There and Back Again: Loss and Reacquisition of Two-Cys Photocycles in Cyanobacteriochromes†
Abstract
Cyanobacteriochromes (CBCRs) are cyanobacterial photoreceptors distantly related to phytochromes. Both families use linear tetrapyrrole (bilin) chromophores that are covalently attached to a conserved Cys residue. CBCRs are more spectrally diverse than phytochromes, with known examples detecting light from the near ultraviolet to the edge of the infrared (370-750 nm). Detection of ultraviolet to blue light by CBCRs is mediated by a second Cys residue, which forms a covalent linkage to the bilin C10 atom. Second linkage formation is best understood in a subfamily possessing a conserved Asp-Xaa-Cys-Phe (DXCF) motif. Some DXCF CBCRs can isomerize their phycocyanobilin (PCB) chromophores into phycoviolobilin (PVB), a property not reported for other lineages. Both the DXCF Cys and PVB formation have been lost during evolution of other CBCR subfamilies. Using phylogenetic analysis and characterization of recombinantly expressed CBCRs, we show that the DXCF Cys residue has also been reacquired during CBCR evolution. Guided by this knowledge, we successfully reintroduced a second cysteine into a red/green CBCR, restoring blue-light sensing and PVB formation with two additional substitutions. Our results validate the roles of these residues in CBCR spectral tuning and thus provide new insight into the molecular basis of their spectral diversity.
Introduction
Photosynthetic organisms often harbor a rich complement of photosensory proteins to allow optimization of metabolism and behavior in response to the light environment 1. For example, land plants utilize phytochrome proteins to detect red and far-red light 2-6. Cyanobacteria also contain phytochromes, which some cyanobacteria use to control responses to far-red light 7-12. In recent years, it has become clear that many aspects of cyanobacterial photobiology are instead regulated by the distantly related cyanobacteriochromes (CBCRs). The phenomenon of complementary chromatic acclimation (CCA), first described over a century ago in the cyanobacterium Oscillatoria sancta, is controlled by CBCRs 13-18. Other CBCRs regulate phototaxis, the transition between motile and sessile lifestyles, and biofilm formation in unicellular cyanobacteria 19-24.
Phytochromes and CBCRs share a conserved GAF domain 25. However, while phytochromes are large photoreceptors usually possessing a PAS-GAF-PHY tridomain photosensory module, CBCRs require only the GAF domain for chromophore binding, spectral tuning, and reversible photoconversion 2, 25-27. Both phytochromes and CBCRs utilize linear tetrapyrrole (bilin) chromophores to detect light. Bilins are covalently attached to the protein via a thioether linkage to a conserved Cys in the CBCR domain 27. This canonical Cys or “first” Cys is often essential for efficient binding of bilin chromophore 28, 29. Absorption of a photon by the bilin π system triggers reversible photoisomerization of the bilin 15,16-double bond in both phytochromes and CBCRs 25, 27, 30-37. After primary photoisomerization of the dark-adapted state, one or more light-independent steps lead to formation of a metastable photoproduct which can revert to the dark state over seconds to days via dark reversion 18, 38.
CBCRs reported to date adopt the 15Z configuration in the dark state and the 15E configuration in the photoproduct. This has been established by characterization of samples in different photostates after acid denaturation: 15E bilins can be photoconverted to the 15Z configuration under such conditions, but not vice versa 39, 40. Characterization of denatured samples has also established that CBCRs can harbor different bilins. For example, CBCRs controlling CCA in response to green and red light contain phycocyanobilin (PCB) adducts 16-18. CBCRs controlling cell–cell aggregation in Thermosynechococcus spp. instead contain phycoviolobilin (PVB) adducts (see Figure S1, for structures of PCB and PVB adducts, and refs. 22, 24, 41, 42). Cyanobacterial cells synthesize PCB and CBCRs initially incorporate PCB, but some CBCRs are able to isomerize PCB into PVB in the absence of other proteins 41-43.
CBCRs exhibit astonishing spectral diversity 9, 22, 27, 42, 44, with published examples exhibiting peak dark-state absorption ranging from the near ultraviolet (386 nm) to the edge of the infrared (740 nm). Multiple CBCR subfamilies have been defined by conserved amino acid motifs, or signature sequences, and by photocycle 9, 27, 29, 44-49. For example, CBCRs acting as CCA regulators possess green-absorbing 15Z dark states and red-absorbing 15E photoproducts, and these properties are regulated by a protochromic triad of amino acids that are characteristic of this subfamily 16-18. Other CBCRs exhibit reverse photocycles, in which the 15Z dark-adapted state absorbs red light and the 15E photoproduct absorbs green light 29, 38. Such red/green photocycles have apparently evolved more than once 48. The first red/green subfamily to be recognized 29 includes the well-characterized proteins AnPixJg2 and NpR6012g4 29, 35-38, 47, 50-58. In this subfamily, spectral tuning of the green-absorbing 15E photoproduct relies on conserved Phe residues to trap a twisted chromophore geometry in which the bilin D-ring is out of conjugation 36, 47. These red/green CBCRs also have characteristic conserved aromatic residues on the 5th and 6th β-strands of the GAF fold (Fig. 1). Red/green CBCRs are most closely related to the insert-Cys CBCRs and red/blue CBCRs 9, 46, both of which share the aromatic signature residues of the red/green CBCRs on the fifth and sixth β strands (Fig. 1).

Other CBCR lineages have different signature sequences. For example, a widespread CBCR lineage has a characteristic Asp-Xaa-Cys-Phe (DXCF) motif not found in red/green CBCRs 45. Such CBCRs typically exhibit dark-adapted states with peak absorption of blue, violet, or near ultraviolet light 21, 41, 42, 45, 49, 59. Bilins intrinsically exhibit peak absorption in the green to red region of the spectrum in the absence of protein structure, and the DXCF Cys residue causes the blue shift of chromophore absorption by forming a second linkage to the bilin C10 atom (Fig. 2). In such cases, initial photoisomerization of the bilin 15,16-double bond is often followed by light-independent elimination of this second thioether linkage, resulting in a red shift of the 15E photoproduct to yield a green-absorbing PVB photostate or an orange-absorbing PCB photostate 42, 45, 60, 61. DXCF CBCRs are widespread, and specialized lineages with characteristic domain architectures, bilin content, and/or photocycles have been identified 49. In the most extensive phylogenetic analysis of CBCRs reported to date, one DXCF lineage was recovered as sister to the red/green subfamily of CBCRs, and both groups were found to be descended from other DXCF proteins 49. This sister DXCF lineage includes several examples that use the same Phe residues found in red/green CBCRs to generate a trapped-twist photoproduct with peak absorption at approximately 500 nm, in the teal region of the spectrum 48, 49. However, the signature aromatic residues of the red/green lineage are absent in such proteins. The descent of red/green CBCRs from DXCF CBCRs demonstrates that, the spectroscopic similarity between the red-absorbing 15Z dark states of red/green CBCRs and phytochromes is an example of convergent evolution.

Convergent evolution can also be seen in detection of shorter wavelengths of light by CBCRs, because DXCF CBCRs are not the only CBCRs to use a second Cys for detection of blue to ultraviolet light. The insert-Cys CBCRs contain a large, variable insertion loop after the second β strand of the GAF domain 9. The inserted sequence contains a conserved Cys residue which is essential for detection of blue, violet, or ultraviolet light by insert-Cys CBCRs and which again forms a second linkage to the bilin C10 atom 9, 62. A similar linkage is also thought to form in the 15E photoproduct state of red/blue CBCRs 46, and yet another second Cys residue is implicated in this lineage (Fig. 1). Green/blue photocycles have been reported in specialized DXCF CBCR lineages, and two-Cys photocycles have even been reported in cyanobacterial and algal phytochromes 9, 49, 63. All such cases characterized to date share peak absorption at ≤460 nm in one or both photostates, a canonical first Cys residue, and a second Cys residue essential for detecting light at short wavelengths.
In the current work, we revisit the proposed evolution of red/green CBCRs from DXCF CBCRs in light of the identification of new hybrid sequences with signature residues of both CBCR lineages. We provide phylogenetic evidence for the reacquisition of the DXCF Cys residue in both red/green and insert-Cys CBCR lineages. In vitro characterization of recombinantly expressed CBCRs established the functional significance of the reacquired DXCF Cys residue for light sensing. Moreover, we demonstrate that it is possible to reconstruct a functional DXCF two-Cys photocycle in a model red/green CBCR. Our studies highlight the relative ease with which CBCR photocycles and bilin isomerization are amenable to engineering and provide new insight into changes in spectral tuning during CBCR evolution.
Materials and Methods
Bioinformatics
We used a published CBCR sequence alignment 49 as an initial guide. BLAST 64 searches of the GenBank and DOE-IMG databases were used to identify additional CBCR sequences, which were added to the alignment by hand to construct an alignment of 217 sequences in total (see Data S1). The alignment was then combined with structural information from the 15Z crystal structures of AnPixJ and TePixJ (PDB accessions 3W2Z, 4FOF, 4GLQ: 33, 35). Secondary structure assignments and solvent accessibility calculated using DSSP 65 were added to the alignment using an in-house script that also removed positions having ≥5% gaps and converted the alignment to PHYLIP format. The resulting alignment of 217 sequences with 163 positions was used to infer a maximum-likelihood phylogeny in PhyML-structure 66, 67 using an EX-EHO substitution model in partition mode with confidence estimated by the approximate likelihood ratio test (aLRT) using four substitution rate categories and calculated estimates for the gamma-shape distribution parameter and the proportion of invariable sites (command-line settings: -m EX_EHO -M PART -a e -c 4 -v e). CBCR phylogenies inferred using this procedure have been shown to be comparable to those obtained using Bayesian methods 49.
Cloning and site-directed mutagenesis of CBCRs
Anacy_3174g6 (amino acids 951–1123 of the full-length Anacy_3174 open reading frame in Anabaena cylindrica PCC 7122) was cloned from genomic DNA prepared from Anabaena sp. PCC 7938 (generous gift of Elsie Campbell and Prof. Jack Meeks, UC Davis) using PCR with appropriate primers that include bases at the 5′ end to create a start codon with an NcoI restriction site and at the 3′ end to create a SmaI site allowing in-frame fusion to the coding sequence for the C-terminal intein-CBD affinity tag. The amplified DNA sequence from Anabaena sp. PCC 7938 was identical to that of Anabaena cylindrica PCC 7122. M595_0799g2 (amino acids 244–414 of the M595_0799 open reading frame from Lyngbya aestuarii strain BL J) and LYNGBM3L_56870g6 (amino acids 979–1175 of the LYNGBM3L_56870 open reading frame from Moorea producens strain 3L) were obtained as synthetic genes (Genscript, Piscataway, NJ) codon optimized for expression in Escherichia coli with flanking NcoI and SmaI restriction sites. M. producens strain 3L was previously classified as Lyngbya majuscula 68, 69. All CBCR genes were then subcloned into the expression plasmid pBAD-Cph1-CBD 70 using unique NcoI and SmaI sites to generate in-frame fusions to the C-terminal intein-CBD tag. Variant proteins were constructed by site-directed mutagenesis using the QuikChange Kit (Stratagene) with appropriate primers in accordance with the manufacturer's directions. Amino acid numbering follows the numbering of the full-length protein rather than the isolated CBCR domain in all cases, and all constructs were verified by nucleotide sequencing.
Purification and characterization of CBCRs
Expression of Anacy_3174g6, M595_0799g2, and LYNGBM3L_56870g6 as intein-CBD fusion proteins in E. coli engineered to produce PCB followed the published procedure 71. Escherichia coli cultures were harvested by centrifugation, and cell pellets were stored at –80°C until use. For purification, pellets were resuspended in 20 mm Na-HEPES (pH 8.0), 500 mm NaCl, 1 mm EDTA and lysed using a microfluidizer. Lysate was clarified by ultracentrifugation prior to loading onto a chitin column at 4°C (New England Biolabs) and washing in accordance with the manufacturer's directions 49. Intein-CBD cleavage was triggered by addition of 50 mm DTT followed by overnight incubation. Peak fractions were dialyzed into TKKG buffer (25 mm TES-KOH pH 7.8, 100 mm KCl, 10% (v/v) glycerol) for spectroscopic characterization. Purified CBCRs were analyzed by SDS-PAGE and zinc blotting (Figure S2). Absorption spectra were acquired on a Cary 50 spectrophotometer at 25°C. Photoconversion was triggered in the absorption cuvette using a xenon source equipped with band-pass interference filters (400 nm/70 nm FWHM, 450 nm/25 nm FWHM, 500 nm/40 nm FWHM, 550 nm/70 nm FWHM, 600 nm/40 nm FWHM, 650 nm/40 nm FWHM: CVI Laser Optics). For denaturation assays, a 100 μL aliquot of protein was diluted with 1 mL of 7 m guanidinium chloride/1% HCl (v/v). Photoconversion of denatured samples was assayed using the xenon lamp and a 320 nm long-pass filter (Schott). All difference spectra are reported as 15Z – 15E.
Results
Loss and reacquisition of the DXCF Cys during CBCR evolution
Despite the similarity between the dark states of red/green CBCRs and phytochromes, phylogenetic analyses demonstrated that red/green CBCRs are more closely related to DXCF CBCRs than phytochromes 48, 49. Red/green CBCRs are also closely related to insert-Cys and red/blue CBCRs 9, 46. In the largest CBCR phylogeny reported to date, two CBCR domains from Lyngbya spp., including M595_0799g2 from Lyngbya aestuarii strain BL J, were recovered as sister to a large lineage comprising red/green, insert-Cys, and red/blue CBCRs 49. These Lyngbya CBCR sequences have both the DXCF Cys residue and signature residues found in red/green CBCRs, such as conserved hydrophobic residues on the fifth and sixth β strands and a DXXL motif rather than a DXCF motif (Fig. 1). The position of these hybrid sequences in phylogenetic analyses thus implied that sequence adaptations associated with the red/green photocycle emerged before loss of the DXCF Cys.
To better understand this transition in CBCR evolution, we first sought to establish whether such hybrid sequences retained a functional DXCF Cys and a two-Cys photocycle. We therefore characterized purified M595_0799g2 as an isolated CBCR domain after recombinant expression in E. coli engineered to synthesize PCB. M595_0799g2 exhibited a dark-adapted state with peak absorption at the edge of the ultraviolet (394 nm, Fig. 3A and Table 1). Illumination with violet light (400 ± 35 nm) resulted in photoconversion to a photoproduct state at slightly longer wavelengths, with an apparent peak in the violet region of the visible spectrum (402 nm). However, photoconversion was shown to be incomplete by an acid denaturation assay, which also revealed the presence of a PCB adduct (Fig. 3B). A variant protein lacking the DXCF Cys residue exhibited peak absorption at much longer wavelengths (Fig. 3C). Illumination resulted in modest formation of a blue-shifted photoproduct. These data thus emphasize the hybrid nature of M595_0799g2: It retains a functional DXCF residue and has a two-Cys photocycle, but it contains the PCB adduct of red/green CBCRs rather than the PVB adduct found in many DXCF CBCRs.

| Protein | Variant | SAR | 15Z peak (nm) | 15E peak (nm) |
|---|---|---|---|---|
| M595_0799g2 | Wild-type | 0.4 | 394 | Incomplete |
| M595_0799g2 | C317A | 0.7 | 616 | Incomplete |
| Anacy_3174g6 | Wild-type | 0.2 | 550 | 424 |
| Anacy_3174g6 | C1027A | 0.03 | 622 | None formed |
| LYNGBM3L_56870g6 | Wild-type | 0.3 | 386 | Incomplete |
| LYNGBM3L_56870g6 | C1185A (– 1st Cys) | 0.2 | 384 | Incomplete |
| LYNGBM3L_56870g6 | C1140A (– insert Cys) | 0.2 | 402, 614 | 416 |
| LYNGBM3L_56870g6 | C1156A (– DXCF) | 0.4 | 386 | 382, 564 |
| NpR6012g4 | Wild-type | 0.9 | 650 | 542 |
| NpR6012g4 | H659C (DTCLQ) | 0.4 | 404, 650 | 406, 540 |
| NpR6012g4 | H659C L660F (DTCFQ) | 0.1 | 406 | Incomplete |
| NpR6012g4 | A622L DTCFQ | 0.08 | ~ 406, 566 | 410 |
- SAR, specific absorbance ratio (peak absorption of long-wavelength bilin band/peak absorption of protein ultraviolet band). Values for wild-type NpR6012g4 are from ref. 38.
This result prompted us to search for additional hybrid sequences. Several such sequences were found, along with additional red/green and insert-Cys sequences. We also observed closely related sequences that have lost the canonical first Cys and hence are predicted to have lost the ability to bind chromophore. These sequences were all combined into an alignment comprising 217 sequences (see Data S1), including diverse DXCF CBCRs and a small outgroup of knotted phytochromes. This alignment was combined with information from available CBCR crystal structures 33, 35 to infer a maximum-likelihood (ML) phylogeny using PhyML-structure 67. A collapsed view of the resulting tree is presented in Fig. 4 (for the full tree, see Data S2). Consistent with previous analyses 9, 45, 49 and with the small size of the CBCR GAF domain, a robust picture of CBCR evolution could not be inferred. However, this analysis nevertheless provided new insight into the evolution and diversification of the red/green CBCR subfamily and its closest relatives.

In the current analysis, red/green and insert-Cys CBCRs are largely separated into two clades that are sister to each other (Fig. 4 and Figure S3). The known red/blue CBCRs, both of which are found in Acaryochloris strains 46, arise within the red/green CBCRs as a derived lineage and are sister to red/green CBCR sequences that are also from Acaryochloris strains (Fig. 5A). The two hybrid sequences from Lyngbya spp., including M595_0799g2, are sister to red/green, insert-Cys, and red/blue CBCRs as expected (Figs 4 and 5B), but we did not recover a single clade of hybrid sequences. We therefore define a monophyletic “expanded red/green” lineage (hereafter, XRG lineage) that includes all such hybrid sequences, canonical red/green CBCRs, insert-Cys CBCRs, and red/blue CBCRs (Fig. 4, dashed box, and Figure S3). The XRG lineage has robust signature residues, including conserved aromatic residues on the 5th and 6th beta strands and a consensus DXXL motif in place of the DXCF motif (Fig. 1). As in our previous analysis 49, a monophyletic lineage of DXCF CBCRs was recovered as sister to the XRG lineage (Fig. 4). This DXCF lineage includes most known teal-DXCF proteins and can be distinguished from the XRG lineage by a DXCF motif and by hydrophilic residues in place of the conserved aromatic residues on the 5th and 6th beta strands (Fig. 1).

Hybrid sequences do not form a monophyletic group within the XRG lineage (Figs 4 and 5B,C). Notably, a small group of hybrid sequences including Anacy_3174g6 was recovered as derived from red/green CBCRs with good support (Fig. 5C). These sequences thus represent an apparent example of reacquisition of the DXCF Cys after the transition to the red/green photocycle. Similarly, we noted that the insert-Cys CBCR LYNGBM3L_56870g6 has also acquired an apparent DXCF Cys residue (Fig. 1). LYNGBM3L_56870g6 is placed as a derived insert-Cys sequence (Fig. 5D), consistent with the presence of the insert-Cys sequence and of XRG signature residues (Fig. 1). This protein thus provides an apparent example of DXCF reacquisition after the transition to insert-Cys CBCRs. We conclude that reacquisition of the DXCF Cys has occurred repeatedly during CBCR evolution. However, phylogenetic analysis alone does not reveal whether DXCF Cys reacquisition has been accompanied by changes in spectral tuning.
Reacquired DXCF Cys residues are functional
We therefore examined two previously uncharacterized CBCRs, Anacy_3174g6 and LYNGBM3L_56870g6. Both are found in larger proteins that have a series of CBCR domains in tandem along with a C-terminal MCP domain. Hence, these proteins have domain architectures similar to those of PtxD, the phototaxis photoreceptor in Nostoc punctiforme 23. Anacy_3174g6 is a hybrid sequence from Anabaena cylindrica strain PCC 7122 and is the last of six GAF domains found in tandem in the full-length Anacy_3174 protein. LYNGBM3L_56870g6 is an insert-Cys CBCR from Moorea producens and is the sixth of seven GAF domains found in tandem in the full-length LYNGBM3L_56870 protein. Both CBCRs were characterized in vitro as isolated CBCR domains after recombinant expression as intein-CBD fusion proteins in E. coli engineered to synthesize PCB 71.
Anacy_3714g6 exhibited facile, reversible photoconversion between a green-absorbing state and a blue-absorbing state (Fig. 6A). The green-absorbing state had a red shoulder that could be photoconverted to a similar blue-absorbing species with red light (Fig. 6B). Denaturation analysis revealed the presence of a mix of PVB and PCB, with PVB as the majority species (Fig. 6C), and demonstrated that the blue-absorbing state was the 15E photoproduct. Therefore, Anacy_3174g6 exhibited a green/blue photocycle. A variant protein lacking the DXCF Cys residue exhibited poor chromophore binding (Table 1). The small amount of holoprotein that was present exhibited peak absorption at 622 nm and did not undergo photoconversion (Fig. 6D). Taken together, these data demonstrate that Anacy_3174g6 has reacquired both second linkage formation and PVB formation late in its evolution, and the reacquired DXCF Cys residue is essential for both PVB formation and the green/blue photocycle.

LYNGBM3L_56870g6 exhibited photoconversion between two states with peak absorption in the ultraviolet to blue (Fig. 7A and Table 1). Denaturation analysis of the lit state demonstrated the presence of PCB and showed that photoconversion was incomplete (Fig. 8A). This analysis established the 15Z photostate in LYNGBM3L_56870g6 as having peak absorption at 386 nm (Table 1), but incomplete conversion precluded identification of the peak wavelength for the 15E photostate. LYNGBM3L_56870g6 therefore exhibited a second linkage in both photostates, resulting in overlapping absorption bands. However, these data do not indicate whether that second linkage requires the insert Cys or the DXCF Cys. We therefore used site-directed mutagenesis to construct variants of this protein lacking the first (canonical) Cys, the insert Cys, or the DXCF Cys.


The variant lacking the first Cys (Cys1185) exhibited reduced chromophore binding (Table 1) but only subtle changes in peak wavelength and lineshape (Fig. 7B). Interestingly, denaturation analysis of this variant revealed two populations with overlapping absorption maxima (Fig. 8B): one with peaks at 686 and 374 nm, and another with peaks at shorter wavelengths (606 and 334 nm). Peak wavelengths for the first population were at longer wavelengths than expected for covalent PCB adducts 48, 72, consistent with loss of the A-ring linkage and hence with loss of covalent binding upon denaturation. Peak wavelengths for the second population were ambiguous but were consistent with the presence of a minority PVB population. These data implicate the first Cys in chromophore binding and in determining the bilin content in LYNGBM3L_56870g6.
The variant protein lacking the insert Cys (Cys1140) exhibited striking differences relative to wild-type LYNGBM3L_56870g6 (Fig. 7C). This variant could readily photoconvert between a violet-absorbing state (416 nm: Table 1) and a mixed state having both violet (402 nm) and orange-red (614 nm) absorption maxima. These results are consistent with the presence of a stable second Cys linkage in the violet-absorbing state, but partial loss of that linkage in the mixed state. Denaturation analysis identified the violet-absorbing state as containing predominantly 15E bilin, whereas the violet and orange-red species instead correspond to two populations containing predominantly 15Z bilin (Fig. 8C,D).
The variant protein lacking the DXCF Cys (Cys1156) also converted between an ultraviolet-absorbing state (386 nm: Fig. 7D) and a mixed state with absorption in the ultraviolet (382 nm) and green (564 nm). By contrast with the ultraviolet-absorbing 15E population observed in the variant lacking the insert Cys, the ultraviolet-absorbing population in the variant lacking the DXCF Cys had very similar spectral properties to those of the 15Z photostate of wild-type LYNGBM3L_56870g6. Denaturation analysis confirmed that this ultraviolet-absorbing species contained predominantly 15Z bilin, whereas the mixed state contained both 15Z and 15E bilin populations Fig. 8E,F). These data demonstrate that both the insert-Cys residue and the DXCF Cys residue are essential for the LYNGBM3L_56870g6 photocycle, making this protein the first-known three-Cys CBCR. We conclude that the DXCF Cys residue is functional in both Anacy_3174g6 and LYNGBM3L_56870g6.
Engineering a second linkage in the red/green CBCR NpR6012g4
The reacquisition of functional DXCF Cys residues in naturally occurring CBCRs led us to consider introduction of such a residue into a red/green CBCR. The DXCL motif seen in hybrid sequences such as M595_0799g2 and Anacy_3174g6 is equivalent to the DTHL of the model red/green CBCR NpR6012g4 (Fig. 1), with His659 as the equivalent to the second Cys residue. We therefore constructed and characterized the H659C variant of NpR6012g4 (hereafter, DTCLQ NpR6012g4). This variant exhibited reduced chromophorylation (Table 1), but the red-absorbing 15Z photostate exhibited additional absorption in the violet region of the spectrum relative to the 15Z photostate of the wild type (Fig. 9A). Surprisingly, the ratio of red to violet absorption in this 15Z photostate was temperature dependent (Fig. 9B), with higher temperature favoring the red-absorbing species (650 nm peak absorption: Table 1) and lower temperature favoring the violet-absorbing species (404 nm). At 10°C, selective illumination of the violet-absorbing population yielded increased green absorption with concomitant bleaching of both violet and red absorption (Fig. 9C). Selective illumination of the red-absorbing population at 40°C (Fig. 9C) resulted in formation of a violet-absorbing species (obscured by the loss of the 15Z violet-absorbing species and of the Soret band of the red-absorbing species) and much lower amounts of a green-absorbing photoproduct (Table 1). These 15E green- and violet-absorbing species again were in thermal equilibrium, but in this case, higher temperatures favored the violet-absorbing species (Fig. 9D).

We next examined the effects of an additional substitution in NpR6012g4. Leu660 is equivalent to the DXCF Phe residue and is immediately C-terminal to the introduced DXCF Cys residue (Fig. 1). The doubly substituted H659C L660F NpR6012g4 variant (hereafter, DTCFQ NpR6012g4) thus has a complete DXCF motif. The L660F substitution has been previously described and had only minor effects on the red/green photocycle of NpR6012g4 48. By contrast, DTCFQ NpR6012g4 exhibited a violet-absorbing state (406 nm, Table 1) with greatly reduced red and green absorption (Fig. 10A), implicating the DXCF Phe residue in stabilizing the second linkage. However, illumination resulted in only slight spectral shifts, implicating loss of activity in this variant. Interestingly, denaturation analysis of DTCFQ NpR6012g4 revealed the presence of both PCB and PVB, whereas DTCLQ NpR6012g4 contained only PCB (Fig. 10B).
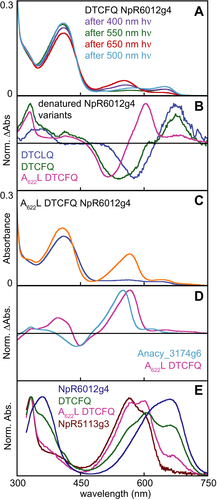
The poor photoactivity of DTCFQ NpR6012g4 prompted us to introduce a third substitution. Ala622 in NpR6012g4 is a conserved small residue in the first β strand of red/green and teal-DXCF CBCRs 47. In other DXCF CBCRs, such as Tlr0924, the equivalent residue is a larger aliphatic residue such as Leu (Fig. 1). Previous studies showed that the A622L substitution had only minor effects on the red/green photocycle of NpR6012g4 47. Remarkably, the triply substituted A622L DTCFQ variant exhibited photoconversion between violet- and green-absorbing states (Fig. 10C). This behavior resulted in a difference spectrum similar to that of Anacy_3174g6 (Fig. 10D), albeit with less efficient photoconversion and chromophore incorporation (Table 1). Denaturation analysis (Fig. 10B & E) demonstrated that PVB content was higher in this variant than in DTCFQ NpR6012g4, showing that the three mutations are sufficient to convert a red/green CBCR into a green/blue sensor. These results demonstrate that PVB content and spectral tuning are both amenable to engineering upon reintroduction of a DXCF motif into NpR6012g4.
Discussion
Reacquisition of the DXCF Cys in red/green and insert-Cys CBCRs
In this work, we examine the evolutionary transition from DXCF CBCRs to red/green CBCRs, in which the DXCF Cys and its second linkage to the chromophore were lost. Phylogenetic analysis allowed us to define a monophyletic expanded red/green (XRG) lineage comprising red/green, insert-Cys, red/blue, and hybrid CBCRs. This analysis is consistent with loss of the DXCF Cys after appearance of signature residues of the XRG lineage, such as the conserved hydrophobic residues on the 5th and 6th beta strands and the DXXL motif (Fig. 1). The subsequent transition from hybrid sequences such as M595_0799g2 to red/green or insert-Cys CBCRs is not fully resolved in the current phylogenetic analysis, but it is clear that this transition must involve loss of the DXCF Cys residue.
This loss has also been reversed during CBCR evolution: Both Anacy_3174g6 and LYNGBM3L_56870g6 are derived sequences that reacquired functional DXCF Cys residues. This reacquisition of the DXCF Cys is analogous to acquisition of a second Cys residue in red/blue CBCRs 46, but in the latter case, a different second Cys residue is used (Fig. 1). Anacy_3174g6 has reacquired both the DXCF Cys residue and PVB formation within the context of a red/green CBCR, resulting in a green/blue photocycle. The variant Anacy_3174g6 protein lacking the DXCF Cys is photochemically inactive. Similar behavior has been observed in DXCF CBCRs 45, 49, 59. The small amount of chromophore still bound in the Anacy_3174g6 variant protein absorbs in the red region of the spectrum, implicating loss of PVB formation in the absence of the DXCF Cys in Anacy_3174g6. Again, this behavior mimics that seen in DXCF CBCRs 42, 45, 59. These data confirm that reacquisition of the DXCF Cys residue in this protein has been accompanied by reacquisition of two hallmarks of DXCF proteins: a two-Cys photocycle and PVB formation.
LYNGBM3L_56870g6 is an apparently unique example of an insert Cys that has reacquired the DXCF Cys residue, making it the first-known 3-Cys CBCR. Characterization of variant proteins demonstrated that all three-Cys residues are important. The canonical first Cys is necessary for maximal bilin binding and for normal bilin content, with apparent PVB formation occurring in its absence. The insert-Cys residue is essential for spectral tuning of the 15Z dark state, whereas the DXCF Cys is essential for spectral tuning of the 15E photoproduct. We therefore propose a Cys-swap photocycle in LYNGBM3L_56870g6, with the insert Cys forming a second linkage in the dark state and the DXCF Cys forming a second linkage in the photoproduct (Fig. 11A). This model differs from previously reported two-Cys photocycles. In many CBCRs, the second linkage is present in the 15Z dark-adapted state (Fig. 2). In other cases, such as Anacy_3174g6, the situation is reversed and the second linkage is present in the 15E photoproduct (Fig. 12A). In contrast to these photocycles, LYNGBM3L_56870g6 has a second linkage present in both photostates. This is also the case in M595_0799g2 (Fig. 12B) and in the insert-Cys CBCR NpR1597g2 9, but in these cases, the same linkage remains stable throughout the photocycle. Instead, LYNGBM3L_56870g6 apparently has different Cys residues forming linkages in the two photostates (Fig. 11A).


The Cys-swap model we propose for LYNGBM3L_56870g6 implies that the 15Z spectrum of the variant lacking the DXCF Cys and the 15E spectrum of the variant lacking the insert Cys would mimic the two photostates of the wild-type protein. A difference spectrum calculated using these two spectra would then mimic that of wild type regardless of differences in the amount of photoconversion. Indeed, a calculated difference spectrum for these two variants is in excellent agreement with that of wild-type LYNGBM3L_56870g6 (Fig. 11B), consistent with the Cys-swap model. Future studies will be required to test this working hypothesis, but it is clear that the reacquired DXCF Cys is again required for the wild-type photocycle of LYNGBM3L_56870g6.
Engineering CBCR spectral responses
Characterization of Anacy_3174g6 and LYNGBM3L_56870g6 implied that tuning of red/green CBCR photocycles via introduction of an appropriately placed Cys residue might be feasible. We therefore introduced such a residue into the model red/green CBCR NpR6012g4. A single amino acid change was sufficient to introduce formation of a thermally labile second linkage, but the resulting DTCLQ variant protein retained considerable red/green activity. We were able to introduce efficient PVB formation and modest green/blue activity with only three substitutions (A622L H659C L660F). Effectively, we were able to reconstitute an inefficient version of the Anacy_3174g6 photocycle in NpR6012g4 by introducing a canonical DXCF motif and one additional substitution. It may prove similarly possible to alter CBCR spectral responses more generally with very few substitutions, a finding which would aid efforts to utilize CBCRs in multichannel optogenetics or synthetic biology. Similarly, the large spectral shifts induced upon loss or reacquisition of the DXCF Cys would be expected to shift the physiological action spectra of the CBCRs involved and hence might provide selective advantages under specific light environments. Future testing of this hypothesis will require establishment of more robust assays for physiological function in tandem CBCRs than are currently available.
Our studies also implicate elimination of the second linkage as a critical point in the evolution or engineering of CBCR photocycles. Many CBCRs are known to exhibit both overlapped photostates and incomplete photoconversion 42, including M595_0799g2 and LYNGBM3L_56870g6 in this work. Incomplete photoconversion in such cases is thought to arise due to establishment of a photoequilibrium between the overlapped photostates 42, with the extent of photoconversion determined by the forward and reverse rates for photoconversion in a given light environment and by the rate of dark reversion. The primary photoisomerization of doubly linked CBCR photostates does not produce a large spectral shift 60, 73. It is therefore possible that any primary 15E photoproduct formed could be photoconverted back to the 15Z state before it can undergo elimination of the second Cys. The low activity observed with DTCFQ NpR6012g4 could arise from such behavior, among other explanations. There is clearly residual blue-absorbing 15Z material present in the A622L DTCFQ triple mutant of NpR6012g4 (Fig. 10C), and photoconversion remains incomplete as judged by the denaturation assay. The subsequent transition from such a case to the efficient green/blue photocycle of Anacy_3174g6 would then be accomplished via specific destabilization of the doubly linked, blue-absorbing 15Z population. Although this concept is currently speculative, we anticipate that time-resolved studies of these variant proteins will aid in testing this hypothesis and in developing future variants with more robust photoconversion.
Acknowledgements
This work has been supported by a grant from the Chemical Sciences, Geosciences, and Biosciences Division, Office of Basic Energy Sciences, Office of Science, United States Department of Energy (DOE DE-SC0002395 to J.C.L.). We thank Elsie Campbell and Jack Meeks for the gift of genomic DNA.




