Surgical Site Infections After Pediatric Liver Transplantation—Impact of a Change in Perioperative Prophylactic Antibiotic Protocol
Funding: The authors received no specific funding for this work.
ABSTRACT
Background
In spite of improved survival rates after pediatric liver transplantation, infections remain major contributors to perioperative morbidity and mortality. This study aimed to understand the impact of type and duration of perioperative antibiotic prophylaxis (PAP) on the occurrence of surgical site infections (SSIs).
Methods
In total, 125 patients who underwent liver transplantation between 2014 and 2020 were retrospectively included. Patients were categorized into two periods based on changes in the standard PAP regimen. Risk factors for SSIs were investigated, including the influence of PAP duration, antibiotic substances used, and abdominal patch placement using multivariable regression models.
Results
SSIs occurred in 23 (19%) of 119 analyzed patients and were not impacted by changes in the PAP regimen. The placement of an abdominal patch was a relevant risk factor for SSIs (odds ratio 3.81; 95% confidence interval [CI] 1.15–12.68). Longer PAP duration reduced the occurrence of SSIs by up to 4.6 percentage points (95% CI 0.0–9.1) per day, with its effect diminishing with longer duration. The choice of antibiotic substances for PAP changed after implementation of the new protocol, with a decline in vancomycin usage from 14% to 3%.
Conclusion
The results of this study emphasize the need for evidence-based PAP regimens tailored to the unique needs of pediatric liver transplant recipients. The occurrence of SSIs remains complex and is influenced by various factors beyond the PAP regimen. Multicentric efforts to develop effective prevention strategies against SSIs in this vulnerable population are warranted.
Abbreviations
-
- CI
-
- confidence interval
-
- DAG
-
- directed acyclic graph
-
- IQR
-
- interquartile range
-
- MDR
-
- multidrug resistance/resistant
-
- MSAS
-
- minimally sufficient adjustment sets
-
- OR
-
- odds ratio
-
- PAP
-
- perioperative antibiotic prophylaxis
-
- PICU
-
- pediatric intensive care unit
-
- SD
-
- standard deviation
-
- SSI
-
- surgical site infection
1 Introduction
Pediatric liver transplantation is often the treatment of choice for chronic liver diseases like biliary atresia and acute liver failure. Because of improvements in care over the last two decades, 1-year survival rates reach up to 95%. As mortality declined, morbidity especially in the early postoperative phase on the pediatric intensive care unit (PICU) remains a substantial problem [1-9]. Patients' reduced preoperative condition, long surgery time, massive transfusions, immunosuppression, and a disturbed gastrointestinal barrier mainly contribute to the early complications.
Bacterial infections like bloodstream infections, pneumonia, or surgical site infections (SSIs) are among the most frequent and severe complications after transplantation [10-17] and directly or indirectly cause about half of early postoperative deaths. SSIs occur in about a quarter of all transplant recipients with the clinical presentation ranging from superficial wound infections to intra-abdominal abscess, peritonitis, or infected necrosis leading to sepsis and requiring additional surgery. Observational studies and registries identified [10, 12, 18] multi-resistant bacteria (MRB) colonization, high-level immunosuppression, and insertion of an abdominal patch as risk factors for SSIs.
At the same time, little is known about protective measures to prevent SSI. In contrast to many other surgical procedures, only few and partially differing recommendations or guidelines on duration or drug choice for perioperative antibiotic prophylaxis (PAP) after pediatric liver transplantation exist. For example, the adults and pediatrics guidelines from 2013 recommended a third-generation cephalosporin plus ampicillin or piperacillin-tazobactam alone [19]. Guidelines from 2019 by the American Society of Transplantation Infectious Diseases Community of Practice stated that ampicillin/sulbactam could be an alternative for 48 h or less, not distinguishing between adults and children [20].
Current clinical practice differs from the mentioned recommendations. A study on regimes in different pediatric transplant centers found a huge variety: from single shot antibiotic to more than a week, and from narrow spectrum to broad-spectrum antibiotic drug [21]. Currently, no data on the clinical value of PAP in children after liver transplantation are available.
In this retrospective single-center study, we assessed the effect of a change in the antibiotic perioperative prophylaxis protocol in pediatric liver transplantation from cefotaxime plus ampicillin without predefined duration to ampicillin/sulbactam with preplanned duration. Additionally, we examined the influence of the duration of the PAP on the occurrence of SSIs.
2 Methods
2.1 Patients
All patients <18 years of age who received a liver transplantation between January 2014 and December 2020 were included. Anonymized data were collected by retrospective chart review and comprised demographics and clinical and outcome information. SSI was adapted from the definition of the Centers for Disease Control and Prevention National Healthcare Safety Network [22] and reported as superficial, deep incisional and organ space infections further classified as cholangitis, intra-abdominal abscess/infected hepatic necrosis, or peritonitis. Infectious complications were assessed per patient and as individual events, that is, an SSI with peritonitis and cholangitis were counted as two events.
2.1.1 Definition of Organ Space Infections
SSIs involving organ/space were classified as follows: (a) Infected ascites/peritonitis was diagnosed if >250 granulocytes/μL collected by puncture or via a proper drain were associated with a positive bacterial culture. Abscess or infected necrosis was ruled out by ultrasound or other imaging. (b) Cholangitis was present when a fever >38°C was associated with a new or aggravated cholestasis, dilated bile ducts upon ultrasound, or if associated with positive bacterial cultures obtained from percutaneous or perioperative drainage. (c) Infected hepatic necrosis was defined by clinical SIRS (systemic inflammatory response syndrome) in accordance with histologically proven necrosis, new or aggravated increase in transaminases, or heterogeneous texture of the graft viewed using ultrasound. An abscess was defined as a collection of fluid drained surgically or aspirated under ultrasound guidance, which showed pus cells upon microscopy and for which the culture yielded one or more organisms, if not already under antibiotic treatment.
2.2 Anti-Infective Strategy and Surveillance
2.2.1 Period 1 (P1, 01/2014–02/2017)
Children after liver transplantation received ampicillin and cefotaxime for PAP if the MRB colonization status was negative. Treatment duration was at the discretion of the treating physician and was influenced by clinical course, presence of an abdominal patch, and course of inflammation biomarkers (mainly C-reactive protein, CrP). Antifungal prophylaxis was administered by oral nystatin during the PICU stay.
2.2.2 Period 2 (P2, 03/2017–12/2020)
In 2017, standard operating procedure (SOP) for perioperative antimicrobial prophylaxis was changed to ampicillin and sulbactam for three reasons: coverage of anaerobic bacteria, simplicity (one antibiotic vs. two before), and avoidance of third-generation cephalosporines. Preplanned duration was limited to 5 days in infants and 2 days in children older than 1 year. Intravenous fluconazole at 4 mg/kg during PICU stay was introduced as antifungal prophylaxis instead of oral nystatin. Adherence to the intended age-specific PAP duration was defined as SOP conformity. The new prophylaxis was integrated in the SOP of the postoperative period in the PICU and was presented to all hepatology and intensive care consultants who are in charge of children after liver transplantation.
In both periods, divergence from usual antibiotic choice for PAP was possible if MDR colonization was present, if the patient was already treated shortly before transplantation, or on the discretion of the responsible physician.
2.2.3 Supportive Nonpharmacological Anti-Infective Measures
Throughout the entire study period, further anti-infective strategies included single room whenever possible, gloves and gown for direct patient care. Skin surgical protocol disinfection (povidone-iodine based) did not change over the time of the study. Surveillance was performed by bacterial/fungal cultures from ascites, pleural effusions, and tracheal secretions on Days 1, 3, and 7 after transplantation, and in case of suspected infection like fever (all if drainage or tube in place). Viral surveillance was performed by weekly measurement of cytomegaly and Epstein–Barr serum viral loads.
2.3 Statistics
Data are presented as median and 25th to 75th percentiles for skewed variables and mean ± standard deviation (SD) for symmetrical distributions. Discrete variables are presented as counts and relative frequencies. Deceased patients were excluded from all analyses because all but one death occurred within 2 days after transplantation.
Adjusted odds ratios (ORs) with 95% confidence intervals (CIs) for SSIs were calculated for the exposures of interest using multivariable logistic regression. A multivariable linear regression model was used to assess the association of PAP duration and SSIs. To identify the minimally sufficient adjustment sets (MSAS) for the regression models, causal diagrams based on the theory of directed acyclic graphs (DAG) were used, as recommended for causal inference in pediatric and critical care research [23-26]. For PAP duration and SOP-conform PAP duration over both periods, MSAS included age, CRP, presence of an abdominal silicone patch, reason for LTX, and preoperative MDR carrier status (Figure S1a). To assess of the impact of an abdominal patch on the occurrence of SSIs, the MSAS was the type of transplanted organ (whole or split organ) (Figure S1b). The MSAS for the type of antibiotic (ampicillin + cefotaxim vs. ampicillin/sulbactam) was age, abdominal patch, and preoperative MDR carrier status (Figure S1c).
Statistical analyses were performed using SAS Enterprise Guide 8.3 (SAS Institute Inc., Cary, NC, USA).
2.4 Ethics
The study protocol was approved by the local ethics committee of the Medical Faculty of the University Duisburg-Essen (20-9732-BO).
3 Results
A total of 125 patients was included (P1: n = 59, P2: n = 66), of which six patients died (P1: n = 1 [2%], P2: N = 5 [8%]) (Table 1). No deceased patient experienced infectious complications. Because all but one patient died within 2 days after transplantation (Table S1), deceased patients were excluded from further analyses. In both periods, survivors showed similar demographics, MDR bacterial colonization, postoperative laboratory results, incidence of abdominal silicone patch for temporary wall closure, underlying disease, and type of transplantation (split versus full organ and deceased versus living related liver transplantation) (Table 1).
| 2014–2016 | 2017–2020 | |
|---|---|---|
| N total | 59 | 66 |
| Died | 1 (2%) | 5 (8%) |
| N analyzed | 58 | 61 |
| Age, median (IQR), years | 1.6 (0.6–8.8) | 2.5 (0.6–9.1) |
| Male | 25 (43%) | 37 (61%) |
| Length of PICU stay, median (IQR), days | 14.5 (8–22) | 11 (7–17) |
| Duration of mechanical ventilation, median (IQR) | 4 (1–7) | 2 (1–6) |
| Living donor transplantation | 23 (40%) | 29 (48%) |
| Deceased donor transplantation | 35 (60%) | 32 (52%) |
| Split organ transplantation (only in deceased donor transplantations) | 8/35 (23%) | 9/32 (28%) |
| Primary disease | ||
| Chronic hepatopathy | 43 (74%) | 46 (75%) |
| Retransplantation | 7 (12%) | 3 (5%) |
| Metabolic disease | 3 (5%) | 5 (8%) |
| Acute liver failure | 5 (9%) | 4 (7%) |
| Others | 1 (2%) | 6 (10%) |
| Postoperative laboratory exams | ||
| Minimum quick value within the first 24 h post liver transplantation (%) (mean ± SD) | 28 ± 11 | 26 ± 10 |
| Maximum serum lactate within the first 24 h post liver transplantation (mmol/L) (mean ± SD) | 8.7 ± 5.3 | 7.0 ± 4.0 |
| Maximum serum CrP within the first 72 h post liver transplantation (mg/dL) (mean ± SD) | 8.2 ± 4.7 | 8.4 ± 4.3 |
- Note: Quick value represents the percentage of normal thromboplastin time.
- Abbreviations: CrP, C-reactive protein; IQR, interquartile range; PICU, pediatric intensive care unit; SD, standard deviation.
The median length of PICU stay declined from 14.5 days in P1 to 11 days in P2, and the median duration of invasive mechanical ventilation declined from 4 to 2 days. In P1, more patients experienced at least one episode of acute rejection than in P2 (Tables 1 and 2).
| 2014–2016 (n = 58) | 2017–2020 (n = 61) | |
|---|---|---|
| Preoperative MDR bacterial colonization | 7 (12%) | 7 (12%) |
| Acute rejection | 19 (33%) | 14 (23%) |
| Abdominal patch | 7 (12%) | 7 (12%) |
| Surgical revisions | ||
| 0 | 29 (50%) | 28 (46%) |
| 1 | 12 (21%) | 21 (34%) |
| >1 | 17 (29%) | 12 (20%) |
| SSI (patients) | 10/58 (17%) | 13/61 (21%) |
| SSI (events per patient) | 0.21 | 0.25 |
| Peritonitis (episodes) | 7 (12%) | 7 (12%) |
| Cholangitis (episodes) | 5 (9%) | 5 (8%) |
| Abscess (episodes) | 0 (0%) | 3 (5%) |
| Time to SSI, median (IQR) | 19 (9–33) | 12 (8–22) |
| Fever episodes | ||
| Unknown | 12 (21%) | 0 (0%) |
| 0 | 6 (10%) | 12 (18%) |
| 1 | 20 (34%) | 26 (43%) |
| ≥2 | 20 (34%) | 23 (38%) |
- Abbreviations: IQR, interquartile range; MDR, multidrug resistant; SSI, surgical site infections.
3.1 Perioperative Prophylaxis
The duration of PAP was 4.5 (IQR 3–7) days in P1 and 4 (IQR 3–6) days in P2. PAP duration complied with the newly implemented SOP in 45% (n = 54) of cases in both periods combined. In P1, 43% (n = 25) of the patients had a treatment duration that complied with the later implemented SOP, compared to 48% (n = 29) after its implementation.
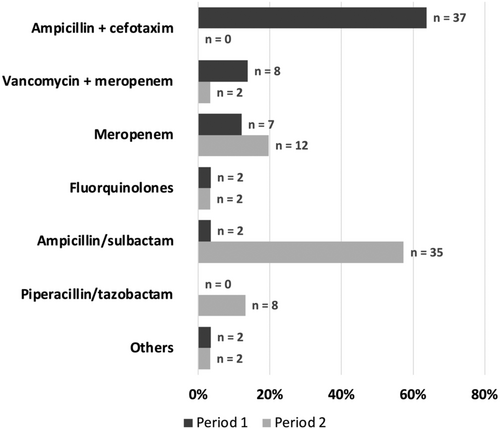
The predominant antibiotic substance or combination for PAP changed from ampicillin + cefotaxime in P1 to ampicillin + sulbactam in P2 (Figure 1). A strong decline was observed for the combination of vancomycin + meropenem, with a complementary increase in meropenem monotherapy and piperacillin + tazobactam. Postoperative vancomycin usage dropped from 14% (n = 8) in P1 to 3% (n = 2) in P2, reflecting and OR for receiving vancomycin as part of PAP of 0.21 (0.04–1.04).
3.2 Surgical Site Infections
In total, 23 patients (19%) experienced an SSI, 10 (17%) in P1 and 13 (21%) in P2, of those 6 patients with ampicillin and cefotaxim prophylaxis (16%), and 8 patients with ampicillin/sulbactam prophylaxis (22%). No incisional wound infections were documented. Median time to SSI was 19 (IQR 9–33) days in P1 and 12 (IQR 8–22) days in P2. Tables 2 and 3 provide data on details of SSI for all patients of the two periods (Table 2) and for those with standard antibiotic prophylaxis (Table 3). Incidence of SSI was similar in the two different populations. Patients who experienced an SSI were older, had longer duration of mechanical ventilation and PICU stays, and slightly shorter duration of PAP. The highest proportion of infection was observed in recipients of split organs from deceased donors (8/17, 47%) (Table 4). Two patients had explorative surgery due to SSI in P1, and three patients in P2. In 20 patients with SSI, the causative pathogen was detected (Table 5). Fourteen of 20 microorganisms (79%) were not susceptible to the antibiotic prophylaxis.
| Ampicillin and cefotaxime (n = 37) | Ampicillin/sulbactam (n = 35) | |
|---|---|---|
| Preoperative MDR bacterial colonization | 2 (5%) | 4 (11%) |
| Acute rejection | 10 (27%) | 10 (29%) |
| Abdominal patch | 5 (14%) | 4 (11%) |
| Surgical revisions | ||
| 0 | 18 (49%) | 17 (49%) |
| 1 | 7 (21%) | 11 (34%) |
| >1 | 11 (29%) | 6 (20%) |
| SSI (patients) | 6/37 (16%) | 8/35 (23%) |
| SSI (events per patient) | 0.21 | 0.26 |
| Peritonitis (episodes) | 3 (8%) | 3 (9%) |
| Cholangitis (episodes) | 3 (8%) | 2 (6%) |
| Abscess (episodes) | 0 (0%) | 3 (9%) |
| Time to SSI, median (IQR) | 18 (9–30) | 12 (8–22) |
| Fever episodes | ||
| Unknown | 5 (14%) | 0 (0%) |
| 0 | 5 (14%) | 5 (14%) |
| 1 | 15 (41%) | 21 (60%) |
| ≥2 | 12 (32%) | 9 (26%) |
- Abbreviations: IQR, interquartile range; MDR, multidrug resistant; PAP, perioperative antibiotic prophylaxis; SSI, surgical site infections.
| Surgical site infection | ||
|---|---|---|
| No: n = 96 (81%) | Yes: n = 23 (19%) | |
| Age, median (IQR), years | 1.7 (0.6–9.2) | 2.6 (0.6–7.7) |
| Male | 49 (51%) | 10 (43%) |
| Duration of mechanical ventilation, median (IQR) | 3 (1–7) | 5 (1–9) |
| Length of PICU stay, median (IQR) | 10.5 (7–18.5) | 15 (12–23) |
| Duration of perioperative antibiotic prophylaxis, median (IQR) | 4 (3–6) | 3 (2–5) |
| Abdominal patch (n = 14) | 6/14 (43%) | 8/14 (57%) |
| Living donor transplant (n = 52) | 43/52 (83%) | 9/52 (17%) |
| Deceased donor transplant (n = 67) | 53/67 (79%) | 14/67 (21%) |
| Split organ (only in deceased donor organ transplants) (n = 17) | 9/17 (53%) | 8/17 (47%) |
| Surgical revisions, median (IQR) | 0.5 (0–1) | 1 (0–3) |
- Note: If not indicated otherwise, all numbers represent n (%).
- Abbreviation: IQR, interquartile range.
| Patient | Period | Material | Pathogen | Susceptible to prophylaxis |
|---|---|---|---|---|
| 11 | 1 | Bile, BC | Enterococcus faecium | No |
| 18 | 1 | Ascites, BC | Stenotrophomonas maltophilia | No |
| 32 | 1 | Ascites | Enterobacter cloacae | Yes |
| 36 | 1 | Intra-abdominal swab, BC | Enterococcus faecium | No |
| 38 | 1 | Ascites | Bacillus cereus | Not tested |
| 47 | 1 | Bile, BC | Enterococcus faecium 1 | No |
| 55 | 1 | Ascites | Enterobacter cloacae 2 | No |
| 56 | 1 | Ascites | Enterococcus faecium | Yes |
| 62 | 2 | Ascites, BC | Escherichia coli | Yes |
| 67 | 2 | Bilioma | Klebsiella oxytoca, Stenotrophomonas maltophilia | No |
| 72 | 2 | Ascites | Staphylococcus aureus | Yes |
| 79 | 2 | BC | Enterobacter cloacae | No |
| 83 | 2 | Ascites | Enterococcus faecium, CNS | No |
| 96 | 2 | Bile, ascites | Enterococcus faecium | No |
| 102 | 2 | BC | Escherichia coli | No |
| 103 | 2 | Ascites, SeptiFast | Enterobacter cloacae | No |
| 106 | 2 | Intra-abdominal swab, ascites | Escherichia coli | No |
| 109 | 2 | Ascites, BC | CNS | No |
| 117 | 2 | Ascites, intra-abdominal swab, BC | Pseudomonas aeruginosa | No |
| 122 | 2 | Abscess drainage | Streptococcus anginosus | Yes |
- Abbreviations: BC, blood culture; CNS, coagulase-negative staphylococci; 2ESBL, extended-spectrum beta lactamase; SeptiFast, bacterial multiplex PCR in blood; 1VRE, vancomycin-resistant enterococcus.
If an abdominal patch was placed, the adjusted OR for SSI was 3.81 (1.15–12.68). SOP-conform PAP duration showed no clear association with the occurrence of SSIs (adjusted OR 0.57 [0.21–1.54]), neither did the introduction of ampicillin + sulbactam instead of ampicillin + cefotaxime (OR 1.13 [0.36–3.52]). Multivariable linear regression revealed a probability reduction of 4.6 percentage points (0.0–9.1) on the occurrence of SSIs per day of PAP within the first 7 postoperative days. Applied without time limit, the reduction in SSIs was 1.1 percentage point (0.8–3.0) per day of PAP.
4 Discussion
In this retrospective single-center study, SSIs occurred in about a fifth of patients and were not impacted by the regimen of PAP. The placement of an abdominal patch was identified as a relevant risk factor for SSIs. The effect of PAP on the reduction in SSIs became smaller with longer duration. In our cohort, efficacy of PAP was limited, as 70% of the identified pathogens were resistant against the prophylaxis we had used. One could argue that this result should prompt prophylaxis with broad-spectrum antibiotics. However, a recent analysis of over 2500 pediatric liver transplantations did not find a benefit of lower SSI rate when broader-spectrum antibiotics were used [27].
Our results confirm that SSIs contribute to morbidity during the early postoperative phase after liver transplantation in children. Similar rates have been observed previously [28, 29]. On the other hand, lower infection rates can be achieved, as reported by Banach et al. [10]: 11% of segmental graft recipients developed an SSI, whereas only 4% of whole graft recipients developed SSI [10]. The same result was observed in our study, with recipients of deceased donor split grafts developing SSIs twice as often as recipients of whole or living donor grafts. According to literature, biliary complications are associated with SSIs [10, 28]. Thus, SSIs might be effectively reduced by surgery technique, potentially including prevention bundles rather than single measures. However, robust evidence on optimal (intraoperative) measures to reduce SSIs are lacking. Results from adult trials cannot be extrapolated directly due to differing risk factors and physiological characteristics [30]. Furthermore, studies from nonimmunocompromised children that failed to identify benefit from PAP [31, 32] cannot be directly transferred to children in the postoperative phase after LTx, which is characterized by high levels of immunosuppressants, often prolonged ventilation, use of vasopressors and sometimes associated wound healing disorders in the early postoperative phase. Prospective randomized controlled trials are warranted to identify optimal prevention bundles against SSIs at all stages and reduce antimicrobial exposure as well as reoperations.
An important cornerstone of balancing harm and benefit from antibiotic exposure is the optimal duration of PAP. In our study, no change in actually applied PAP duration was achieved. Compliance with the newly introduced recommendation on PAP duration remained below 50%, suggesting that additional considerations determined the duration of PAP administration regardless of the SOP. A recently published multicenter study across Europe found great heterogeneity regarding the duration of PAP, with a range from single dose PAP to 10 days [19]. In one study, standardization of perioperative antibiotic regimen in pediatric liver transplantation, gram-negative broad-spectrum antibiotic treatment for >48 h declined from 77% to 44% after implementation without an increase in infectious complications [31]. Our study found that prolonged PAP duration did not substantially reduce SSIs. A group of patients that might benefit from prolonged PAP duration are patients with an abdominal patch, which was an important risk factor for SSIs in this study.
Besides the duration, the choice of antibiotic substance for PAP is important. In this study, the change in PAP regimen resulted in a strong shift toward ampicillin/sulbactam instead of ampicillin + cefotaxime. Furthermore, the exposure to a combination of reserve antibiotics, that is, vancomycin + meropenem declined in favor of meropenem monotherapy or piperacillin/tazobactam without an increase in SSIs. Even stronger effects regarding vancomycin treatment with preserved outcomes were achieved in a study with higher baseline vancomycin usage (50%), which dropped to 7% after antibiotic regimen standardization [33]. Careful choice of antibiotics is especially warranted to minimize the exposure to hepato- and ototoxic medication, which is acknowledged for vancomycin but has been also reported for trimethoprim and amoxicillin/clavulanic acid in pediatric liver transplant recipients [34, 35].
While it is well-acknowledged that unwarranted use of broad-spectrum antibiotics contributes to the selection of multidrug-resistant (MDR) bacteria [20], recipients of solid organ transplantations are predisposed for infections with MDR bacteria due to previous exposure to antibiotics of donors and recipients, prolonged hospital stays, and immunosuppression [36]. In transplant patients with sepsis, MDR organisms were identified as causative pathogens in 48% of cases and conferred increased odds for mechanical ventilation and higher degrees of organ failure [26]. Alike, MDR carrier status and high levels of immunosuppression were independent risk factors for infections, SSIs, severe sepsis, and septic shock [11, 12]. The synthesis of these findings suggests that MDR carriers might benefit from individually tailored antibiotic regimens, as discussed in the literature [11, 37] and already practiced in some European liver transplant centers [19]. However, a study tempering enthusiasm for this approach was published by Cardile et al. on adult LTX recipients who were carriers of carbapenem-resistant Klebsiella pneumoniae [38]: In the standard antibiotic treatment group, microbiota richness after liver transplant patients was increased and harmful Enterobacteriaceae and Klebsiella spp. reduced compared with the group receiving targeted MDR antibiogram-based antibiotic treatment. The authors conclude that it might be appropriate to administer standard prophylaxis and reserve targeted regimens for complications [27], a suggestion that is supported by a study where MDR-associated complications occurred much later than viral or culture-negative infections [29]. Use of an abdominal patch was associated with an increased risk of SSI, possibly explained by the need for re-surgery and a dysfunctional barrier to the skin microbiome as in our patients there was no VAC dressing used with abdominal patch.
The main limitation of this study is the retrospective nature, which prevented reliable assessment of underlying reasons for treatment choices and changes in ongoing therapy. Furthermore, due to the single-center design, unobserved center-specific characteristics that influence the occurrence of SSIs and/or treatment outcomes cannot be ruled out or adjusted. Altogether, protocol violations were frequent making it even more difficult to draw substantial conclusions from our data. Reasons for choice of a different antibiotic prophylaxis were not always documented but included MDR carrier status, long hospital stay before transplantation, and recurrent culture-negative cholangitis.
In summary, this study showed that SSIs contribute substantially to postoperative morbidity in pediatric liver transplant recipients, which remained unaffected by a change in PAP regimen. The results imply that overriding considerations determined PAP duration, whereas the standard substances used were effectively modified. Vancomycin usage was further reduced in a setting with already infrequent routine usage and low proportion of MDR carriers. The failure to detect an impact of the substances used for PAP on the occurrence of SSIs add to the growing awareness that evidence-based PAP regimens tailored to this specific patient population or even to individuals' status of organ dysfunction and preoperative MDR carrier status are urgently warranted but not available. Multicentric joint efforts at international level are therefore needed to minimize pharmacotoxicity deriving from antimicrobial treatment and accompanying treatments including sedation and anesthesia during the vulnerable early postoperative phase.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.