in view of the low germination percentage observed following pericarp removal, we cannot rule out the possibility of physiological dormancy resulting from the high ABA content (~4000 pmol·g−1 DW) in B123 embryos (P. Roselló, A. Vigliocco, N. Riera, A. Andrade, M. Calafat, L. Molas, S. Alemano, unpublished data). In B91, it is possible that pericarp presence may improve germination, since germination was ~13% higher than that of cypselas without a pericarp. This effect may be related to regulation of water uptake and gas exchange (Miyajima 1996; Adkins et al. 2002). Another factor that could negatively influence B91 germination is vulnerability of the unprotected embryo because the pericarp imitates the function of a seed coat by providing structural protection. This has been reported in crambe seed (Crambe abyssinica cv. FMS Brilhante; Cardoso et al. 2014) and Abelia × grandiflora (André) Rehd. (glossy abelia; Scheiber & Robacker 2003).
The rate of dormancy release during dry storage of sunflower is strongly affected by temperature in combination with relative humidity (Bazin et al. 2011a,b). We found that the transition from dormant to non-dormant state in B123 seeds at day 33 was affected by storage temperature, i.e. storage at low temperature (−20 °C) was a major factor in maintaining dormancy. In previous studies, dormancy was maintained by storage of sunflower seed at temperatures between −15 °C and −30 °C (Oracz et al. 2008; Bazin et al. 2011ab), and storage of barley seed at −20 °C (Leymarie et al. 2008). The optimal conditions for dry after-ripening treatment often involve a long period of mild ambient temperatures (Ali-Rachedi et al. 2004). In the present study, storage of B123 seeds at room temperature (25 °C) was effective in promoting dormancy release. Oracz et al. (2008) reported that dry storage at 20 °C released sunflower dormancy, while Benech-Arnold et al. (2006) found that dormancy of barley declined slowly during storage at 20 °C and relative humidity 50%.
Phytohormones are important signalling molecules that communicate environmental changes to seeds during germination or dormancy. The metabolism of various phytohormones is interdependent: a change in one hormone eventually alters the content of others. Hormones found in the pericarp are generally provided from the mother plant during seed maturation; in some cases, hormones leak from the embryo during late embryogenesis (Finkelstein et al. 2002). In the present study, hormone profiles differed between lines B123 and B91 and were affected by storage conditions and the cypsela imbibition process. Such hormone levels would be associated with the presence of parenchymal tissue – in the fruit end and in parenchyma cells ray cells – cytoplasmic content, which would indicate the existence of remaining functional activity in the pericarp cell protoplasts towards the end of grain-filling; Cochrane (1985) reported similar results for barley. Also, we cannot rule out that when the seed is rehydrated, hormones could be extruded from the embryo into the pericarp; these hormone levels detected in the pericarp might affect germination. For example, previous studies in rose achenes demonstrated that high concentrations of ABA in the pericarp and testa could inhibit germination (Jin et al. 1995).
Storage at low temperature (−20 °C) appeared to affect JAs content; a decreased content of OPDA (the JA precursor) and JA itself was followed by an increase in the hydroxylated derivative 12-OH-JA, suggesting activation of enzymes involved in the JA biosynthesis pathway. Previous studies indicate that low-hydrated seeds contain ‘pockets’ of higher hydration in which enzymatic catalysis and transcription can occur (Leubner-Metzger 2005; Holdsworth et al. 2007). Müller et al. (2009) reported that changes related to low hydration vary depending on the species and ecotype, and such changes affect responses to environmental signals. The observed effect of low temperature on JAs content suggests that dormant seeds perceive low temperature as a stress factor. We recently observed (Garbero et al. 2011) that cold treatment modified leaf JAs content in different ecotypes of Digitaria eriantha.
A series of preparatory processes occur prior to visible radicle protrusion during imbibition (Finch-Savage & Leubner-Metzger 2006) and seed dry storage (Holdsworth et al. 2007). Genomic approaches using Arabidopsis seed have focused primarily on the transition from imbibition phase II to III (Finch-Savage et al. 2007). The physiological basis of hormonal changes that occur during imbibition phases I and II remains poorly understood. In the present study, a reduction in SA content was triggered after the start of imbibition in B123 pericarps. Similarly, SA content in dry seeds of dormant A. thaliana ecotype Cvi declined gradually following imbibition (Preston et al. 2009). We cannot rule out the possibility that non-enzymatic production of SA catabolic compounds (e.g. dihydroxybenzoates: 2,3-DHBA and 2,5-DHBA) in the pericarp is activated by imbibition. We observed that the kinetics of SA accumulation in B123 pericarp were similar on days 0 and 33, suggesting that this hormone is not the primary mediator of dormancy maintenance.
We observed high ABA accumulation in B123 pericarps during the early hours of imbibition. De novo ABA biosynthesis during imbibition of dormant seeds (but not non-dormant seeds) has been demonstrated in H. annuus (Le Page-Digivry & Garello 1992), Nicotiana plumbaginifolia (Grappin et al. 2000), Hordeum vulgare (Wang et al. 1995) and A. thaliana ecotype Cvi (Ali-Rachedi et al. 2004). Such de novo ABA biosynthesis is considered a mechanism for dormancy maintenance. In B123 cypselas stored for 33 days at 25 °C, ABA accumulation in the pericarp resulted from embryo-mediated ABA extrusion and allowed cypsela germination. This process may have been stimulated primarily through the mild storage temperature. Embryo-mediated active ABA extrusion was proposed as a mechanism in Beta vulgaris (Hermann et al. 2007), where passive ABA uptake via the pericarp was slow, whereas active extrusion from the seed via the pericarp was a rapid process. Thus, ABA extrusion might be mediated actively via the seed and pericarp.
The JAs were accumulated differentially during the course of imbibition. The role of JAs during seed germination has been demonstrated in many studies (Preston et al. 2009; Dave et al. 2011; Linkies & Leubner-Metzger 2012). In A. thaliana, JA and JA-Ile content changed during imbibition phase I and differed between non-dormant Col and dormant Cvi accessions (Preston et al. 2009). In the dry state, JA content in non-dormant seeds was ~ten-fold higher than in dormant seeds. The high JA content declined rapidly during the early phase of imbibition and was ~ten-fold lower at 5 h. Similarly, we observed a decrease in JA content during imbibition in pericarps of non-dormant B91 cypselas at days 0 and 33 at 25 °C. In contrast to the findings of Preston et al. (2009), we found the highest JA content in pericarps of dormant B123 cypselas; this declined during the course of imbibition from day 0 to 33 at 25 °C. It is therefore possible that a decrease in JA content may occur in the pericarp of both dormant and non-dormant lines and lead to germination. In a study of Wassilewskija (Ws) wild-type and mutant cts-2 of A. thaliana, Dave et al. (2011) observed reduced levels of OPDA and JA upon imbibition of after-ripened seeds. We found a higher OPDA content in the pericarp of dry sunflower cypselas on days 0 and 33 at 25 °C than that reported in Dave et al. (2011) for Arabidopsis, but we observed a similar overall decrease of OPDA content during imbibition. The increase of OPDA in pericarps during imbibition at low temperature storage suggests an activation of enzymes involved in fatty acid release. Further studies are clearly needed to eluciadate the mechanisms whereby JAs regulate seed dormancy and germination.
In view of the high SA content observed in pericarps of dormant B123, we studied the effect of exogenous SA on germination and early growth. We also examined the effect of exogenous JA, whose role in seed dormancy, particularly in sunflower, is poorly known. The role of SA in seed germination is controversial. There are conflicting reports that it can either inhibit germination or increase seed vigour. Some of the apparently contradictory effects may be related to different SA concentrations applied in various assays or studies. Application of exogenous SA to B123 cypselas increased the length of the primary root and hypocotyl, suggesting that SA extrusion and accumulation in the pericarp may affect vegetative growth of the seedling. Exogenous SA has been reported to stimulate growth in soybean (Gutiérrez-Coronado et al. 1998), wheat (Shakirova et al. 2003) and maize (Gunes et al. 2007). In the soybean study, SA treatment increased shoot growth ~20% and root growth ~45%; in the wheat study, SA treatment of seedlings increased cell division in the root apical meristem. Similarly, we found that exogenous SA (1 mm) increased B123 primary root growth 31% and hypocotyl growth 38% at day 10, but had no effect on germination. Application of JAs or methyl-JAs has been reported to inhibit root growth in A. thaliana (Staswick et al. 1992), Oryza sativa (Wang et al. 2002) and Allium cepa. However, the roles of JAs in many aspects of growth regulation remain unclear and may differ among species. In our study, neither JA concentration applied had a notable effect on early growth of dormant B123. In contrast, Corti Monzón et al. (2011) reported that JA inhibited primary and lateral root growth in sunflower via an auxin-independent pathway.
In conclusion, our study extends knowledge on the pericarp anatomical structure and the ways in which storage conditions and early imbibition differentially affect hormone content in pericarps of sunflower in dormant and non-dormant inbred lines B123 and B91, respectively. Also, under our conditions, is possible that the anatomical and physiological characteristics of the pericarp might be related to different degrees of dormancy of the two analysed lines. Further studies are necessary to elucidate the contributions of the seed coat (particularly the micropylar region) and the embryo.
Acknowledgements
This work was supported by a grant from SECyT-UNRC to S.A. The authors are grateful to Dr. S. Anderson for English editing of the manuscript.