Sensory Rhodopsin I and Sensory Rhodopsin II Form Trimers of Dimers in Complex with their Cognate Transducers†
Abstract
Archaeal photoreceptors consist of sensory rhodopsins in complex with their cognate transducers. After light excitation, a two-component signaling chain is activated, which is homologous to the chemotactic signaling cascades in enterobacteria. The latter system has been studied in detail. From structural and functional studies, a picture emerges which includes stable signaling complexes, which assemble to receptor arrays displaying hexagonal structural elements. At this higher order structural level, signal amplification and sensory adaptation occur. Here, we describe electron microscopy data, which show that also the archaeal phototaxis receptors sensory rhodopsin I and II in complex with their cognate transducers can form hexagonal lattices even in the presence of a detergent. This result could be confirmed by molecular dynamics calculations, which revealed similar structural elements. Calculations of the global modes of motion displayed one mode, which resembles the “U”-”V” transition of the NpSRII:NpHtrII complex, which was previously argued to represent a functionally relevant global conformational change accompanying the activation process [Ishchenko et al. (2013) J. Photochem. Photobiol. B 123, 55-58]. A model of cooperativity at the transmembrane level is discussed.
Introduction
The strategies of signal propagation in eukaryotes and prokaryotes display significant differences. Whereas in eukaryotic cells, sensing occurs mainly via spacial gradients, in prokaryotes—because of their small size—temporal gradients provide the signal. One of the best elucidated example of such a system concerns chemotaxis signaling networks in bacteria (for recent reviews, refer to 1-3). Chemotactic receptors (methyl-accepting chemotaxis proteins, MCPs) form dimers and consist of an extracellular receptor domain, the membrane domain and a long rod-shaped cytoplasmic domain. After receptor activation, the signal travels along the dimeric complex, featuring largely an α-helical coiled-coil structure 4, via HAMP domains, responsible for signal conversion and inversion 5, to the adaptation domain, which undergoes reversible methylation/demethylation and thereby increases the dynamic range of signal reception considerably. Finally, the signal is transmitted to the highly conserved kinase-activating domain 6. These membrane proteins form trimers of dimers 7, which assemble to networked arrays 8 providing a template to which the His kinase CheA and the coupling protein CheW are recruited. Li and Hazelbauer 9 concluded that a 2:2:1 organization (two trimers of dimers: two CheW: one CheA dimer) is the core structural and functional unit. Amplification of the incoming signal 10 occurs on the level of these arrays probably governed by cooperative mechanisms 9, 11.
These arrays of chemoreceptors typically cluster at the cell poles close to the flagellar motors. In a collaborative manner, different chemoreceptors team up 11 enabling the bacteria to integrate diverse incoming signals. In recent years, the structure of the arrays and the core complex has been elucidated in detail. The emerging picture displays hexagonal core complexes consisting of MCP trimers of dimers and the P3 and P5 domains of CheA as well as CheW, which can assemble further to generate an indefinitely large array 12, 13. It was proposed that asymmetry within an allosteric unit confers a mechanism of amplification 9. It should be noted that the array geometry at the extracellular side of the plasma membrane is not known at higher resolution.
Archaeal chemo- and phototaxis are thought to be based on similar mechanisms, because the underlying signal transduction chain shows high homology to that of the Escherichia coli system 14. Similar to chemoreceptors from E. coli, the archaeal chemoreceptors consist of a periplasmic receptor domain, a membrane domain and a long rod-shaped cytoplasmic domain connected to the second membrane spanning helix (TM2) by two consecutive HAMP domains. The cytoplasmic domain harbors methylation sites, which are involved in adaptation to constant stimuli. The signaling domain at the cytoplasmic tip constitutes the binding site for CheW and CheA.
Conversely, to the enterobacterial system, which has been thoroughly investigated, only few detailed information about archaeal taxis signal transduction systems is available, with Halobacterium salinarum being the best studied system 15. The genome of this halophilic archaeon codes for 10 homologues of Che proteins, two archaeal CheF proteins and 18 homologues to eubacterial MCPs 16. This inventory is similar to that of B. subtilis and seems to be more complex than that of the E. coli system. On the other hand, the activation of CheA by repellent stimuli is observed for both, E. coli and H. salinarum, but is different to that of B. subtilis.
On a molecular level, most information for the archaeal signal transduction network is available for the phototaxis systems. These receptor complexes consist of two microbial rhodopsins: sensory rhodopsin I (SRI) and sensory rhodopsin II (SRII) whose primary functions are related to photophilic and photophobic responses of the bacteria, respectively (for recent reviews on microbial rhodopsins see 17, 18). SRI and SRII form 2:2 complexes with their cognate transducers HtrI and HtrII 19-21). The architecture of the transducers as well as those of the other archaeal chemoreceptors is quite similar to that of enterobacterial MCPs with the exception of the number of HAMP domains. In contrary to the MCPs from enterobacteria with only one HAMP domain, those of archaea possess two HAMP domains 16, 22, 23.
It has been shown for the SRII-transducer complex from Natronomonas pharaonis (NpSRII-NpHtrII) that light excitation triggers defined conformational changes at the protein interface between NpSRII and NpHtrII (reviewed in 17, 18) and adverse dynamical changes in the two HAMP domains 24. It appears that the mechanism of signal transfer is governed by alternating the dynamics of consecutive segments transduction of the cytoplasmic domain of the transducer dimer. How this dynamic behavior modulates the activity of CheA is still unknown. This model of an alternating sequence of dynamic shifts has also been proposed for signal transmission in chemoreceptors 1, 8, 25. It seems that not only structural but also functional similarities exist between enterobacterial and archaeal chemoreceptors.
If these striking similarities of bacterial and archaeal signal transduction are important features of signal transfer and amplification, arrays of signaling molecules should also be present in archaeal phototaxis. Indeed, recent electron microscopy data from Briegel et al. 26 showed that archaeal chemoreceptor arrays have similar dimensions and architecture as those in bacteria. However, the membrane area of phototaxis receptor complexes is much larger than that of bacterial chemoreceptors consisting only of MCPs. On the other hand, sequence data of the phototaxis transducers HtrII from H. salinarum (HsHtrII) revealed possibly dual functionality, because HsHtrII possesses additionally an extracellular receptor domain—like MCPs—while forming a tight complex with HsSRII 27. The question arises whether these sensory rhodopsin-transducer complexes can also assemble into larger functional arrays. Here, we present molecular dynamics data on the SRII/HtrII complex from N. pharaonis (NpSRII:NpHtrII), which indicate that this is indeed the case and that the NpSRII:NpHtrII transmembrane arrays suggest a mechanism for their cooperativity. To substantiate these calculations in a preliminary analysis, electron microscopy (EM) experiments on samples of trimers of dimers of HsSRI-HtrI and NpSRII:NpHtrII complexes were performed. These EM data clearly showed hexagonal structures indicating a similar architecture as those of bacterial chemoreceptors.
Materials and Methods
Expression and purification of NpSRII:NpHtrII and its C-terminally truncated version NpSRII:NpHtrII(1-114)
Sensory rhodopsin II and its cognate transducer NpHtrII were expressed and purified from E. coli as described before 28-31. Membranes were solubilized in 2% (w/v) n-dodecyl-β-D-maltopyranoside (DDM, Anatrace) in buffer A (300 mm sodium chloride, 50 mm NaPi, pH 8). Purifications of C-terminally hexa His-tagged proteins were performed by immobilized metal ion affinity chromatography 32 on Ni-NTA resin (Qiagen) using a linear gradient from 30 to 250 mm imidazole in buffer B (300 mm sodium chloride, 50 mm sodium phosphate, 0.05% (w/v) DDM, pH 8). It was followed by exclusion chromatography on a HiLoad 16/600 Superdex 200 pg column (GE Healthcare) with a flow rate of 0.5–1 mL min−1 (300 mm NaCl, 50 mm NaPi, 0.05% (w/v) DDM; pH 8).
For the formation of the NpSRII:NpHtrII complex, the purified proteins were combined with two-fold molar excess of NpSRII and incubated for 12 h at 4°C in buffer B. Subsequently, the mixture was applied to preparative size exclusion chromatography on a HiLoad 16/600 Superdex 200 pg column at 4°C using the same buffer (Figure S2). Fractions containing solely NpSRII and NpHtrII were selected for EM.
Negative stain transmission electron microscopy of NpSRII:NpHtrII and NpSRII:NpHtrII(1–114)
Purified fractions of NpSRII:NpHtrII and NpSRII:NpHtrII(1–114) complexes were examined by negative stain electron microscopy. Colloidon/carbon-coated copper grids were treated in a glow discharger (Femto version A, Diener electronic). After incubation with the protein solution, grids were washed three times in double-distilled water and stained with an aqueous 0.7% (w/v) uranyl formate solution 33. Images were acquired using a JEOL JEM 1400 transmission electron microscope with an acceleration voltage of 120 kV. Processing of data acquired for the NpSRII:NpHtrII complex by negative stain electron microscopy was done using the EMAN2 34 and SPARX 35 program suites.
Expression and purification of HsSRI-HtrI
The strategy for cloning, expression and purification of the full-length fusion protein HsSRI-HtrI is essentially the same as for the already described version HsSRI-HtrI1–52 31, 36, 37. A shortened sopI gene from H. salinarum, which lacks downstream sequences for the last 15 amino acids, was fused upstream of the full-length HsHtrI gene using the megaprimer PCR method 38. A coding sequence for six histidines was added to give a His-tag at the C-terminus of the fusion protein. Both chimera constructs were inserted into the expression vector pET11a (Novagen) under the control of the T7 promotor. Expression was done in E. coli BL21(DE3) RP cells at 37°C in double yeast tryptone medium supplemented with 200 mg L−1 ampicillin. As soon as OD600 of 0.6–0.8 was reached, the cells were fed with 1 mm isopropyl-β-D-thiogalactopyranoside and 12 μm of all-trans retinal (Sigma) for 4 h and then harvested by centrifugation (10 000 g).
The pelleted cells were resuspended in 500 mm NaCl, 50 mm 2-(N-morpholino)ethanesulfonic acid (MES) at pH 6.0, and 2 mm EDTA and disrupted in a French press. The insoluble membrane fraction was harvested by centrifugation (130 000 g, 1 h, 4°C) and solubilized overnight at 4°C in 4 m NaCl, 50 mm MES at pH 6.0 and 2% (w/v) DDM (Anatrace). Still insoluble material was removed by an additional centrifugation. The solubilized HsSRI-HtrI variants were further purified on a Ni-NTA agarose column (Qiagen), which was equilibrated with buffer containing 4 m NaCl, 50 mm MES at pH 6.0 and 0.05% (w/v) DDM. Three washing steps were performed: first with 10 bed volumes of the plain buffer, second with buffer plus 20 mm imidazole and finally again with the plain buffer. Elution was done in buffer at pH 4.5, and the purified proteins were immediately diluted with buffer at pH 6.8 to adjust the final pH in the range of 5.5–6.0. Shortly before usage, the HsSRI-HtrI proteins were separated on a gel filtration column (HiLoad 16/60 Superdex 200 pg, GE Healthcare) to get rid of aggregated protein.
Negative stain transmission electron microscopy of HsSRI-HtrI
Gel filtration fractions containing detergent-solubilized HsSRI-HtrI variants were adsorbed for 10–60 s to parlodion carbon-coated copper grids rendered hydrophilic by glow discharge at low pressure in air. Grids were washed with three drops of double-distilled water and stained with two drops of 0.75% (w/v) uranyl formate. Electron micrographs were recorded at a magnification of 50 000× on Eastman Kodak Co. S0-163 sheet films with a Hitachi H-7000 electron microscope operated at 100 kV. Negatives were digitized with a Heidelberg Primescan D 7100 at 4 Å per pixel resolution.
Protein–protein docking
The crystal structure of the NpSRII:NpHtrII dimer in the ground state (PDB access code 1H2S) was taken as an initial structure for the lattice building. We employed the protein–protein docking program M-ZDOCK 39 to predict cyclically symmetric multimeric conformations of the trimers of NpSRII:NpHtrII dimers and, then, of the hexamers of the obtained trimers of dimers. The M-ZDOCK algorithm uses a grid-based fast Fourier transform approach for searching for the best conformation in a space of symmetric (Cn) multimers consisting of a given number n of protomers. The obtained conformations are ranked according to ZDOCK 2.3.2 scoring function 40, which includes atomic contact energy (ACE) potential, shape complementarity and electrostatics terms.
All-atom molecular dynamics simulations
All-atom molecular dynamics simulations were carried out in Gromacs 5.0.5 using the CHARMM36 force field with CMAP corrections and TIP3P water. The initial structure of the transmembrane part of the NpSRII:NpHtrII dimer (the full NpSRII and residues 23 to 82 of NpHtrII) was placed into a 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine: 1-hexadecanoyl-2-(9Z-octadecenoyl)-sn-glycero-3-phospho-(1’-rac-glycerol) (POPE:POPG =3:1) model membrane and solvated using the CHARMM-GUI web server 41. The appropriate numbers of Na+ and Cl− ions were placed into the simulation box to neutralize the system and set the ionic strength to 0.15 m. Simulations were done in the NPT ensemble controlled by means of a Nose-Hoover thermostat (Tref = 303 K, τT = 2 ps) and a Parrinello-Rahman barostat (isotropic pressure, pref = 1 atm, τp = 2 ps, compressibility = 4.5 · 10–5 bar−1); the time step equaled 2 fs; a Verlet cutoff scheme 42 and PME 43 were used for nonbonded interactions. The initial equilibration (5 ns with the heavy atoms of the lipids and the protein restrained by the harmonic potential with k = 1000 kJ · mol−1 · nm−2) was followed by the lipids relaxation simulation (200 ns, the protein backbone restrained with the harmonic potential of the same strength) and, then, by the production simulation (1 μs).
Calculations of the global modes of motion
 (1)
(1) (2)
(2)Equivalently to the PCA approach, H is diagonalized and the obtained eigenvectors correspond to the collective low-frequency molecular modes (i.e. normal modes), while the eigenvalues characterize the energy cost of corresponding displacements.
The normal modes obtained from ANM are independent on force field selection and tiny structural details. They derive from the overall topology of macromolecules and describe global, low-frequency modes of motions potentially accessible for a system.
The similarity of collective modes acquired by PCA and ANM can be estimated by calculating their pairwise overlap, which is given by the correlation cosine of corresponding eigenvectors 44.
Here, PCA and ANM were performed using the ProDy toolkit 47.
Results and Discussion
Electron microscopy of trimers of dimers
For the electron microscopy experiments, different samples of HsSRI-HsHtrI and NpSRII:NpHtrII complexes were purified and analyzed. The list of samples included NpSRII complexed to full-length transducer and to a shortened transducer (NpHtrII1–114). Further constructs encompassed HsSRI fused genetically to its cognate transducer. Also in this case, a full-length and a shortened HsHtrI1–52 transducer was used (the constructs are shown in Figure S1). From the elution profile of NpSRII:NpHtrII complexes, a peak was observed were one would expect trimers of dimers to elute (Figure S2).
Figures 1 and 2 display selected examples of particles and class averages obtained by EM for HsSRI-HtrI and NpSRII:NpHtrII. The electron micrograph predominantly shows rounded hexagonal structures with diameters of roughly 10 nm. Generally, the samples appear to be heterogeneous, showing different shapes and sizes of objects. It is obvious that this general shape is observed for all samples. As also the shortened transducer forms similar structures (see Fig. 1, lower panels for HsSRI-HtrI1–52), it seems that solely the membrane domain is responsible for forming the observed particles.


The full-length transducer NpHtrII in complex with the receptor NpSRII gave better results allowing for a preliminary classification (sectors of electron micrographs of NpSRII:NpHtrII and NpSRII:NpHtrII1–114 are shown in Figure S3). In Fig. 2, eight representative classes for the complex NpSRII:NpHtrII are shown. Panels G and H show a top view of the complexes. Here, the electron density represents a cluster of six spheres arranged in a ring with high extra electron density in the center. The diameter of the arrangement is about 7–8 nm. One could interpret this result by an arrangement of three dimers forming a hexagonal ring. The central electron densities presumably stem from the transducer cytoplasmic domain. Similar observations can be made for HsSRI-HtrI (full length) in comparison with the truncated transducer (1–52, see Fig. 1). Other views of a possible cytoplasmic domain are depicted in panels C and E where an extra density protrudes out of the ring-like arrangement. Overall, the data are not accurate enough to warrant further processing. However, a major conclusion can be drawn: the complex of NpSRII with its transducer forms trimers of dimers in a hexagonal topology (see in particular Fig. 2H) even in detergents. This latter observation indicates quite strong binding, which cannot be disrupted by a mild detergent like DDM.
Molecular modeling of the transmembrane arrays
Reconstruction of the transmembrane arrays
Taking the EM data into account, we started with the prediction of the trimer-of-dimers conformations using rigid-body protein–protein docking, using the available structural data for the NpSRII:NpHtrII complex, as described in the Methods section. The two highest ranked conformations were termed “Y”-shaped and “O”-shaped trimers (Z-DOCK score = 44.27 and 39.48, respectively, with higher score value meaning better match) because their general topologies resemble these letters. In the “Y”-shaped conformation, only one monomer per NpSRII:NpHtrII dimer interacts with the other two dimers within the trimer. The interface consists of helices A and B of one monomer and helix D of the corresponding partner (Figure S4). Contrarily, in the “O”-shaped trimer, both monomers of each dimer contact to the adjacent dimers, leading to a ring-like topology with helices A and B of one monomer interacting with helix E of another one (Fig. 3).

These alternative conformations of trimer-of-dimers were subjected to another round of symmetrically restrained protein–protein docking calculations with C6 symmetry. The condition of hexameric symmetry was imposed to obtain lattice conformations, which are congruent with the hexagonal arrangement observed in the EM micrographs and that of the cytoplasmic domains of the chemoreceptor clusters 26. The resulting lattice model for the “O”-shaped trimer-of-dimers is shown in Fig. 3 along with the determined intrahexagonal interface. The latter is represented by the contacts between helix A of one monomer with helices D and E of its partner. In the alternative lattice model based on the “Y”-shaped trimer, the intertrimer contacts are formed by helices A and B and helix D as shown in Figure S4.
Overall, the “O”-conformation is in good agreement with the ring-like topology obtained by EM for trimers of dimers. In addition, the putative inter- and intratrimer interfaces determined for this conformation are consistent with the distribution of lipid molecules, which are bound to the protein in the available crystal structures of NpSRII (Fig. 4). We assumed that the regions of the protein surface, which are important for protein–protein interactions, should have lower affinity to lipids and vice versa. In accordance with this assumption, in the crystal structure of NpSRII obtained without the cognate transducer (PDB access code 3QAP, 48) there are no resolved lipids in the vicinity of helices F and G, which form the interface with the NpHtrII transducer. Another region lacking bound lipids is an area comprising helices D and E. These helices are involved in formation of both, the interdimer and the intertrimer contacts in the “O” model.

The transmembrane array is concave
The distance between two neighboring trimers of dimers within a hexameric cell of the tightly packed transmembrane lattice equals 9.5 nm for the “O”-shaped model (and 9.2 nm for the alternative “Y” lattice). This value is slightly larger compared to similar distances measured between the neighboring cytoplasmic tips of receptors in the honeycomb lattices formed by chemoreceptors with the CheA/CheW baseplate (varying between 6.9 nm 49 and 8.0 nm 26), which is believed to be highly conserved across multiple bacterial and archaeal species and thus unlikely to exceed these limits 26. We hypothesized that this difference can represent an intrinsic property of the receptors, which assemble in concave transmembrane lattices. The concavity of the receptor arrays facilitates their localization preferably at the cell poles 50, 51. A rough estimation of the curvature of the membrane-inserted arrays is given in Fig. 5. The calculated inverse membrane curvature equals 150–300 nm, which is in agreement with the experimentally determined curvature values (~400 nm) in the polar regions of the cylinder-shaped bacterial cells 52.

Global motions of the NpSRII:NpHtrII dimer suggest a model of receptor cooperativity
Above, we have shown that the organization of the cytoplasmic tips of the transducers in hexagonal arrays, which form a platform for binding the histidine-kinase CheA and the adapter protein CheW, necessitates formation of similarly highly ordered lattices of the NpSRII:NpHtrII membrane domain. Such densely packed two-dimensional clusters consisting of identical subunits potentially can function in a highly cooperative manner, which might be the basis for signal amplification 53-55. If the activation of a single receptor causes significant changes of its overall structure and/or dynamics, these changes can allosterically spread throughout the lattice, leading to a cooperative behavior 56,which might account for the cooperativity in the cytoplasmic baseplate 57. However, the existence of equally dense lattices of the transmembrane domains suggests that they can act in a similar way, thus providing an additional layer for signal amplification.
In order to study plausible cooperativity at the transmembrane level, we applied principal component analysis (PCA) to a 1-μs-long unbiased MD trajectory of a model of the transmembrane part of the NpSRII:NpHtrII1-82 dimer (the length of transducer in the 1H2S X-ray structure) in a lipid bilayer to reveal possible global motional modes of the NpSII:NpHtrII dimer. The collective motions detected by PCA (principal components, PCs) were compared with the global modes predicted by normal mode analysis (NMA) (shown in Fig. 6).
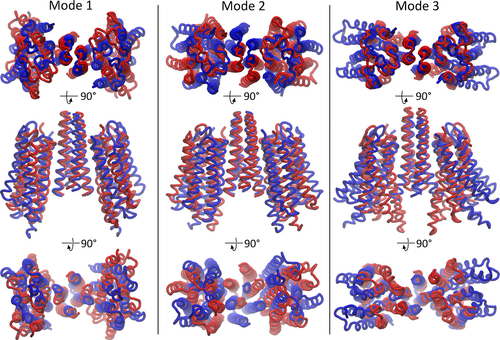
Both methods point to the presence of similar global motions as confirmed by the high overlap values between the PCA and ANM modes (Table S1).
The first two normal modes correspond to global twisting and wagging of the SRII molecules. The third normal mode largely overlaps with the PC1 of PCA analysis. This motional mode is of special interest as it closely resembles the transition between the “U”- and “V”-shaped conformations of the NpSRII:NpHtrII complex, which was previously argued to represent a functionally relevant global conformational change accompanying the activation process 58, 59. Indeed, the histogram of the trajectory projection on the PC1 as well as the histograms of the intermonomer SRII-SRII angle (between helices F) and distance (between the COMs of the cytoplasmic segment of helix F in both monomers) distributions are clearly bimodal indicating the presence of two conformations. While one of them remains structurally close to the “V”-shaped conformation, another one can be attributed to the “U”-shaped conformer (see Figure S5).
Interestingly, the membrane insertion energies, calculated for the “U”- and “V”-shaped dimers with the help of the PPM web server 60, differ by only ~5 kT (−503.3 and −516.3 kJ mol−1, respectively), suggesting that the two conformations can easily interconvert (e.g. upon absorption of a photon, which energy is about 20 times higher). However, the mode of interaction of the transmembrane region with the HAMP domain seems to be different in the “U”- and “V”-shaped conformations 61. The latter implies feasible coupling between conformation of the HAMP and orientation of the SRII, which may facilitate a global cooperative response of the receptor:transducer clusters upon their activation.
Conclusions
Based on MD calculations and EM data, we have constructed a plausible model of the transmembrane lattice formed by the NpSRII:NpHtrII phototaxis complexes. It has been experimentally shown, that for the cytoplasmic baseplate, this kind of receptor arrays is conserved in bacteria and archaea 26. In principle, the hexameric arrangement of the cytoplasmic domains of receptors/transducers implies a similar dense organization of the transmembrane regions of the photosensory complexes. Indeed, our computational analysis revealed two alternative building blocks of the transmembrane lattice, one of which (“O”-shaped trimer-of-dimers) represents a conformation similar to the experimentally determined one. Interestingly, the distribution of the co-crystallized lipids around NpSRII 48 displayed low affinity to sites around helices F and G as well as D and E. The former site is involved in the interface between the receptor and its transducer, while helices D and E form inter- and intradimer contacts in the “O” model of the transmembrane lattice.
Analysis of the MD simulation of the truncated NpSRII:NpHtrII1-82 dimer revealed the presence of global motions involving reorientation of the NpSRII molecules (Fig. 6 and Figure S5 and Table S1). One of these global modes resembles the conformational change (a relative orientation of NpSRII molecules within a dimer reminiscent of the “U”-”V” shape change), which was previously attributed to the activation process based on the alternative symmetries of the receptor complex revealed by X-ray crystallography 58. Such large amplitude global motions, which at the same time are associated with a subtle free energy change, can allosterically spread across the two-dimensional transmembrane lattice triggering a cooperative activation of multiple copies of the receptors, which can provide an additional level for signal amplification.
For the best-studied system, the chemotactic arrays of enterobacteria most of the structural and functional information are available for the cytoplasmic domain and the cytoplasmic baseplate (see e.g. 13, 62). Signal transfer from the membrane and activation of CheA is thought to follow an alternating static–dynamic mechanism (63, reviewed in 25). In the CheA “on” state, the protein interaction region adopts a dynamic state, which changes to a static state in the “off” state. Similar results were obtained for the archaeal phototaxis receptor/transducer complex 63, 64. The allosteric coupling of these dynamic transitions into hexagonal arrays is thought to occur through a unique interface interaction between CheW and the P5 domain of CheA 65-67. However, it is still an open question about the molecular mechanism of this allosteric coupling. Is the spread of information only accomplished via the cytoplasmic baseplate of the array or is the membrane domain also involved in the cooperativity of bacterial chemo(photo)sensory arrays? The present data provided evidence that the membrane domains of sensory rhodopsins in complex with their cognate transducers can form hexagonal lattices and show cooperativity at the transmembrane level. How the coupling between membrane domain and baseplate is accomplished, by statistical–thermodynamic models 68 or other models has still to be elucidated.
Acknowledgements
This work has been supported by the Deutsche Forschungsgemeinschaft (SFB944, P10 to HJS), by the DAAD program “Ostpartnerschaften”, by the Ministry of Education and Science of the Russian Federation (RFMEEI61615X0044), and by the Russian Foundation for Basic Research (RFBR, grant 17-04-01219). Computational facilities of the Lomonosov Supercomputing Center of Moscow State University were used. We thank Rouslan Efremov and Christos Gatsogiannis for initial advice and discussion about the EM-experiments and Ramona Justinger (Forschungszentrum Jülich) for excellent technical support.




