Proteomic analysis of residual proteins in blades and petioles of fallen leaves of Brassica napus
Abstract
Brassica napus L. is an important crop plant, characterised by high nitrogen (N) levels in fallen leaves, leading to a significant restitution of this element to the soil, with important consequences at the economic and environmental levels. It is now well established that the N in fallen leaves is due to weak N remobilisation that is especially related to incomplete degradation of foliar proteins during leaf senescence. Identification of residual proteins in a fallen leaf (i.e. incompletely degraded in the last step of the N remobilisation process) constitutes important information for improving nutrient use efficiency. Proteome analysis of the vascular system (petioles) and blades from fallen leaves of Brassica napus was performed, and the 30 most abundant residual proteins in each tissue were identified. Among them, several proteins involved in N recycling remain in the leaf after abscission. Moreover, this study reveals that some residual proteins are associated with energy metabolism, protection against oxidative stress, and more surprisingly, photosynthesis. Finally, comparison of blade and petiole proteomes show that, despite their different physiological roles in the non-senescing leaf, both organs redirect their metabolism in order to ensure catabolic reactions. Taken together, the results suggest that a better degradation of these leaf proteins during the senescence process could enable improvements in the N use efficiency of Brassica napus.
Introduction
Leaf senescence is a genetically programmed developmental process that constitutes the ultimate phase in leaf development (Lim et al. 2007). At the whole plant level, the main function of leaf senescence is to enable nutrient recycling, especially of nitrogen (N) from the ageing leaves to other organs (Smart 1994; Masclaux-Daubresse et al. 2008). Senescence symptoms characterised by leaf yellowing occur in a highly coordinated order, starting from the leaf margins toward the base of the leaf. Thus, cells near the vascular bundle are the last to senesce. It has been well documented that this ultimate phase of development leading to leaf abscission results in a massive coordinated degradation of macromolecules transported out of senescing leaves (Masclaux-Daubresse et al. 2010). Senescence-related cell changes are first detected in the chloroplasts (Dodge 1970), whereas the mitochondria and nucleus remain intact until advanced senescence (Smart 1994; Inada et al. 1998). The breakdown of chloroplasts is associated with a decrease in photosynthesis that occurs alongside chlorophyll degradation (Humbeck et al. 1996; Lu & Zhang 1998). This chloroplast breakdown is an important step in N recycling, since up to 80% of total leaf N is stored in this organelle. Thus, it is well established that degradation of RubisCO, the main leaf protein representing up to 50% of soluble proteins in a fully expanded leaf, is crucial for nutrient recycling. Other changes in cellular catabolism, such as hydrolysis of membrane lipids, membrane proteins and nucleic acids, are also associated with the leaf senescence process. In contrast, some proteins are synthesised (or not hydrolysed) to mediate degradation and transport of degradation products and maintain cellular energy metabolism during senescence. Recently, proteomic studies have shown that the identified senescence-up-regulated proteins can be grouped into several functional categories, such as metabolism, respiration, stress responses and proteolysis (Wilson et al. 2002; Schiltz et al. 2004; Swidzinski et al. 2004; Zhao et al. 2005; Hebeler et al. 2008; Desclos et al. 2009). Among these, proteolysis plays a key role in leaf N recycling. Currently, proteases involved in protein degradation associated with leaf senescence are not yet clearly identified. However, some studies have shown that specific proteases such SAG12 (senescence-associated gene 12) and aspartate proteases remain present until the last stages of leaf senescence (Etienne et al. 2007; Desclos et al. 2008). These data suggest a major role for these proteases in N mobilisation from the ageing leaves to storage or newly developing tissues (for review: Roberts et al. 2012). Currently, both the onset and progression of leaf senescence are considered to be major levers for improving N Use Efficiency (NUE) and yield in field crops (Bogard et al. 2011; Masclaux-Daubresse & Chardon 2011). Thus, since the crucial role of leaf senescence in crop production is now accepted, key leaf senescence regulatory networks still need to be elucidated, especially for crops characterised by weak N remobilisation such as Brassica napus.
Oilseed rape (B. napus L.) is the second largest oleaginous crop in the world (Carré & Pouzet 2014) and is characterised by a low NUE. Compared to cereals, this crop requires a higher amount of nutrients (Hocking et al. 1997; Rathke et al. 2006) and has a higher critical N demand (Colnenne et al. 1998), but only 50% of the N from fertiliser is used for seed filling (Schjoerring et al. 1995). Previous studies have shown that the low NUE of oilseed rape is mainly due to weak leaf N remobilisation efficiency from leaves to sink tissues during senescence (Rossato et al. 2001). Indeed, during the vegetative stage, fallen leaves may contain high concentrations of residual N, which can exceed 2% of dry matter (Rossato et al. 2001; Malagoli et al. 2005a). Thus, no large restitution of organic N to the soil means that it is not directly available to plants without first being mineralised and has a significant impact on both crop yield and environmental balance (Dejoux et al. 2000). Some studies have focused on the weak N remobilisation during B. napus foliar senescence. Thus, Tilsner et al. (2005) clearly demonstrated that mRNA transcript level of genes encoding amino acid transporters (especially BnAAP1 and BnAAP2) increases in the senescing B. napus leaf, allowing an optimal transfer of amino acids to phloem. This work suggests that the weak of N remobilisation observed in B. napus could be primarily related to poor hydrolysis of foliar proteins. Because oilseed rape occupies an important place in the agricultural landscape, a better understanding of the proteolytic processes associated with leaf senescence may lead to improved NUE in oilseed rape and consequently enhance the agro-environmental balance (Gregersen et al. 2013). Recently, Desclos et al. (2009) have shown that 55 proteins involved in metabolism, energy, detoxification, stress response, proteolysis and protein folding are accumulated (induced or not degraded) during leaf senescence. Assuming that a N level in fallen leaves is due to the incomplete degradation of proteins, it is therefore desirable to deepen our knowledge of the residual proteins in fallen leaves. Thus, study of the fallen leaf proteome will allow further information to be obtained about the hypothesis that some proteins are difficult to degrade during senescence-related proteolysis.
In this context, the aim of this work was to identify the major residual proteins in fallen leaves of B. napus during the vegetative stage. Because (i) the vascular system (especially the petiole and midrib) senesces later than leaf blades (Gan & Amasino 1997), and (ii) the vascular system is essential for the onset of blade senescence (Mishra & Gaur 1970, 1977), but also because petioles and blades have different physiological roles in the non-senescing leaf (photosynthesis and transport for blade and petiole, respectively), we have undertaken proteome studies of both organs. Thus, the 30 most abundant residual proteins in the blades and vascular systems (petioles) of fallen B. napus leaves were detected using two-dimensional electrophoresis (2-DE) and identified with mass spectrometry. The consequences of the presence of residual proteins in the blade and petiole of B. napus fallen leaves are discussed in relation to improving the NUE of this crop.
Material and Methods
Plant material
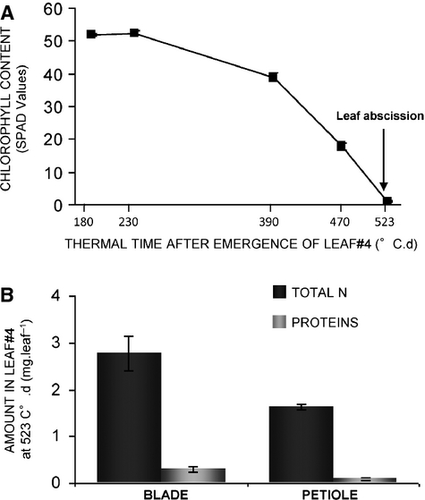
Seeds of B. napus (cv Capitol) were surface-sterilised in 80% ethanol for 30 s followed by 20% sodium hypochlorite for 20 min. After several washes in demineralised water, seed germination was carried out on foam rubber (Hortis Pinpot growing medium, Agrimedia). Just after emergence of the third leaf, seedlings were transplanted to hydroponic conditions into a 20-l plastic tank. Plants were grown under greenhouse controlled conditions with a thermoperiod of 20 °C (day) and 18 °C (night) and a photoperiod of 16 h. Natural light was supplemented with high pressure sodium lamps (MASTER GreenPower T400W; Philips, Amsterdam, the Netherlands), supplying average photosynthetically active radiation (PAR) of 280 μmol photons m−2·s−1 at the top of the canopy. The aerated nutrient solution contained 1 mm KNO3, 3 mm CaCl2, 1 mm K2SO4, 0.5 mm MgSO4, 0.4 mm KH2PO4, 0.15 mm K2HPO4, 0.2 mm Fe-Na-EDTA, 14 μm H3BO3, 5 μm MnSO4, 3 μm ZnSO4, 0.7 μm (NH4)6Mo7O24, 0.7 μm CuSO4 and 0.1 μm CoCl2, and was renewed every 7 days. The leaf rank number was ordered from the oldest to the youngest leaf. After 14 days of growth corresponding to the rosette stage, leaf rank #4 was studied and the age of this leaf was expressed in thermal time (base 5 °C). According to Wenying & Kaikua (2012), a SPAD-502 chlorophyll meter (Minolta, Tokyo, Japan) was used as a relevant non-destructive method to monitor chlorophyll content during the lifespan of leaf rank #4. Ten measures were performed on three biological replicates (i.e. three individual leaf rank #4 from three individual plants). For each biological repetition, an individual mean SPAD value (n = 10) was obtained. At each time of the experiment, the three individual means were used to calculate the mean ± SE (n = 3). Thereafter, leaf rank #4 samples (three biological repetitions) were weighed and the blade separated from the petiole (and midrib), frozen in liquid nitrogen and stored at -80 °C until further analysis. An aliquot of each blade and petiole was freeze-dried, ground to a fine powder and weighed for dry matter determination. Results are presented as mean for three leaf (triplicate) batches ± SE of the mean.
Determination of total N amount
The total N amount was quantified with a continuous flow isotope mass spectrometer (IsoPrime; GV Instrument, Manchester, UK) linked to an elemental analyser (EA 3000; EuroVector, Milan, Italy). The total N amount was an average of three replicates comprised of leaves from three different plants.
Total protein extractions
Protein extraction, 2-DE and image analysis of 2-DE were performed as described in Desclos et al. (2009). For total protein extraction, frozen leaf blade or petiole samples (1.5 g fresh weight) from three replicates were ground in a mortar with liquid nitrogen and resuspended in 2 ml cold acetone containing 10% TCA. After centrifugation at 16,000 g for 3 min at 4 °C, the supernatant was discarded and the pellet rinsed. The pellet was re-suspended in 200 μl R2D2 rehydration buffer (5 m urea, 2 m thiourea, 2% 3-[(3-cholamidopropyl) dimethyl-ammonio]-1-propane-sulphonate, 2% N-decyl-N,N-dimethyl-3-ammonio-1-propane-sulphonate, 20 mm dithiothreitol, 5 mm Tris (2-carboxyethyl) phosphine and 0.5% IPG buffer, pH 4–7 (GE Healthcare, Saclay, France)). The total protein concentration was determined using the method of Bradford (1976) with bovine serum albumin as standard protein. Results are presented as means for the three leaf batches (triplicate) ± SE (n = 3).
Two-dimensional electrophoresis and image capture
The total protein extract was prepared in R2D2 rehydration buffer (125 μg in 330 μl) and was first separated according to charge in an electrofocusing PROTEAN IEF system (Bio-Rad, Marne la Coquette, France), at 20 °C, using 18-cm gel strips forming an immobilised linear pH gradient from 4 to 7 (GE Healthcare, Rouen, France). Each strip was rehydrated at 50 μA·gel−1 for 14 h. Isoelectric focusing ran for 15 min at 250 V, 2 h at 500 V, and then at 10,000 V for the time required to achieve 50 kV. After electrofocusing, the strips were immediately equilibrated in the equilibration buffer (75 mm Tris-HCl, 3% (w/v) SDS, 300 mm Tris base) containing DTT (65 mm), followed by a second incubation in equilibration buffer containing iodoacetamide (50 mm) and bromophenol blue (0.5%). SDS-PAGE was carried out on 12% polyacrylamide (w/v) gels (20 cm × 20 cm) using an Investigator system (Millipore, Saint-Quentin Fallavier, France) at 300 mV. Gels were stained using the silver staining procedure described in Desclos et al. (2009). Gels were scanned with the ProXPRESS 2-D proteomic imaging system (Perkin Elmer, Courtaboeuf, France) before image analysis.
Image analysis of 2-DE
After image acquisition, 2-DE gels were analysed using Progenesis SameSpots Software version 3.0 (Nonlinear Dynamics, Newcastle-upon-Tyne, UK) according the manufacturer's protocol. Gels from three independent biological replicates were used. A 2-DE gel that was the most representative of the three replicates was chosen as reference gel, and spots of others gels were then aligned with it. Spot detection, warping and matching were performed automatically with the software. Matching was automatic but verified manually; artefacts as well as spots that could not be confidently verified as true matches were disregarded rather than manually edited, and misalignments were corrected by manual warping when appropriate. Molecular weight and isoelectric point (pI) were calculated using Samespots software (Nonlinear Dynamics) calibrated with commercial molecular mass standards (precision protein standards unstained; Bio-Rad) run in a separate marker lane on the 2-DE gel. The relative abundance of each protein is expressed as a percentage and was calculated as follows: volume of the single spot/total volume of all spots. An arbitrarily relative abundance threshold of 0.4 was chosen to perform sequencing and identification of the 30 most representative residual proteins for each tissue. Taken together, the total abundance of the 30 selected representative proteins comprised 28.27 ± 1.56% and 37.00 ± 3.20% of total residual proteins in blades and petioles, respectively.
Protein identification with liquid chromatography-electrospray ionization-tandem mass spectrometry (ESI-LC MS/MS)
Excised spots were washed several times with water and dried for a few minutes. Trypsin digestion was performed overnight with a dedicated automated system (MultiPROBE II; PerkinElmer). The gel fragments were subsequently incubated twice for 15 min in a H2O/CH3CN solution to allow extraction of peptides from the gel pieces. Peptide extracts were then dried and dissolved in starting buffer for chromatographic elution, consisting of 3% CH3CN and 0.1% HCOOH in water. Peptides were enriched and separated using Lab-on-a-Chip technology (Agilent, Massy, France) and fragmented using an on-line XCT mass spectrometer (Agilent). The fragmentation data were interpreted using the DataAnalysis program (version 3.4; Bruker Daltonic, Billerica, MA, USA). For protein identification, tandem mass spectrometry peak lists were extracted and compared with the protein database using the MASCOT Daemon (version 2.1.3; Matrix Science, London, UK) search engine. The searches were performed with a maximum of one missed cleavage for the trypsin and with no fixed modification, but variable modifications for oxidation of methionine and carbamidomethylation and carboxymethylation of cysteines. Tandem mass spectrometry spectra were searched with a mass tolerance of 1.6 Da for precursor ions and 0.8 for MS/MS fragments.
The ESI-LC MS/MS data were converted into MGF (Mascot Generic Format) files that were searched further for proteins with the MASCOT Daemon software. Only peptides matching an individual ion score >53 were considered. Proteins with two or more unique peptides matching the protein sequence were automatically considered as a positive identification. Among the positive matches based on one unique peptide, the fragmentation spectrum from each peptide was manually interpreted using the conventional fragmentation rules. In particular, we looked for a succession of at least five y- and/or b-ions, specific immonium ions, specific fragment ions (proline and glycine), and signatures of any modifications carried by the peptides. Measured peptides were searched in the NCBInr protein sequence database viridiplantae.
Results
Senescence parameters of fallen B. napus leaves
All measurements in this study were made on samples collected from B. napus leaf rank #4. The study began 14 days after emergence of leaf rank #4, corresponding to 180°Cd (thermal time, base 5). In order to monitor leaf senescence progression of this leaf rank, kinetic evolution of chlorophyll content, which is a well-established plant senescence marker (Masclaux et al. 2000; He et al. 2005), was established using a chlorophyll SPAD meter (Fig. 1A). Leaf chlorophyll content measurements showed that the level in leaf #4 did not change during the progression from 180°Cd to 230°Cd, which corresponds to the mature stage. Chlorophyll content declined significantly from 230°Cd to 523°Cd, corresponding to leaf senescence progression (Fig. 1A). At 523°Cd, leaf rank #4 had fallen (abscission) and the level of chlorophyll was close to 0, resulting in complete chlorophyll degradation associated with leaf senescence (Fig. 1A). From this experiment it can be concluded that the lifespan of leaf rank #4 was 523°Cd. After abscission (523°Cd), petioles (and midrib) and blades were separated. Total N and amounts of residual protein were determinate in both (Fig. 1B). The residual amounts of total N were 2.78 ± 0.38 mg and 1.63 ± 0.06 mg in blades and the vascular system, respectively. The amounts of residual protein were 0.31 ± 0.06 mg·leaf−1 in blades and 0.10 ± 0.02 mg·leaf−1 in petioles (Fig. 1B). These results show incomplete N recycling in both tissues from leaf rank #4 during leaf senescence. As suggested by the amount of residual protein (Fig. 1B), this incomplete N recycling in leaf #4 may be explained by incomplete degradation of some leaf proteins. In order to identify these residual proteins in blades and petioles, proteomic analyses of both organs from fallen leaf #4 were performed.
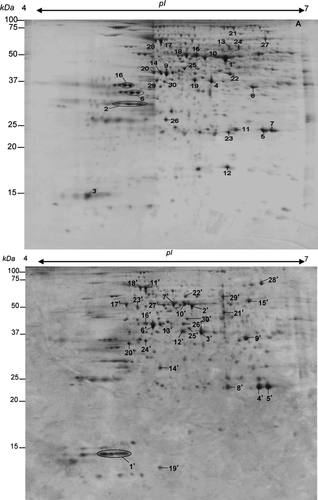
Residual proteins in blades of fallen leaves from B. napus
The 2-DE gels from blade protein extracts were imaged and analysed using SameSpots Software version 3.0 (Nonlinear Dynamics). A total of 500 proteins were reproducibly detected on 2-DE gels of blades from fallen leaf rank #4. For protein analysis, the volumes for each spot obtained from three biological analyses were averaged. This work focused on the 30 most abundant residual proteins in blades of fallen leaf #4 (Fig. 2A). The relative abundance of these 30 proteins, which was close to 28% of the total blade proteins, confirmed that they correspond to major proteins in fallen leaves. Their identification was performed using an ESI-LC MS/MS strategy with criteria described in Material and Methods. Thirty proteins were successfully sequenced and identified, except for spot 26, which matched an unnamed protein from Vitis vinifera. Among them, aspartic proteases (Table 1; spots 2 and 3) and BnSAG12 cysteine protease (Table 1; spots 5 and 7), two of the most abundant residual proteins with a total relative abundance close to 3.84 and 3.58, respectively, were identified in a previous study of the B. napus senescing leaf proteome (Desclos et al. 2009). Among these data, some spots were designated with the same protein name and accession number (Table 1). This was the case for spots 5 and 7 (both corresponding to Senescence-associate 12, SAG12), but also for spots 6 and 16 (DREPP plasma membrane polypeptide family protein), spots 15 and 18 (mitochondrial F1 ATP synthase beta subunit), spots 14 and 20 (phosphoglycerate kinase) and spots 13 and 24 (myrosinase). These similar residual proteins were located at different positions on the gel with different molecular weight, pI or both, suggesting that they might be different isoforms and/or partial degradation products of these proteins.
| spot no. | relative abundance (%) | exp. pI/Mw | theo. pI/Mw | PMa | SCb (%) | protein name/organism /NCBI accession no. | functional classification/subcellular localisation |
|---|---|---|---|---|---|---|---|
| 1 | 2.68 ± 0.09 | 6.0/56.1 | 5.9/52.9 | 12 | 29 | RubisCO large subunit /Brassica oleracea / gi|1346967 | 02. Energy / C |
| 2 | 2.27 ± 0.05 | 5.0/34.5 | 5.1/54.2 | 3 | 17 | Aspartic protease / Brassica napus / gi|1326165 | 06. Protein Destination & Storage / V |
| 3 | 1.57 ± 0.07 | 4.6/12.7 | 4.4/9.5 | 3 | 47 | Aspartic protease / Brassica oleracea / gi|872030 | 06. Protein Destination & Storage / C,V |
| 4 | 1.50 ± 0.09 | 5.9/44.0 | 5.6/37.7 | 6 | 34 | Nitrilase 1 /Brassica rapa / gi|121550795 | 11. Disease/Defence / C |
| 5 | 1.78 ± 0.04 | 6.4/25.9 | 7.0/38.3 | 3 | 13 | Senescence-specific cysteine protease BnSAG12 /Brassica napus / gi|5823018 | 06. Protein Destination & Storage / V |
| 6 | 1.27 ± 0.10 | 5.0/39.0 | 5.0/24.6 | 3 | 12 | DREPP plasma membrane polypeptide family protein / Arabidopsis thaliana / gi|15235363 | 11. Disease-Defence / Ch, V |
| 7 | 1.80 ± 0.05 | 6.5/25.8 | 7.0/38.3 | 3 | 12 | Senescence-specific cysteine protease BnSAG12 /Brassica napus / gi|5823018 | 06. Protein Destination & Storage / V |
| 8 | 1.32 ± 0.02 | 6.3/41.5 | 6.1/35.5 | 7 | 34 | Malate dehydrogenase putative / Arabidopsis thaliana / gi|15219721 | 02. Energy / C, M |
| 9 | 1.05 ± 0.05 | 5.4/47.8 | 5.2/53.6 | 14 | 41 | ATP synthase subunit beta (ATPase subunit beta) / Brassica napus / gi|75336517 | 02. Energy / Ch, M |
| 10 | 0.92 ± 0.04 | 5.8/57.2 | 5.5/47.4 | 13 | 42 | Enolase / Brassica rapa / gi|34597330 | 02. Energy / C, Ch |
| 11 | 0.82 ± 0.07 | 6.1/25.9 | 5.2/27.1 | 5 | 24 | Triose phosphate isomerase / Arabidopsis thaliana / gi|414550 | 02. Energy / C, Ch |
| 12 | 0.79 ± 0.03 | 6.0/17.3 | 8.9/28.2 | 5 | 28 | Peptidylprolyl isomerase /Arabidopsis thaliana / gi|11762200 | 06. Protein Destination & Storage / C, Ch, M |
| 13 | 0.78 ± 0.05 | 6.1/63.4 | 6.3/62.2 | 6 | 15 | Myrosinase, thioglucoside glucohydrolase /Brassica napus / gi|414103 | 20. Defence & Secondary metabolism / V |
| 14 | 0.84 ± 0.11 | 5.3/48.0 | 5.9/50.1 | 14 | 43 | Phosphoglycerate kinase / Arabidopsis thaliana / gi|15230595 | 02. Energy /C, Ch |
| 15 | 0.71 ± 0.02 | 5.7/58.7 | 6.5/63.3 | 18 | 49 | Mitochondrial F1 ATP synthase beta subunit / Arabidopsis thaliana / gi|17939849 | 02. Energy / M, Ch |
| 16 | 1.30 ± 0.09 | 5.0/42.2 | 5.0/24.6 | 1 | 5 | DREPP plasma membrane polypeptide family protein / Arabidopsis thaliana / gi|15235363 | 11. Disease & Defence / PM |
| 17 | 0.68 ± 0.08 | 5.3/68.7 | 5.1/68.8 | 24 | 55 | VHA-A (Vacuolar ATP synthase subunit A) / Arabidopsis thaliana / gi|15219234 | 02. Energy / V |
| 18 | 0.85 ± 0.01 | 5.6/60.9 | 6.5/63.3 | 20 | 54 | Mitochondrial F1 ATP synthase beta subunit / Arabidopsis thaliana / gi|17939849 | 02. Energy / Ch, M |
| 19 | 0.65 ± 0.07 | 5.7/45.0 | 5.5/42.1 | 8 | 33 | Phosphoglycerate kinase PGK / Arabidopsis thaliana / gi|15219412 | 02. Energy / C, Ch |
| 20 | 0.62 ± 0.01 | 5.3/48.8 | 5.9/50.1 | 7 | 20 | Phosphoglycerate kinase / Arabidopsis thaliana / gi|15230595 | 02. Energy / C, Ch |
| 21 | 0.51 ± 0.05 | 6.1/80.2 | 5.8/98.1 | 13 | 15 | Aconitate hydratase / Arabidopsis thaliana / gi|4586021 | 02. Energy / M |
| 22 | 0.50 ± 0.03 | 6.1/47.8 | 5.6/42.8 | 11 | 33 | Glyceraldehyde-3-phosphate dehydrogenase B subunit / Arabidopsis thaliana / gi|336390 | 02. Energy / C, Ch |
| 23 | 0.48 ± 0.05 | 6.0/25.4 | 5.5/29.4 | 8 | 40 | Carbonic anhydrase 1 (CA1) / Arabidopsis thaliana / gi|30678347 | 02. Energy / Ch |
| 24 | 0.48 ± 0.01 | 6.2/63.4 | 6.3/62.2 | 11 | 24 | Myrosinase, thioglucoside glucohydrolase / Brassica napus / gi|414103 | 20. Defence & Secondary metabolism / V |
| 25 | 0.45 ± 0.07 | 5.6/50.8 | 5.8/51.6 | 4 | 11 | Translation elongation factor Tu / Arabidopsis thaliana / gi|15237059 | 05. Protein synthesis / Ch, M |
| 26 | 0.43 ± 0.03 | 5.4/29.2 | 5.6/24.7 | 2 | 6 | Unnamed protein product / Vitis vinifera / gi|15735370 | 13. Unclassified |
| 27 | 0.43 ± 0.03 | 6.4/70.4 | 6.0/116.6 | 4 | 5 | Alpha-mannosidase / Arabidopsis thaliana / gi|10177664 | 06. Protein Destination & Storage / V |
| 28 | 0.42 ± 0.01 | 5.3/69.3 | 5.2/68.7 | 15 | 34 | V-type proton ATPase catalytic subunit A / Brassica napus / gi|2493122 | 02. Energy / V |
| 29 | 0.41 ± 0.06 | 6.4/50.9 | 5.7/44.4 | 6 | 15 | Phosphoribulokinase PRK / Arabidopsis thaliana / gi|15222551 | 02. Energy / Ch |
| 30 | 0.40 ± 0.08 | 5.3/41.8 | 5.7/39.1 | 7 | 26 | Pyruvate dehydrogenase (acetyl-transferring) / Arabidopsis thaliana / gi|15241286 | 02. Energy / M |
- For each protein, value is mean ± SE (n = 3). The subcellular localisation of each protein was performed by compiling data from different databases i.e. Pubmed (www.ncbi.nlm.nih.gov/pubmed), Information Hyperlink Over Proteins (IHOP: http://www.ihop-net.org/UniPub/iHOP/) and Wolf PSORT online software (http://wolfpsort.org). C: Cytosol, Ch: Chloroplast (or Plastid), M: Mitochondrion, PM: Plasma membrane, V: Vacuole. a, Number of peptides matched; b, Percentage of sequence coverage.
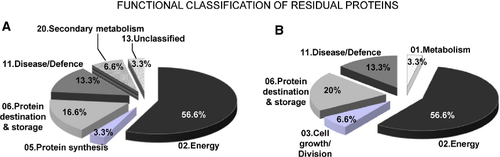
Thirty proteins were classified into functional classes according to the scheme of Bevan et al. (1998) and the KEGG PATHWAY Database (www.genome.ad.jp/kegg/pathway/html). Functional classification (Fig. 3A) showed that residual blade proteins belonged to different categories: 02. Energy (56.6%), 06. Protein destination and storage (16.6%), 11. Disease/Defence (13.3%), 20. Defence & Secondary metabolism (6.6%), 05. Protein synthesis (3%) and 13. Unclassified (3.3%). The largest functional category included 17 proteins involved in energy metabolism. Thus, among 17 proteins belonging to this 02. Energy functional class, seven proteins (spots 10, 11, 14, 19, 20, 22 and 30) are involved in glycolysis and gluconeogenesis, three proteins (spots 9, 15 and 18) belong to the electron transport chain, three (spots 1, 23 and 29) are involved in photosynthesis, two (spots 8 and 21) correspond to enzymes of the TCA cycle and two (spots 18 and 28) are involved in the H+-vacuolar gradient (Table 1). From these data, it is noteworthy that the RubisCO large subunit (spot 1) involved in photosynthesis was the most abundant protein in fallen B. napus leaves. The second largest functional category, Protein destination and storage, was represented as protein hydrolytic activity, such as senescence-specific cysteine protease (SAG12), two different aspartic proteases and an alpha-mannosidase involved in degradation of glycoproteins (Table 1).
Residual proteins in petioles of fallen leaves from B. napus
Petiole proteome analysis was performed using the same experimental approach as the blade proteome. The petiole residual proteome comprised a total of 234 proteins. The identity of the 30 most abundant residual proteins and an annotated reference map are provided in Table 2 and Fig. 2B. The relative abundance of these 30 proteins was close to 37% of the total proteins of the B. napus petiole. As previously observed in the blade proteome, some identified proteins (spots 4' and 5'; spots 11' and 14') were designated with the same name and accession number and possibly correspond to different isoforms and/or products of degradation. Using functional classification previously described in Bevan et al. (1998), residual of petiole proteins were classified as follows (Fig. 3B): 02. Energy (56.6%), 06. Protein destination and storage (20.0%), 11. Disease/Defence (13.3%), 03. Cell growth/Division (6.6%) and 01. Metabolism (3.3%) (Fig. 3B). As previously observed for the blade, the major part of the residual proteins of the petiole (i.e. 17/30 proteins) belong to the 02. Energy functional class. Among them, seven (spots 2', 8', 12', 14', 16', 24' and 27') are involved in glycolysis and gluconeogenesis, four (spots 7', 15', 17' and 23') belong to the electron transport chain, three (spots 10', 11'and 18') are involved in the H+-vacuolar gradient and three (spots 9', 25' and 26') are enzymes of the TCA cycle.
| spot no. | relative abundance | exp. pI / Mw | theo. pI / Mw | PMa | SCb (%) | protein name / organism /NCBI accession no. | functional classification / subcellular localisation |
|---|---|---|---|---|---|---|---|
| 1' | 4.45 ± 0.38 | 4.6/11.3 | 5.1/20.1 | 2 | 9 | Lipid-associated family protein / Arabidopsis thaliana / gi|18399899 | 11. Disease/Defence / Ch |
| 2' | 2.95 ± 0.13 | 5.6/59.7 | 5.5/47.3 | 12 | 43 | Enolase / Brassica rapa / gi|34597330 | 02. Energy / C |
| 3' | 2.74 ± 0.25 | 5.7/44.9 | 5.6/38.4 | 8 | 34 | Nitrilase-like protein / Brassica napus /gi|14211396 | 11. Disease & Defence / C |
| 4' | 2.56 ± 0.09 | 6.4/24.2 | 7.0/38.3 | 2 | 10 | Senescence-specific cysteine protease BnSAG12/ Brassica napus / gi|5823018 | 06. Protein Destination & Storage / V |
| 5' | 2.40 ± 0.15 | 6.6/26.6 | 7.0/38.3 | 3 | 12 | Senescence-specific cysteine protease Bn SAG12 / Brassica napus / gi|5823018 | 06. Protein Destination & Storage / V |
| 6' | 2.14 ± 0.19 | 5.2/47.8 | 5.3/41.6 | 13 | 50 | Actin / Brassica napus / gi|4139264 | 03. Cell Growth/Division / C |
| 7' | 1.44 ± 0.06 | 5.4/61.7 | 6.5/63.3 | 21 | 50 | Mitochondrial F1 ATP synthase beta subunit / Arabidopsis thaliana / gi|17939849 | 02. Energy / Ch, M |
| 8' | 1.43 ± 0.12 | 6.1/24.0 | 5.2/27.1 | 6 | 38 | Triose phosphate isomerase / Arabidopsis thaliana / gi|414550 | 02. Energy / C, Ch |
| 9' | 1.39 ± 0.08 | 6.3/43.5 | 6.1/35.5 | 8 | 34 | Malate dehydrogenase putative / Arabidopsis thaliana / gi|15219721 | 02. Energy / C, M |
| 10' | 1.20 ± 0.10 | 5.6/61.7 | 6.2/59.7 | 22 | 54 | ATP synthase beta chain 2 / Arabidopsis thaliana / gi|18415911 | 02. Energy / Ch, M |
| 11' | 1.18 ± 0.04 | 5.1/73.2 | 5.2/68.7 | 20 | 45 | V-type proton ATPase catalytic subunit A / Brassica napus / gi|2493122 | 02. Energy / T |
| 12' | 1.15 ± 0.11 | 5.5/45.1 | 5.5/42.1 | 10 | 37 | Phosphoglycerate kinase, putative / Arabidopsis thaliana / gi|21536853 | 02. Energy / C, Ch, M |
| 13' | 1.11 ± 0.09 | 5.3/48.7 | 5.4/41.8 | 8 | 45 | Actin 8 / Arabidopsis thaliana / gi|1669389 | 03. Cell Growth/Division / Cytosol |
| 14' | 0.97 ± 0.02 | 5.3/30.0 | 5.2/27.1 | 6 | 38 | Triose phosphate isomerase / Arabidopsis thaliana / gi|414550 | 02. Energy / C, Ch |
| 15' | 0.90 ± 0.04 | 6.4/64.0 | 6.0/55.1 | 15 | 36 | ATPase subunit 1 / Brassica napus / gi|112253900 | 02. Energy / Ch, M |
| 16' | 0.80 ± 0.06 | 5.1/49.3 | 5.9/50.1 | 10 | 30 | Phosphoglycerate kinase / Arabidopsis thaliana / gi|15230595 | 02. Energy / C, Ch, M |
| 17' | 0.78 ± 0.05 | 4.9/61.3 | 4.9/54.7 | 10 | 29 | Nucleotide-binding subunit of vacuolar ATPase / Arabidopsis thaliana / gi|166627 | 02. Energy / T |
| 18' | 0.77 ± 0.06 | 5.0/74.1 | 5.4/68.9 | 9 | 18 | Vacuolar H+-ATPase / Malus x domestica / gi|131573315 | 02. Energy / T |
| 19' | 0.71 ± 0.02 | 5.3/9.8 | 4.9/12.9 | 1 | 11 | CCH copper chaperone / Arabidopsis thaliana / gi|15228869 | 06. Protein Destination & Storage / C |
| 20' | 0.72 ± 0.05 | 4.9/40.7 | 5.1/40.8 | 5 | 20 | Anthocyanidin synthase /Brassica oleracea / gi|29423729 | 11. Disease/Defence / C |
| 21' | 0.67 ± 0.05 | 6.0/56.1 | 7.6/52.5 | 14 | 46 | Monodehydroascorbate reductase / Brassica oleracea / gi|46093473 | 11. Disease & Defence / C, Ch, M, P |
| 22' | 0.63 ± 0.04 | 5.6/67.0 | 6.3/81.8 | 1 | 1 | Subtilase family protein / Arabidopsis thaliana / gi|22331076 | 06. Protein Destination & Storage /V |
| 23' | 0.63 ± 0.05 | 5.0/58.5 | 5.1/53.7 | 12 | 34 | ATP synthase CF1 beta subunit / Brassica napus / gi|262400757 | 02. Energy / Ch, M |
| 24' | 0.59 ± 0.02 | 5.1/40.7 | 5.7/39.1 | 5 | 20 | Pyruvate dehydrogenase (acetyl-transferring) /Arabidopsis thaliana / gi|15241286 | 02. Energy / M |
| 25' | 0.59 ± 0.04 | 5.6/46.7 | 6.3/45.3 | 9 | 24 | Succinyl-CoA ligase beta-chain / Arabidopsis thaliana / gi|15225353 | 02. Energy / M |
| 26' | 0.58 ± 0.05 | 5.5/49.0 | 5.8/47.0 | 3 | 10 | Putative dehydrogenase / Solanum lycopersicum / gi|24850453 | 02. Energy / M |
| 27' | 0.57 ± 0.03 | 5.3/60.7 | 5.8/47.3 | 8 | 26 | Enolase / Brassica napus / gi|34597332 | 02. Energy / C, Ch |
| 28' | 0.55 ± 0.04 | 6.5/78.3 | 6.0/116.5 | 3 | 4 | Alpha-mannosidase / Arabidopsis thaliana / gi|10177664 | 06. Protein Destination & Storage / V |
| 29' | 0.42 ± 0.03 | 6.0/69.4 | 6.3/59.1 | 3 | 7 | Mitochondrial processing peptidase beta subunit / Arabidopsis thaliana / gi|15232845 | 06. Protein Destination & Storage / M |
| 30' | 0.40 ± 0.04 | 5.6/51.7 | 5.4/42.8 | 6 | 19 | S-Adenosylmethionine synthetase 2 / Brassica juncea / gi|75309777 | 01. Metabolism / C |
- For each protein, value is mean ± SE (n = 3). The subcellular localisation of each protein was performed by compiling data from different databases i.e. Pubmed (www.ncbi.nlm.nih.gov/pubmed), Information Hyperlink Over Proteins (IHOP: http://www.ihop-net.org/UniPub/iHOP/) and Wolf PSORT online software (http://wolfpsort.org). C, Cytosol; Ch, Chloroplast (or Plastid); M, Mitochondrion; P, Peroxisome; V, Vacuole. a, Number of peptides matched; b, Percentage of sequence coverage.
Comparison of residual proteins in blades and petioles
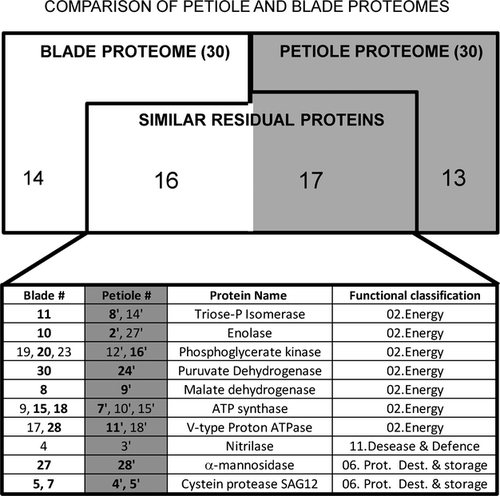
While the functional classification of residual proteins is very similar for blades and petioles (Fig. 3A and B), an individual comparison of residual proteins identified for both organs was performed (Fig. 4). Two residual proteins are considered as common to both organs if they are strictly identical (i.e. with the same accession number, in bold in the Table of Fig. 4) but also if they correspond to an isoform harbouring the similar metabolic function. As an example, the same triose phosphate isomerase (identical accession number) was identified in both organs (spot 11 and 14', respectively) and another isoform of this enzyme has been also identified in the petiole proteome (spot 14'). From this comparison, nine residual proteins were identified as strictly identical in the proteome of both organs: triose phosphate isomerase (spots 11 and 14'), enolase (spots 10 and 2'), phosphoglycerate kinase (spots 20 and 16'), pyruvate dehydrogenase (spots 30 and 24'), malate dehydrogenase (spots 8 and 9'), ATP synthase (spots 15, 18 and 7'), V-type H+ ATPase (spots 28 and 11'), α-mannisodase (spots 27 and 28') and cysteine protease SAG12 (spot 5, 7, 4' and 5'). Otherwise, common residuals proteins (i.e. seven for blades and eight for petioles) corresponding to different isoforms of proteins identified from blade or petiole proteomes: triose phosphate isomerase (spot 14'), enolase (spot 27'), phosphoglycerate kinase (spots 19, 23 and 12'), ATP synthase (spots 9, 10' and 15'), V-type H+ATPase (spot 17 and 18') and nitrilase (spots 4 and 3'). It should be emphasised that these common residual proteins (strictly identical and isoforms) belong to only three functional classes, i.e. 02. Energy, 06. Protein destination & storage and 11. Disease/Defence.
Discussion
Brassica napus is an important crop plant, characterised by a high N level in fallen leaves, which leads to a significant return of this element to the soil, with important environmental and economic consequences (Malagoli et al. 2005a). It is now well established that this residual N in fallen leaves is due to weak N remobilisation, which is essentially related to incomplete degradation of foliar proteins during leaf senescence. The N remobilised during protein degradation in the oldest leaves can be translocated to young leaves or storage tissue (Feller & Fischer 1994; Rossato et al. 2002). As a consequence, the amount of protein in mature B. napus leaves declines progressively during the final steps of development due to massive protein degradation by proteases associated with senescence (Gombert et al. 2006; Etienne et al. 2007). Indeed, considering that N and soluble protein amounts in the B. napus mature leaf can reach 40–45 mg·leaf−1 (Etienne et al. 2007), results presented in Fig. 1B confirm that these compounds are strongly degraded during senescence of leaf rank #4. Nevertheless, the presence of residual N and proteins in fallen leaves suggests that some proteins are difficult to degrade during senescence. This hypothesis is reinforced by previous works showing that not all foliar proteins degrade at the same rate as the majority of proteins during N remobilisation in B. napus leaves (Desclos et al. 2009). Thus, it is widely accepted that these poorly degraded proteins represent the major pool of inorganic N that is returned to the soil, and therefore constitute a potential lever to improve the efficiency of nutrient use in this crop (Desclos et al. 2009).
The aim of this study was to improve our knowledge of this residual pool of proteins through proteomic analysis of the petioles and blades from fallen leaves of B. napus. Up to now, no studies have focused on the identification of residual proteins that remain during the last step of leaf N remobilisation. This study focused on the final step of development of mature leaves of B. napus (leaf rank #4). First, as presented in Fig. 1A, the decrease in chlorophyll content begins after 250 °Cd and continues to 0 ± 1.2 until abscission of the leaf (523°Cd). These results are consistent with previous studies indicating that chloroplasts are among the first cellular organelles that become disorganised during leaf senescence, while the mitochondria and nuclei remain intact for longer in order to maintain energy metabolism and senescence-associated gene expression (Lim et al. 2007). Moreover, this chloroplast breakdown is important for N recycling because around 75% of leaf N is located in these organelles (Hörstensteiner & Feller 2002), mostly in the form of the light-harvesting complex of photosystem II in the thylakoid membranes and RubisCO in the stroma. RubisCO is an abundant chloroplast protein whose degradation may explain the decrease in photosynthetic capacity of cells that is associated with leaf senescence (Hörstensteiner & Feller 2002). However, other works have indicated that the amount of RubisCO exceeds by far the requirements for photosynthesis, and postulate that it also serves as the main N reserve in vegetative tissues (Roberts et al. 2012). Second, according to these studies, a proteomic analysis of blades and petioles from fallen leaves of B. napus was performed in order to (i) verify whether both functional and storage RubisCO were completely degraded during senescence, and (ii) identify other major residual proteins in these both organs with different physiological roles in the non-senescent leaf.
Thus, in each tissue (blade and petiole), the 30 most abundant proteins, representing a total relative abundance around 30% of residual proteins, were identified. Surprisingly, our data reveal that RubisCO is the most representative residual protein, with a relative abundance of about 2.68% in blades (Table 1; spot 1). This reveals that this major plastidial protein is largely but not completely degraded during leaf senescence, which could be explained by the high abundance of RubisCO, representing 50% of the total soluble proteins in leaves (Mae et al. 1983). Moreover, according to recent studies, this poor degradation of chloroplastic proteins could be explained by the need to induce specific proteolytic systems at different stages of leaf senescence. Indeed, studies of B. napus and other plants have shown that degradation of some chloroplastic proteins requires FtsH proteases, which are induced relatively late in senescence (Desclos et al. 2009; Roberts et al. 2012). In addition, the degradation of RubisCO requires plastidial aspartic proteases, such as CND41 (Kato et al. 2004), but also the formation of small vacuoles involved in autophagy (Ishida & Yoshimoto 2008). Thus, this specific autophagy pathway involved in the degradation of stromal proteins, such as RubisCO, requires that the central vacuole contain serine, aspartic and cysteine proteases. The transfer of plastid proteins (such as RubisCO) from chloroplasts to the central vacuole is possible through the formation of Senescence-Associated Vesicles (SAVs) and/or RubisCO-Containing Bodies (RBCs), i.e. autophagosomes developed from the chloroplast envelope (Ishida & Yoshimoto 2008; Merkulova et al. 2014). Previous reports have indicated that SAVs are small vacuoles containing stromal proteins (e.g. RubisCO and glutamine synthetase 2) and harbouring a high level of protease activity, especially, SAG12 (Otegui et al. 2005; Martínez et al. 2008), a papain-like cysteine protease strongly induced during leaf senescence (Buchanan-Wollaston & Ainsworth 1997; Gan & Amasino 1997). In the present study, BnSAG12 senescence-associated cysteine protease (spots 5, 7, 4' and 5'), two aspartic proteases with vacuolar localisation (spots 2 and 3) and an alpha-mannosidase (spots 27 and 28'), which is a vacuolar enzyme involved in degradation of glycoproteins, were identified. In addition, a vacuolar pH of about 5 is necessary in plants to promote breakdown of macromolecules by different acid hydrolases. A previous work found that acidification of these cell compartments requires Vacuolar proton-translocating ATPases (V-ATPases), which are especially efficient under glucose deprivation (Diakov & Kane 2010). In our study, some V-ATPases were identified in both tissues (spots 9, 17 and 28 in blades and 11' and 18' in petioles). Thus, in response to carbohydrate starvation that occurs during senescence (Smart 2007; Hörstensteiner & Feller 2002), V-ATPases might take part in the acidification of lytic vacuoles. All these data are in agreement with previous work indicating that stromal proteins are preferentially degraded via the trafficking of SAVs (or RCBs), which is associated with autophagy. Because V-ATPases and vacuolar hydrolases (such as BnSAG12, aspartic proteases and alpha-mannosidase) were found in the proteome of fallen leaves, this autophagy process (known to begin at the early stage of natural leaf senescence; Guiboileau et al. 2012) could remain active up to the final stage of senescence.
Using functional classification previously described in Bevan et al. (1998), this study shows a high proportion (57%) of residual proteins in petioles and blades known to be involved in energy metabolism (Fig. 3A and B). Among them, some enzymes involved in the glycolysis pathway and the TCA cycl,e such as triose-phosphate isomerase (spots 11, 8' and 14'), glyceraldehyde-3-P dehydrogenase (spot 22), phosphoglycerate kinase (spots 14, 19, 20 and 12'), enolase (spots 10, 2' and 27'), pyruvate dehydrogenase (spots 30 and 24'), malate dehydrogenase (spots 8 and 9'), succinyl-Coenzyme A dehydrogenase (spot 25') and aconitate hydratase (spot 21) were identified. Moreover, pyruvate dehydrogenase, an enzyme linking glycolysis and the TCA cycle (spots 29 and 24'), and some subunits of mitochondrial F1-ATP synthases (spots 9, 15, 18, 10', 23') involved in the production of ATP, were also identified in both tissues. All these data are consistent with (i) previous proteomic studies of leaf senescence showing that the energy-related proteins have a lower rate of degradation than the majority of soluble proteins (Desclos et al. 2009), and (ii) studies showing that mitochondria remain intact up to late time points in senescence in order to produce the energy (ATP) required for catabolism of macromolecules (Lim et al. 2007).
In addition, in both tissues, around 13% of major residual proteins were classified in the Disease/Defence and Secondary metabolism functional classes (Fig. 3A and B). Among them, two nitrilases (spots 4 and 3') were identified in both tissues from fallen B. napus leaves. This is in agreement with a study in Arabidospis by Piotrowski et al. (2001), which showed that nitrilase activity (especially NIT4 isoform) is up-regulated in senescing leaves to facilitate detoxification of cyanides co-produced during ethylene synthesis (Howden & Preston 2009). Among other proteins classified in Defence or Secondary metabolism, some, such as monodehydroascorbate reductase (spot 21'), peptidylprolyl isomerase (spot 12) and anthocyanidin synthase (spot 20'), are known to be involved directly or indirectly in protection against oxidative stress. In addition, the CCH copper chaperone belonging to 06. Protein destination and storage is involved in mobilisation of copper from metalloprotein degradation, and is also involved in protection against oxidative stress associated with defence mechanisms in Arabidopsis, tomato and poplar (Himelblau et al. 1998; Mira et al. 2002; Lee et al. 2005). The presence of these proteins is in agreement with the oxidative burst from chloroplasts, mitochondria and peroxisomes, which occurs during leaf senescence (for review: Zentgraf & Hemleben 2008). Thus, reactive oxygen species (ROS) are considered as essential signals to promote senescence by inducing senescence-associated genes and protein degradation (Niewiadomska et al. 2009). However, some enzymes, e.g. glyceraldehyde-3-P dehydrogenase and phosphoglycerate kinase involved in energy production, are highly sensitive to ROS and require oxidative protection systems to ensure their enzyme functioning in the cell (Marri et al. 2014). All these results are consistent with studies showing that during leaf senescence progression, plants develop a complex network to manage ROS (including ROS-scavenging and ROS-producing proteins) to finely balance redox potential and ROS production (for review: Zentgraf & Hemleben 2008).
Finally, this study reveals that many residual proteins identified in blades and petioles are similar and/or belong to the same functional classes (Figs 3 and 4). Since blades and petioles have a different physiological role in the non-senescing leaf (photosynthesis and transport for blades and petioles, respectively), these results are surprising and underline that, during senescence, both organs redirect their metabolism to ensure essential energy production, proteolysis and protection against oxidative stress. Thus, some residual proteins identified in this present study might be essential to enable efficient senescence. In contrast, many proteins involved in photosynthesis, such as RubisCO (spot 1), carbonic hydratase CA1 (spot 23) or phosphoribulokinase (PRK, spot 29) that are not related to senescence, were nevertheless found in the residual proteome of blades. This is in agreement with a previous study showing that some chloroplast proteins, like PRK, are especially difficult to degrade during natural or induced senescence (Crafts-Brandner et al. 1996). However, PRK seems to be a particular case because previous studies have demonstrated that the amount of residual proteins in B. napus could be significantly reduced during senescence induced by abiotic stress such as N deprivation (Gombert et al. 2006; Etienne et al. 2007). Taken together, these data suggest that the amount of some residual proteins might be reduced substantially in fallen leaves of B. napus without affecting the natural senescence efficiency. Previously, using a modelling approach, Malagoli et al. (2005b) showed that an increase in leaf lifespan of B. napus would allow an improvement in NUE. From this study, perhaps a leaf with a longer lifespan could increase the period of mobilisation and reduce the amount of residual proteins in fallen leaves. Thus, it will be interesting to compare proteomic profiles of fallen leaves from B. napus genotypes with contrasted leaf lifespan in order to verify absence or reduced amounts of many (or all) residual proteins in leaves with a longer lifespan.
Conclusions
Using a proteomic approach, this study clearly reveals that some leaf proteins are not completely degraded during the process of senescence in B. napus. Analysis of residual proteins in the blades and petioles of fallen leaves showed that the major proteins are involved in energy metabolism, plant defence reactions (especially protection against oxidative stress) and proteolysis (belonging to 06. Protein destination & storage). To our knowledge, this study is the first to propose an inventory of major residual proteins that are difficult to degrade during leaf senescence in B. napus (e.g. proteins involved in energy production and catabolic reactions). Thus, it has provided interesting candidate proteins (e.g. proteases belonging to 06. Protein destination & storage functional class) for future experiments using transgenic/mutagenic approaches that could specify their role in the N remobilisation associated with leaf senescence. More surprisingly, this study highlights that RubisCO, a chloroplast protein usually considered a prime target during senescence, is the major residual protein, with high relative abundance especially in blades of fallen leaves. Considering that leaves are the major source of N and that the low NUE of B. napus must be explained by an accumulation of residual leaf proteins, it might be interesting to compare the residual amount of RubisCO in fallen leaves from different genotypes with contrasting NUE. Indeed, if a good correlation between NUE and the residual amount of RubisCO in B. napus fallen leaves could be established, the amount of residual RubisCO could become an indicator for identifying new B. napus genotypes with improved NUE.
Acknowledgements
The authors thank Dr P. Laîné for permission for M. Desclos-Theveniau to undertake this study. We also thank J. Bonnefoy and M.P. Bataillé for technical help in IRMS analysis. The authors also acknowledge Dr Laurence Cantrill for proofreading and English correction.