Fibrotic focus: An important parameter for accurate prediction of a high level of tumor-associated macrophage infiltration in invasive ductal carcinoma of the breast
Abstract
Our group and others have previously reported that a fibrotic focus is a very useful histological factor for the accurate prediction of the outcome of patients with invasive ductal carcinoma of the breast. We classified 258 cases of invasive ductal carcinoma into those with and those without a fibrotic focus to investigate whether the presence of a fibrotic focus was significantly associated with the degree of tumor-associated macrophage (CD68, CD163 or CD204-positive) infiltration or whether the presence of tumor-associated macrophage infiltration heightened the malignant potential of invasive ductal carcinoma with a fibrotic focus. Multiple regression analyses demonstrated that a fibrotic focus was the only factor that was significantly associated with a high level of CD68-, CD163- or CD204-positive tumor-associated macrophage infiltration. The combined assessment of the presence or absence of a fibrotic focus and a high or a low level of CD204-positive tumor-associated macrophage infiltration clearly demonstrated that CD204-positive tumor-associated macrophage infiltration had a significant prognostic power only for patients with invasive ductal carcinoma with a fibrotic focus in multivariate analyses; CD204-positive tumor-associated macrophages might only exert a significant effect on tumor progression when a fibrotic focus is present within the invasive ductal carcinoma of the breast.
Recently, the prognostic significance of tumor-associated macrophage infiltration for the accurate prediction of outcome has been reported for patients with carcinomas arising in several organs.1-3 Tumor-associated macrophages derived from circulating monocytes originating from the bone marrow or spleen are commonly recognized as the two ends of a spectrum of activation (M1 and M2) in tumors.4 In a tumor setting M1 tumor-associated macrophages are thought to promote anti-tumor immunity; they secrete high amounts of pro-inflammatory cytokines and promote TH1 cell responses.5 In contrast, M2 tumor-associated macrophages produce low amounts of pro-inflammatory cytokines and higher amounts of the anti-inflammatory cytokine interleukin-10; they stimulate angiogenesis and tissue repair in addition to suppressing cytotoxic T cell function and they are thought to promote tumor progression and to be associated with a poor prognosis. M2 macrophages have important functions in wound healing and angiogenesis, express high levels of immunosuppressive cytokines (such as interleukin-2, or interleukin-10), and also express scavenger receptors such as CD163 or CD204.6, 7 Recently, CD163 or CD204-positive tumor-associated macrophages have been reported to be correlated with tumor progression and poor patient outcome in patients with malignant tumors in several organs.8-10
Our group and others have previously reported that a fibrotic focus is a very useful histological predictor for the accurately prediction of the outcome of patients with invasive ductal carcinoma of the breast.11-20 The characteristics of tumor-stromal fibroblasts forming a fibrotic focus or a high tumor angiogenesis ratio have been suggested to heighten the malignant potential of invasive ductal carcinoma with a fibrotic focus13, 21, 22; other reports have clearly revealed that the presence of a fibrotic focus is closely associated with intratumoral hypoxia in invasive ductal carcinoma of the breast.13, 17, 23 Furthermore, a cDNA microarray analysis clearly demonstrated specific biological characteristics of invasive ductal carcinomas with a fibrotic focus.15
The purpose of the present study was to investigate whether tumor-associated macrophage infiltration plays an important role in heightening the malignant potential of invasive ductal carcinoma of the breast with a fibrotic focus.
MATERIALS AND METHODS
Patients and histological examinations
The subjects of this study were 258 consecutive patients with invasive ductal carcinoma of the breast who did not receive neoadjuvant therapy and were surgically treated at the Saitama Medical University International Medical Center between January 2007 and December 2009. All the patients were Japanese women, ranging in age from 29 to 92 years old (median, 55 years). All the invasive ductal carcinomas diagnoses were made preoperatively based on the results of a needle biopsy. A partial mastectomy had been performed in 189 patients, a modified radical mastectomy had been performed in 67, and a standard radical mastectomy had been performed in 2. Levels I, II, and ± III of axillary lymph node dissection had been performed in all the patients. Among the 258 patients, 243 patients had received adjuvant therapy (no adjuvant therapy, 11 patients; unknown, 4 patients). Among these patients, 122 received endocrine therapy, 36 received chemotherapy, 69 received chemoendocrine therapy and 16 received trastuzumab with an endocrine therapy regimen and a chemotherapy regimen. All the tumors were classified according to the pathological UICC-TNM (pTNM) classification.24 The protocol (14–031) for this study was reviewed by the institutional review board of the Saitama Medical University International Medical Center.
For the pathological examination, the surgically resected specimens were fixed in 10% formalin. Well-known clinicopathological factors and the presence of a fibrotic focus were evaluated (Table 1). Patients were grouped according to age (≤39 years and >39 years).25-27 Briefly, the fibrotic foci were surrounded by a highly cellular zone of infiltrating ductal carcinoma cells and occupied variable percentages of the tumor area (Fig. 1a,b).11, 12 The foci were located at almost the exact center of the tumor and consisted of fibrous bands expanding radially into the surrounding area, giving them a scar-like appearance.
| No. of patients (%) | |||||
|---|---|---|---|---|---|
| Tumor recurrence | Tumor-related death | ||||
| Present | Present | ||||
| Cases | 22 (9) | HR; 95% CI P-value | 9 (4) | HR; 95% CI P-value | |
| Adjuvant therapy | 254 | ||||
| None | 11 | 3 (27) | 1.0 | 2 (18) | 1.0 |
| Yes | 243 | 19 (8) | 0.2; 0.06–0.7 | 7 (3) | 0.1; 0.03–0.6 |
| 0.010 | 0.010 | ||||
| Age (years) | 258 | ||||
| ≤39 | 17 | 3 (18) | 1.0 | 1 (6) | 1.0 |
| >39 | 241 | 19 (8) | 0.4; 0.1–1.4 | 8 (3) | 0.6; 0.07–4.4 |
| 0.170 | 0.583 | ||||
| Estrogen receptor/progesterone receptor status | |||||
| Negative | 47 | 7 (15) | 1.0 | 5 (11) | 1.0 |
| Positive | 211 | 15 (7) | 0.4; 0.2–1.1 | 4 (2) | 0.2; 0.04–0.6 |
| 0.063 | 0.007 | ||||
| HER2 category | |||||
| 0, 1+ or 2+NA | 215 | 15 (7) | 1.0 | 6 (3) | 1.0 |
| 2+A or 3+ | 43 | 7 (16) | 2.5; 0.9–6.1 | 3 (7) | 2.5; 0.6–9.9 |
| 0.051 | 0.188 | ||||
| Ki-67 labeling index (%) of tumor cells | |||||
| ≤14 | 72 | 1 (1) | 1.0 | 0 | 1.0 |
| >14 | 186 | 21 (11) | 8.8; 1.2–65.1 | 9 (5) | NA |
| 0.034 | |||||
| Fibrotic focus | 258 | ||||
| Absent | 159 | 4 (3) | 1.0 | 0 | 1.0 |
| Present | 99 | 18 (18) | 7.8; 2.6–22.8 | 9 (9) | NA |
| <0.001 | |||||
| Histologic grade | |||||
| Grade 1 | 64 | 2 (3) | 1.0 | 0 | 1.0 |
| Grade 2 | 104 | 4 (4) | 1.3; 0.2–7.0 | 2 (2) | 1.0 |
| 0.781 | |||||
| Grade 3 | 90 | 16 (18) | 6.2; 1.4–26.8 | 7 (8) | 6.7; 1.4–31.8 |
| 0.015 | 0.018 | ||||
| Invasive tumor size (mm) | |||||
| ≤20 | 99 | 3 (3) | 1.0 | 0 | 1.0 |
| >20 to ≤50 | 146 | 15 (10) | 3.6; 1.0–12.4 | 7 (5) | 1.0 |
| 0.043 | |||||
| >50 | 13 | 4 (31) | 12.1;2.7–54.0 | 2 (15) | 6.1; 1.3–29.2 |
| 0.001 | 0.024 | ||||
| Skin invasion | |||||
| Absent | 232 | 16 (7) | 1.0 | 5 (2) | 1.0 |
| Present | 26 | 6 (23) | 4.2; 1.6–10.7 | 4 (15) | 8.3; 2.2–31.0 |
| 0.003 | 0.002 | ||||
| Tumor necrosis | |||||
| Absent | 186 | 12 (7) | 1.0 | 2 (1) | 1.0 |
| Present | 72 | 10 (14) | 2.2; 0.9–5.2 | 7 (10) | 9.1; 1.9–43.7 |
| 0.059 | 0.006 | ||||
| Blood vessel invasion | |||||
| Absent | 121 | 5 (4) | 1.0 | 2 (2) | 1.0 |
| Present | 137 | 17 (12) | 3.2; 1.2–8.8 | 7 (5) | 3.2; 0.7–15.3 |
| 0.022 | 0.146 | ||||
| Lymph vessel invasion | |||||
| Absent | 152 | 9 (6) | 1.0 | 3 (2) | 1.0 |
| Present | 106 | 13 (12) | 2.1; 0.9–5.0 | 6 (6) | 2.9; 0.7–11.5 |
| 0.079 | 0.129 | ||||
| UICC pN category | |||||
| pN0 | 181 | 10 (6) | 1.0 | 2 (1) | 1.0 |
| pN1 | 44 | 4 (9) | 1.7; 0.5–5.4 | 1 (2) | 2.0; 0.2–21.4 |
| 0.383 | 0.560 | ||||
| pN2 | 23 | 4 (17) | 3.8; 1.2–12.3 | 3 (13) | 13.5; 2.2–78.6 |
| 0.025 | 0.004 | ||||
| pN3 | 10 | 4 (40) | 9.7; 3.0–30.8 | 3 (30) | 33.9; 5.5–197.1 |
| <0.001 | <0.001 | ||||
| CD204-positive tumor-associated macrophage infiltration | |||||
| Low | 124 | 3 (3) | 1.0 | 2 (2) | 1.0 |
| High | 134 | 18 (13) | 4.4; 1.5–13.0 | 7 (5) | 3.3; 0.7–15.9 |
| 0.007 | 0.136 | ||||
| CD68-positive tumor-associated macrophage infiltration | |||||
| Low | 129 | 6 (5) | 1.0 | 3 (2) | 1.0 |
| High | 129 | 16 (12) | 2.5; 0.9–6.4 | 6 (5) | 1.8; 0.5–7.4 |
| 0.055 | 0.388 | ||||
| CD163-positive tumor-associated macrophage infiltration | |||||
| Low | 130 | 13 (10) | 1.0 | 2 (2) | 1.0 |
| High | 128 | 9 (7) | 0.7; 0.3–1.6 | 7 (6) | 3.6; 0.7–17.1 |
| 0.407 | 0.113 | ||||
| UICC pTNM stage | |||||
| I | 86 | 3 (3) | 1.0 | 0 | 1.0 |
| II | 122 | 9 (7) | 2.2; 0.6–8.0 | 3 (2) | 1.0 |
| 0.245 | |||||
| III | 50 | 10 (20) | 6.8; 1.9–24.9 | 6 (12) | 9.3; 2.4–37.4 |
| 0.004 | 0.002 | ||||
- HR, hazard ratio; CI, confidence interval; hormone receptor positive, positive for estrogen receptor or progesterone receptor if ≥ 1% of tumor cell nuclei are immunoreactive; hormone receptor negative, negative for estrogen receptor or progesterone receptor if < 1% of tumor cell nuclei are immunoreactive; HER2 2+NA, HER2 2+ but not gene amplification; HER2 2+A, HER2 2+ with gene amplification; pN0, no nodal metastasis, but including lymph node with isolated tumor cell clusters (single tumor cells or small clusters of cells not more than 0.2 mm in greatest dimension); pN1, 1 to 3 nodal metastases; pN2, 4 to 9 nodal metastases; pN3, 10 or more nodal metastases; en, endocrine therapy; ch, chemotherapy; NA, not available. Low category of tumor-associated macrophage infiltration represents the median values or lower of tumor-associated macrophage infiltrations; the high category of tumor-associated macrophage infiltration represents values higher than the median value of tumor-associated macrophage infiltrations; the median values of CD204-positive, CD68-positive and CD163-positive tumor-associated macrophage infiltration are listed in Table 2.
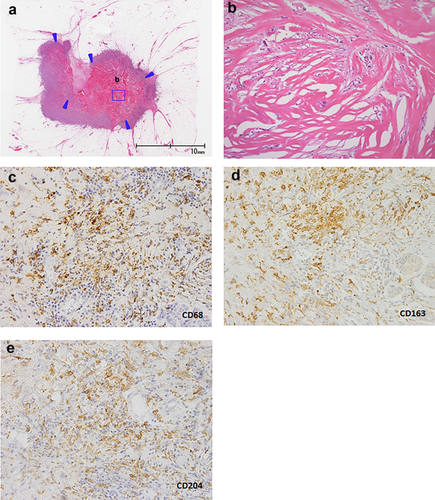
The following antibodies were used for the immunohistochemistry analysis: anti-estrogen receptor rabbit monoclonal antibody (SP1; Roche, Tucson, Arizona, USA), anti-progesterone receptor rabbit monoclonal antibody (1E2, Roche), anti-HER2 rabbit monoclonal antibody (4B5, Roche), mouse monoclonal antibody MIB-1 (1:50; DAKO, Glostrup, Denmark), anti-CD68 mouse monoclonal antibody (KP1, DAKO, 1:50), anti-CD163 mouse monoclonal antibody (10D6, 1:400; Leica, Newcastle Upon Tyne, United Kingdom), and anti-CD204 mouse monoclonal antibody (SRA-E5, 1:100; TGI, Kobe, Hyogo, Japan). We defined the estrogen receptor status and the progesterone receptor status in the tumor cells according to the ASCO/CAP guideline.28 HER2 expression in the tumor cells was also categorized according to the ASCO/CAP guideline.29 The MIB-1 labeling index of the stroma-invasive tumor cells was set at a threshold of 14%.30 Tumor-associated macrophage infiltration was assessed as the average number of tumor-associated macrophages in three hot spots under high-power fields within the tumor (Fig. 1c–e).31-33 When assessing the tumor areas to be used for counting the number of tumor-associated macrophage infiltrations in each tumor, we did not assess areas that had been subjected to a needle biopsy prior to surgery to avoid any influence of the needle biopsy on tumor-associated macrophage infiltration within the tumor.34 The investigators (HS and TH) were blinded to the patients clinicopathological data during the assessment.
Statistical analysis and patient outcome
The univariate correlation analyses were performed using an ANOVA; factors with a significant correlation in the univariate analyses were entered into a multivariate analysis using multiple regression. Survival was evaluated using a median follow-up period of 74.3 months (range: 2.8 to 102.5 months) until March 2016. Tumor recurrence and tumor-related death were observed in 23 and 9 of the 258 patients with invasive ductal carcinoma, respectively. The factors that were significantly associated with tumor recurrence in the univariate analyses were entered into multivariate analyses using the Cox proportional hazard regression model. Since the number of patients who died of the disease was less than 10, a multivariate analysis for tumor-related death could not be performed in this study (Table 1). As for tumor-associated macrophage infiltration, the median values were used as cut-off values for each tumor-associated macrophage infiltration (Table 2). A median value or lower was classified as a low level of tumor-associated macrophage infiltration, and a higher than median value was classified as a high level of tumor-associated macrophage infiltration in this study. The disease-free survival curve and the overall survival curve were drawn using the Kaplan-Meier method. All the analyses were performed using Statistica/Windows software (version 10; StatSoft, Tulsa, OK, USA).
| Tumor-associated macrophage (TAM) infiltration values | ||||||
|---|---|---|---|---|---|---|
| CD68-positive TAM | CD163-positive TAM | CD204-positive TAM | ||||
| Range | 7–842 | Range | 60–982 | Range | 4–709 | |
| Average | 284 | Average | 332 | Average | 246 | |
| Median | 283 | Median | 304 | Median | 229 | |
| Multiple regression analysis | ||||||
| Range | β | Range | β | Range | β | |
| Average | SE | Average | SE | Average | SE | |
| P-value | P-value | P-value | ||||
| Fibrotic focus (mm) | ||||||
| Absent | 7–812 | 0.296 | 60–834 | 0.226 | 4–709 | 0.307 |
| 234 | 0.059 | 296 | 0.060 | 203 | 0.058 | |
| <0.001 | <0.001 | <0.001 | ||||
| Present | 7–842 | 134–982 | 52–689 | |||
| 362 | 389 | 315 | ||||
| Histologic grade | ||||||
| Grade 1 | 7–711 | 0.169 | 60–834 | 0.081 | 4–709 | 0.211 |
| 206 | 0.070 | 266 | 0.072 | 175 | 0.069 | |
| 0.016 | 0.261 | 0.002 | ||||
| Grade 2 | 7–716 | 80–982 | 16–674 | |||
| 264 | 320 | 277 | ||||
| Grade 3 | 96–842 | 148–824 | 105–689 | |||
| 362 | 392 | 319 | ||||
| Estrogen receptor/progesterone receptor status | ||||||
| Negative | 20–842 | –0.120 | 60–982 | –0.217 | 6–689 | –0.171 |
| 364 | 0.064 | 434 | 0.065 | 332 | 0.063 | |
| 0.060 | 0.001 | 0.007 | ||||
| Positive | 7–829 | 71–834 | 4–709 | |||
| 266 | 309 | 227 | ||||
| Invasive tumor size (mm) | ||||||
| ≤20 | 7–711 | 0.106 | 60–834 | –0.010 | 6–709 | 0.026 |
| 232 | 0.062 | 295 | 0.059 | 207 | 0.057 | |
| 0.086 | 0.861 | 0.649 | ||||
| >20–≤50 | 7–829 | 80–982 | 4–674 | |||
| 313 | 362 | 275 | ||||
| >50 | 96–842 | 106–625 | 105–689 | |||
| 344 | 275 | 224 | ||||
| Tumor necrosis | ||||||
| Absent | 7–842 | 0.010 | 60–982 | 0.086 | 4–709 | 0.045 |
| 259 | 0.063 | 304 | 0.065 | 222 | 0.062 | |
| 0.880 | 0.187 | 0.476 | ||||
| Present | 7–829 | 166–824 | 26–674 | |||
| 346 | 401 | 306 | ||||
| Ki-67 labeling index (%) of tumor cells | ||||||
| ≤14 | 7–693 | 0.086 | 60–826 | 0.107 | 6–577 | 0.022 |
| 214 | 0.064 | 266 | 0.065 | 192 | 0.063 | |
| 0.180 | 0.104 | 0.725 | ||||
| >14 | 7–842 | 85–982 | 4–709 | |||
| 311 | 357 | 267 | ||||
| Skin invasion | ||||||
| Absent | 7–829 | 0.045 | 60–982 | – | 4–709 | – |
| 278 | 0.057 | 330 | 242 | |||
| 0.436 | ||||||
| Present | 7–842 | 161–625 | 105–689 | |||
| 331 | 350 | 283 | ||||
| Nodal status | ||||||
| Absent | 7–812 | –0.003 | 60–982 | – | 4–709 | – |
| 265 | 0.061 | 325 | 235 | |||
| 0.955 | ||||||
| Present | 54–842 | 103–826 | 18–689 | |||
| 325 | 348 | 272 | ||||
- SE, standard error; –, not significant in univariate analysis.
RESULTS
Factors significantly associated with macrophage infiltration
The presence of a fibrotic focus was the only factor that was significantly associated with a high level of CD68-, CD163- or CD204-positive tumor-associated macrophage infiltration in the multivariate analyses (Table 2). The histologic grade was associated with a significant high level of CD68-positive tumor-associated macrophage infiltration and CD204-positive tumor-associated macrophage infiltration, while a negative estrogen and progesterone receptor status was associated with a significantly high level of CD163-positive tumor-associated macrophage and CD204-positive tumor-associated macrophage infiltration in the multivariate analyses. The invasive tumor size, the Ki-67 labeling index of the tumor cells, the presence of tumor necrosis, the presence of skin invasion and the presence of nodal metastasis were not associated with a significantly high level of tumor-associated macrophage infiltration in the multivariate analyses.
Univariate and multivariate analyses for patient outcome
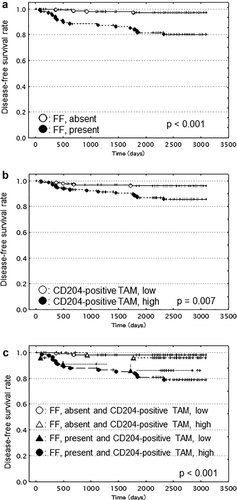
The presence of a fibrotic focus (Fig. 2a) and a Ki-67 labeling index >14% significantly increased the hazard ratios for tumor recurrence in the univariate analysis, and no tumor-related deaths were observed among cases without a fibrotic focus or among cases with a Ki-67 labeling index ≤14% (Table 1). Adjuvant therapy, histologic grade 3, an invasive tumor size >50 mm, the presence of skin invasion, UICC pN2 category, UICC pN3 category and UICC pTNM stage III were significantly associated with tumor recurrence and tumor-related death in the univariate analyses (Table 1). An invasive tumor size >20 to ≤50 mm, the presence of blood vessel invasion, and a high level of CD204-positive tumor-associated macrophage infiltration (Fig. 2b) were associated with significantly increased hazard ratios for tumor recurrence in the univariate analyses (Table 1). A negative estrogen receptor/progesterone receptor status and the presence of tumor necrosis were associated with significantly increased hazard ratios for tumor-related death in the univariate analyses (Table 1). Age, a high level of CD68-positive tumor-associated macrophage infiltration, a high level of CD163-positive tumor-associated macrophage infiltration, HER2 category and lymph vessel invasion were not significantly associated with tumor recurrence or tumor-related death in the univariate analyses (Table 1).
In the multivariate analyses, since significantly strong associations between the presence of a fibrotic focus and tumor-associated macrophage infiltration were observed (Table 2), the outcome predictive powers of the presence of a fibrotic focus and tumor-associated macrophage infiltration were separately evaluated using the following two models to avoid the mutual influence of these factors: model 1 included the fibrotic focus, and model 2 excluded the fibrotic focus.
Overall (n = 258), the presence of a fibrotic focus (P = 0.008) and a UICC pN3 category (P = 0.044) significantly increased the hazard ratios for tumor recurrence in the model 1 multivariate analysis. A high level of CD204-positive tumor-associated macrophage infiltration (P = 0.138), a high level of CD68-positive tumor-associated macrophage infiltration (P = 0.316), the invasive tumor size (>20 to ≤50 mm, P = 0.395; >50 mm, P = 0.083), the presence of skin invasion (P = 0.059), the histologic grade (grade 2, P = 0.678; grade 3, P = 0.358), the presence of blood vessel invasion (P = 0.546) and a Ki-67 labeling index >14% (P = 0.083) failed to increase the hazard ratio for tumor recurrence in the model 1 multivariate analysis significantly. In the model 2 multivariate analysis, histologic grade 3 (P = 0.001), the presence of skin invasion (P = 0.002) and a UICC pN3 category (P = 0.002) significantly increased the hazard ratios for tumor recurrence; a high level of CD204-positive tumor-associated macrophage infiltration (P = 0.089), a high level of CD68-positive tumor-associated macrophage infiltration (P = 0.513), the invasive tumor size (>20 to ≤50 mm, P = 0.684; >50 mm, P = 0.143), histologic grade 2 (P = 0.501), the presence of blood vessel invasion (P = 0.183) and a Ki-67 labeling index >14% (P = 0.169) failed to increase the hazard ratio for tumor recurrence in the model 2 multivariate analysis significantly.
The model 1 multivariate analysis demonstrated that the presence of a fibrotic focus was associated with significantly increased hazard ratios for tumor recurrence in the subgroups except for the nodal status negative subgroup and the nodal status positive subgroup; in contrast, a high level of CD204-positive tumor-associated macrophage infiltration was associated with significantly increased hazard ratios only for tumor recurrence in the nodal status positive subgroup (Table 3). The model 2 multivariate analyses demonstrated that a high level of CD204-positive tumor-associated macrophage infiltration was associated with significantly increased hazard ratios for tumor recurrence in the UICC pTNM stage III subgroup, and the nodal status positive subgroup (Table 3).
| Hazard ratio | |||
|---|---|---|---|
| 95% confidence interval | |||
| P-value | |||
| pTNM stage | Nodal status | ||
| I and II (n = 208) | III (n = 50) | Negative (n = 181) | Positive (n = 77) |
| Model 1 | |||
| Fibrotic focus, present (Referent category: Fibrotic focus, absent) | |||
| 4.9 | 8.5 | 3.9 | 8.9 |
| 1.1–20.7 | 1.1–67.9 | 0.9–15.7 | 0.6–140.3 |
| 0.033 | 0.042 | 0.056 | 0.116 |
| CD204-positive tumor-associated macrophage infiltration value, high (Referent category: CD204-positive tumor-associated macrophage infiltration value, low) | |||
| 0.6 | 3.8 | 0.5 | 11.5 |
| 0.2–2.5 | 0.4–34.3 | 0.1–2.2 | 14.1–86.3 |
| 0.521 | 0.239 | 0.356 | 0.019 |
| Model 2 | |||
| CD204-positive tumor-associated macrophage infiltration value, high (Referent category: CD204-positive tumor-associated macrophage infiltration value, low) | |||
| 1.0 | 8.4 | 0.8 | 12.7 |
| 0.2–3.9 | 1.1–65.9 | 0.2–3.5 | 2.1–71.1 |
| 0.997 | 0.044 | 0.711 | 0.005 |
Prognostic classification based on fibrotic focus and tumor-associated macrophage infiltration
In the present study, since the presence of a fibrotic focus was significantly associated with CD204-positive tumor-associated macrophage infiltration (Table 2), we attempted to create a classification consisting of the presence/absence of a fibrotic focus and the level of CD204-positive tumor-associated macrophage infiltration to predict patient outcomes more accurately (Table 4). A univariate analysis clearly demonstrated that a classification based on the presence/absence of a fibrotic focus and the level of CD204-positive tumor-associated macrophage infiltration was significantly associated with tumor recurrence (Table 4, Fig. 2c).
| Fibrotic focus and CD204-positive tumor-associated macrophage classification | ||||
|---|---|---|---|---|
| No. of patients (%) | ||||
| Tumor recurrence | ||||
| Cases | Present | Hazard ratio | 95% Confidence interval | P-value |
| 258 | 22 (9) | |||
| Fibrotic focus absent and CD204 low | ||||
| 106 | 2 (2) | 1.0 | ||
| Fibrotic focus absent and CD204 high | ||||
| 53 | 2 (4) | 2.0 | 0.3–14.4 | 0.480 |
| Fibrotic focus present and CD204 low | ||||
| 24 | 3 (13) | 7.6 | 1.3–45.3 | 0.026 |
| Fibrotic focus present and CD204 high | ||||
| 75 | 15 (20) | 11.2 | 2.5–47.9 | 0.001 |
- Trend hazard ratio, 2.2; Trend 95% confidence interval, 1.5–3.4; Trend P-value, <0.001.
Next, we performed multivariate analyses to clarify the prognostic significance of a classification consisting of the presence/absence of a fibrotic focus and the level of CD204-positive tumor-associated macrophage infiltration both overall and for each of the subgroup categories. The factors that were entered in the multivariate analyses were the same factors that had been entered in the multivariate analyses (model 1 and model 2) mentioned above. In all the cases, a multivariate analysis showed that the presence of a fibrotic focus and a high level of CD204-positive tumor-associated macrophage infiltration were associated with significantly increased hazard ratios for tumor recurrence (P = 0.004). The presence of a fibrotic focus and a low level of CD204-positive tumor-associated macrophage infiltration was not significantly associated with tumor recurrence (P = 0.088), nor was the absence of a fibrotic focus and a high level of CD204-positive tumor-associated macrophage infiltration (P = 0.624). The multivariate analyses clearly demonstrated that a high level of CD204-positive tumor-associated macrophage infiltration together with the presence of a fibrotic focus was associated with significantly increased hazard ratios for tumor recurrence in all the subgroups (Table 5); a low level of CD204-positive tumor-associated macrophage infiltration and the presence of a fibrotic focus significantly increased the hazard ratios for tumor recurrence only among the cases with UICC pTNM stages I and II cases and a high level of CD204-positive tumor-associated macrophage infiltration and the absence of a fibrotic focus was not significantly associated with tumor recurrence in any of the subgroups (Table 5).
| Hazard ratio | |||
|---|---|---|---|
| 95% confidence interval | |||
| P-value | |||
| pTNM stage | Nodal status | ||
| I and II (n = 208) | III (n = 50) | Negative (n = 181) | Positive (n = 77) |
| FF absent and a low level of CD204-positive TAM infiltration | |||
| RC | RC | RC | RC |
| FF absent and a high level of CD204-positive TAM infiltration | |||
| 2.5 | RC | 0.8 | RC |
| 0.2–31.4 | 0.1–12.7 | ||
| 0.485 | 0.927 | ||
| FF present and a low level of CD204-positive TAM infiltration | |||
| 8.9 | RC | 3.3 | 4.8 |
| 1.8–44.6 | 0.8–14.3 | 0.2–120.7 | |
| 0.007 | 0.113 | 0.344 | |
| FF present and a high level of CD204-positive TAM infiltration | |||
| 4.9 | 13.4 | 8.5 | 62.2 |
| 1.2–19.5 | 1.7–107.5 | 1.3–46.8 | 2.4–1641.2 |
| 0.025 | 0.014 | 0.003 | 0.014 |
- FF, fibrotic focus; TAM, tumor-associated macrophage; RC, referent category. Among the UICC pTNM stage III cases, since there were no cases with tumor recurrence among the cases without a fibrotic focus absent and with a high level of CD204-positive tumor-associated macrophage infiltration or among the referent category cases, these two cases and cases with a fibrotic focus and a low level of CD204-positive tumor-associated macrophage infiltration were used as the referent category in the multivariate analysis. Among the cases with nodal metastasis, since no cases with tumor recurrence were observed in the referent category cases, cases without a fibrotic focus and with a high level of CD204-positive tumor-associated macrophage infiltration as well as the referent category cases were used as the referent category in the multivariate analysis.
DISCUSSION
The presence of a fibrotic focus was the only factor that was significantly associated with a high level of CD68-, CD163- and CD204-positive tumor-associated macrophage infiltration in the present study. The presence of a fibrotic focus is indicative of the biological characteristics of tumor-stromal fibroblast, while other factors that were significantly associated with tumor-associated macrophage infiltration in the univariate analyses or multivariate analyses reflected the biological characteristics of the tumor cells only. These findings strongly suggest that tumor-stromal fibroblasts forming a fibrotic focus play a very important role in a high level of tumor-associated macrophage infiltration in invasive ductal carcinoma of the breast. Breast carcinoma with fibrotic focus-like lesion has been previously reported as scar cancer by Fisher et al.35 Fisher et al. divided scar cancers into five subtypes according to the histological features of the sclerotic foci, and the histological features of a fibrotic focus that were similar to those of type 2 or type 3 scar cancer according to Fisher's classification were as follows: type 2, having either edema or a dense acellular hyalinised core with tumor cells only rarely observed within the scar; and type 3, having an evident radiating core and with tumor cells present within the scar as well as at its periphery. These findings strongly suggest that a fibrotic focus is almost histologically equivalent to a scar that plays a very important role in the wound-healing process within the tumor. In addition, the presence of a fibrotic focus is significantly associated with angiogenesis, lymphoangiogenesis and hypoxia in invasive breast carcinomas,13, 22, 23, 36 and these three factors play very important roles in the wound-healing process.37-40 Thus, the radially expanding fibrosclerotic appearance of a fibrotic focus and the biological characteristics of invasive ductal carcinomas with a fibrotic focus strongly suggest that the formation of a fibrotic focus within the tumor is almost equivalent to the process of scar formation in the wound healing process. During the wound healing process, macrophages secrete several growth factors, growth factor receptors, cytokines or chemokines and these secreted factors induce the proliferation of fibroblasts; these fibroblasts secrete collagen fibers to form scars and heal wounds. Since invasive ductal carcinomas with a fibrotic focus exhibit significantly higher frequencies of the expressions of growth factors and their receptors than those without a fibrotic focus,15, 41 tumor-associated macrophages probably play important roles in the high levels of growth factors and their receptors that are often observed in invasive ductal carcinoma with a fibrotic focus of the breast. Therefore, the presence of a fibrotic focus is a very useful histological indicator for the accurate prediction of invasive ductal carcinomas with ample tumor-associated macrophage infiltration.
CD204-positive tumor-associated macrophage infiltration was superior to CD68-positive macrophage infiltration or CD163-positive tumor-associated macrophage infiltration for the prediction of tumor recurrence among patients with invasive ductal carcinoma, exhibiting a prognostic significance in some subgroup categories in the present study. Furthermore, CD204-positive tumor-associated macrophage infiltration had an excellent power for the accurate prediction of tumor recurrence in UICC pTNM stage III category after the mutual influence of CD204-positive tumor-associated macrophage infiltration and the presence of a fibrotic focus had been eliminated; thus, among tumor-associated macrophages CD204-positive tumor-associated macrophage infiltration is probably the best predictor of outcome among patients with invasive ductal carcinoma of the breast, and our results support the prognostic significance of CD204-positive macrophage infiltration in other types of carcinomas.9, 10 As for breast cancer, although some studies have indicated the prognostic significance of CD163-positive tumor-associated macrophage infiltration or CD68-positive tumor-associated macrophage infiltration,1, 42, 43 these studies did not perform a comparative evaluation with CD204-positive tumor-associated macrophage infiltration. CD68 is a broad macrophage marker that cannot discriminate the M2 phenotype from others.44 Reportedly, no correlation was observed between tumor-associated macrophages with CD163 surface expression and two previously published M2 gene signatures when examined using the hierarchal clustering of genome-wide expression profiling data, whereas the M1 markers were unchanged or elevated; in addition, the global gene expression profiles demonstrated a mixed-polarization phenotype that was unrelated to the M1/M2 classification.45 These findings strongly suggest that M1 polarized tumor-associated macrophages, M1/M2 polarized hybrid tumor-associated macrophages or non-M1/M2 polarized tumor-associated macrophages that prevent tumor progression were present among the CD68-positive tumor-associated macrophages or the CD163-positive tumor-associated macrophages, resulting in the finding that both kinds of tumor-associated macrophages failed to show a significant association with the tumor progression of invasive ductal carcinoma of the breast in the present study. Therefore, we can conclude that CD204-positive tumor-associated macrophage infiltration has a superior prognostic power for the accurate prediction of the outcome of patients with invasive ductal carcinoma, compared with CD68-positive tumor-associated macrophage infiltration or CD163-positive tumor-associated macrophage infiltration.
The combined assessment of the presence or absence of a fibrotic focus and a high or a low level of CD204-positive tumor-associated macrophage infiltration clearly demonstrated that CD204-positive tumor-associated macrophage infiltration, especially a high level of CD204-positive tumor-associated macrophage infiltration, had a significant prognostic power only for patients with invasive ductal carcinoma with a fibrotic focus; this finding strongly suggests that the collaboration of tumor-stromal fibroblasts forming a fibrotic focus and CD204-positive tumor-associated macrophages enhances the tumor progression of invasive ductal carcinoma, since the degree of CD204-positive tumor-associated macrophage infiltration was not correlated with the tumor progression of invasive ductal carcinoma without a fibrotic focus in the present study. Although the integrated action among tumor-stromal fibroblasts forming a fibrotic focus and tumor-associated macrophages in addition to invasive tumor cells probably influences the tumor microenvironment and accelerates the malignant potential of invasive ductal carcinoma with a fibrotic focus of the breast, the tumor-stromal fibroblasts forming a fibrotic focus probably play a more important role than tumor-associated macrophages or invasive tumor cells in accelerating the malignant potential of the tumor, since collaboration between tumor-stromal fibroblasts not forming fibrotic focus and tumor-associated macrophage did not accelerate the malignant potential of invasive ductal carcinoma of the breast.
In conclusion, the presence of a fibrotic focus was the best histological indicator for the accurate prediction of the degree of tumor-associated macrophage infiltration in invasive ductal carcinoma of the breast; in addition, CD204-positive tumor-associated macrophages can promote tumor progression only on invasive ductal carcinoma of the breast under the presence of a fibrotic focus within them.
ACKNOWLEDGEMENTS
The present study was supported by the Gakunai-grant (27-B-1-07) from Saitama Medical University and Hidaka Research Project (26-D-1-27) from Saitama Medical University International Medical Center. We are grateful for Mrs. Kyoko Shimizu her excellent technical assistance.
DISCLOSURE STATEMENT
None declared.




