Successful use of eculizumab in immediate ANCA vasculitis recurrence in a pediatric kidney transplant
Abstract
Background
Kidney transplantation is an acceptable therapy end-stage kidney disease secondary to antineutrophil cytoplasmic antibody-associated vasculitis with risk of disease recurrence ranging from 3% to 17%. Standard posttransplant immunosuppression is the mainstay of therapy after recurrence. Recently, new medications focused on complement regulation and avoidance of steroids have been shown to be effective in treating antineutrophil cytoplasmic antibody (ANCA) vasculitis with no studies in the pediatric population.
Methods
We report a 5-year-old patient with immediate recurrence of positive myeloperoxidase (MPO)-ANCA vasculitis after deceased donor kidney transplant and the novel use of eculizumab to salvage the graft.
Results
Eculizumab and transition to ravulizumab has been successful in improving graft function and maintenance of disease remission after immediate MPO-ANCA vasculitis recurrence posttransplant.
Conclusions
Complement inhibitors may be used in addition to standard immunosuppression postkidney transplant in a pediatric patient with MPO-ANCA vasculitis recurrence without higher rates of infections.
Abbreviations
-
- ANCA
-
- antineutrophil cytoplasmic antibody
-
- AAV
-
- associated vasculitis
-
- C5a
-
- complement component 5a
-
- C5b
-
- complement component 5b
-
- ESKD
-
- end-stage kidney disease
-
- MAC
-
- membrane attack complex
-
- MPO
-
- myeloperoxidase
-
- POD
-
- postoperative day
1 BACKGROUND
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a rare cause of systemic, small-vessel vasculitis in children. Despite its rarity, AAV is more likely to relapse in children compared with adults resulting in more damage over time, specifically kidney damage.1 In pediatric patients, end-stage kidney disease (ESKD) results in poor quality of life, decreased life expectancy, and high rates of mental health issues.2 Kidney transplantation has been shown to improve quality of life and provide survival benefits to this population.2 Newer drug therapies, including the complement 5a inhibitor, avacopan, have provided hope for a glucocorticoid-sparing and potentially better way to treat AAV, however, as with many new medical therapies, this treatment has not been studied and is not readily accessible to the pediatric population.3-5
2 METHODS
We report a pediatric patient with immediate recurrence of positive myeloperoxidase (MPO)-ANCA vasculitis after deceased donor kidney transplant with the novel use of eculizumab to salvage the graft.
3 CASE
A young boy presented with ESKD secondary to crescentic glomerulonephritis from MPO-positive AAV diagnosed at 30 months of age by serologies and kidney biopsy. Initial biopsy showed pauci-immune glomerulonephritis with 76% crescents and 22% of the noncrescentic glomeruli were globally sclerosed. Initial therapy consisted of methylprednisolone and rituximab (375 mg/m2 × 4 doses) followed by prednisolone and mycophenolate which failed to induce remission. He received cyclophosphamide transitioning to azathioprine and continued prednisolone. Despite these therapies, he continued to have intermittent episodes of gross hematuria and elevated MPO and ANCA IF titers. He received additional pulse steroids and rituximab infusions and was restarted on cyclophosphamide. Repeat biopsy 1 year after presentation showed 25/37 global glomerulosclerosis and marked interstitial fibrosis with no active disease and he was maintained on rituximab and low-dose prednisone. Even with no active disease, the lowest MPO level achieved was 117 AU/mL, and ANCA IFA titers of 1:80 approximately 1 year prior to transplant. MPO/PR3 (ANCA) antibodies were measured utilizing a semi-quantitative multiplex bead assay performed at ARUP laboratory, 19 AU/mL or less was reported as negative, 20–25 AU/mL was reported as equivocal, and 26 AU/mL or greater was reported as positive. IFA titers were measured utilizing semi-quantitative indirect fluorescent antibody through the ARUP laboratory with a cutoff value of 1:20.
The patient was maintained with three times weekly hemodialysis for 2 years leading to transplant. One month before the transplant, he had another episode of gross hematuria with a stable MPO level of 257 AU/mL and ANCA IFA titer of 1:1280. He underwent cystourethroscopy and bilateral retrograde ureteropyelograms because of the hematuria and concern for a neoplasm secondary to cyclophosphamide use and immunosuppression. This showed normal anatomy and bilateral retrogrades. Hematuria did not recur, and he had no extra-kidney symptoms of vasculitis. Five days before the transplant, the MPO level was 225 AU/mL and the ANCA IFA titer was 1:640.
He underwent a deceased after circulatory death kidney transplant at age 5 from a 29-year-old male donor with a kidney donor profile index (KDPI) of 8%. This was an 8-antigen mismatch transplant with 19 min of warm ischemia time. Final T-cell crossmatches performed by flow cytometry were compatible with B-cell crossmatch performed by flow cytometry equivocal due to rituximab interference. B-cell crossmatch from a previous sample from 1 month before transplant was compatible. Thymoglobulin 3.7 mg/kg and methylprednisolone with a rapid taper over 5 days were used for induction. Maintenance immunosuppression consisted of tacrolimus with an initial trough goal of 10–12 started postoperative day (POD) 1 and mycophenolate 1200 mg/m2 started on the day of transplant. Prior to transplantation, his serum creatinine was 9.22 mg/dL. He had significant hypertension posttransplant requiring a nicardipine drip initially and then a transitioned to amlodipine, labetalol, clonidine, and losartan by postoperative day 2. He followed our standard protocol for hydration, immunosuppression, bacterial, fungal, viral prophylaxis, and pain control. On the afternoon of POD 2, serum creatinine nadired at 0.34 mg/dL. Five days after transplant the patient developed gross hematuria, and 6 days after that, creatinine increased to 0.89 mg/dL. On POD 10, the MPO level was 75 AU/mL. Creatinine continued to increase as shown in Figure 1.
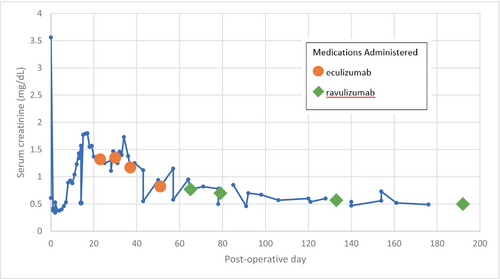
His fluid goal was increased as creatinine increased to ensure dehydration with a large graft in a small patient was not contributing to rising creatinine. Because of continued gross hematuria and rising creatinine, a kidney biopsy was obtained on POD 14 which showed 14% cellular crescents mostly segmental with one possibly circumferential and 1–2 segments with necrotizing lesions. The tubules showed significant injury with many red cells and granular casts, as well as flattened or sloughed epithelium. There was no evidence of acute T-cell-mediated or antibody-mediated rejection. In addition to no signs of thrombotic microangiopathy in the biopsy, atypical hemolytic uremic syndrome (HUS) was not suspected as blood counts and platelets were normal, and schistocytes were minimal. The following day, apheresis was started with a total of 5 treatments completed every other day. In addition, we gave a 750/m2 dose of rituximab after the first apheresis treatment to ensure we maximized immunosuppression. On POD 17, the MPO level was 92 AU/mL. POD 18, MPO level was 80 AU/mL with ANCA IFA titer 1:320. CD19 and CD20 B-lymphocyte counts were 0 cells/μL when checked on POD18, 3 days after rituximab administration, therefore, no additional rituximab was given.
Despite this treatment, serum creatinine remained elevated and the patient continued to have gross hematuria. During apheresis treatments, additional therapeutic options were discussed due to the patient's history of resistant disease with use of standard treatment prior to transplantation. At the time, there were no case reports of use of eculizumab for AAV in pediatric patients or kidney transplant patients, but a handful of case reports in adult patients where eculizumab was utilized off-label in scenarios with a lack of improvement or clinical worsening while receiving standard of care.6-8 Data had been recently published with promising results utilizing avacopan, a complement protein 5a (C5a) receptor inhibitor, for treatment and maintenance of remission of AAV, however, there was no data in pediatric patients or kidney transplant patients.3 To reach the same endpoint of prevention of C5a receptor activation, we opted to target earlier in the complement cascade through the blockade of complement protein 5 (C5) utilizing the complement inhibitor eculizumab, preventing the formation of C5a and C5b altogether. Our patient received prophylactic penicillin VK and had not been vaccinated for meningitis prior to transplantation since he was receiving immunosuppressive therapies including rituximab.
The first dose of eculizumab was given on POD23 after completing 5 treatments of every other day apheresis. Serum creatinine had plateaued at 1.3 mg/dL during that time, and LDH and haptoglobin were normal. Standard dosing for eculizumab consisted of induction 600 mg weekly for 2 doses (administered POD23 and POD30), maintenance with 600 mg at week 3 (administered POD37), then 600 mg every 2 weeks (beginning with administration POD51). Upon discharge from the hospital on POD 25, serum creatinine was 1.25 mg/dL. On POD30, the MPO level was 20 AU/mL with ANCA IFA titer <1:20.
The patient received their first maintenance dose of eculizumab on POD37. Serum creatinine prior to his first eculizumab maintenance dose was 1.17 mg/dL. The patient's first urine sample with RBC < 30/HPF was collected POD51, 36 days after the one-time dose of rituximab, and 27 days after the last plasmapheresis treatment. The patient had received three doses of eculizumab prior to the normal urine. On POD51, eculizumab dose #4 was administered. On POD 57, the MPO level was 34 AU/mL with ANCA IFA titer <1:20. Shortly thereafter, the patient was switched to ravulizumab to decrease the frequency of maintenance infusions. A ravulizumab loading dose of 900 mg was administered POD65 followed by the maintenance dosing of 2100 mg beginning with POD79 and continuing every 8 weeks. Serum creatinine prior to a first maintenance dose of ravulizumab was 0.77 mg/dL. With a successful transition to ravulizumab, serum creatinine continued to trend down. Within the month following the first maintenance ravulizumab infusion, serum creatinine ranged from 0.57 to 0.85 mg/dL and the patient returned to school. By month 5 of complement inhibitor therapy, the patient reached his baseline function with serum creatinine ~0.5 mg/dL. At 17 months posttransplant, the patient continues to receive maintenance ravulizumab infusions and penicillin VK prophylaxis, is up to date with meningococcal vaccinations, and has had no known infectious complications to date including negative screenings for Epstein–Barr virus (EBV), cytomegalovirus (CMV), and serum BK virus, all monitored per our center's routine posttransplant protocols.
4 DISCUSSION
Our experience with this case suggests that complement inhibition through the use of eculizumab may have played a large role in the improvement and normalization of kidney function with maintenance of complete remission of disease in this pediatric patient. Therapy with rituximab is known to induce remission, studied at a dosing of 375 mg/m2 once weekly for 4 weeks in conjunction with glucocorticoids.9, 10 In the RAVE trial, 87.5% of relapses occurred after B cells became re-detectable, and one-third of the rituximab-treated patients relapsed between 6- and 18-month posttherapy initiation, with the majority reconstituting their B cells between 9- and 12-month posttherapy initiation. Among the patients with relapsing disease (n = 101), one-third in the rituximab group had complete remission by 6 months, with only 37% remaining in remission by 18 months.9 Although we did not serially monitor B cells for our patient, they were undetectable when labs were drawn at 3 months posttransplant and had reconstituted when checked again at 6 months posttransplant, 161 days after administration of the one-time rituximab dose. The patient-specific data presented here, along with probability of remaining in remission published in the RAVE trial,9 suggest that it is unlikely our patient has remained in remission due to one-time dose of rituximab and corticosteroid maintenance therapy alone.
Discovery of involvement of the alternative complement pathway in AAV and glomerulonephritis, first hypothesized by Xiao et al., has provided new targets for the treatment of ANCA-associated disease. Activation of neutrophils through ANCA leads to release of factors that bind and activate the alternative complement pathway, leading to the recruitment and activation of additional neutrophils, with the ultimate result of severe inflammation and kidney injury.8 Eculizumab and ravulizumab, terminal complement inhibitors, bind to the complement protein C5 to inhibit cleavage into complement component 5a (C5a) and complement component 5b (C5b). C5a, a proinflammatory anaphylatoxin, is a key complement component in the AAV disease process, as it is highly active in recruitment of neutrophils.6 C5a is the specific target of the complement inhibitor avacopan, which was FDA approved through orphan drug designation in October 2021 for adjunctive treatment for adult patients with severe active AAV in addition to the standard of care including glucocorticoids.11 Due to our patient's age and lack of data in both pediatric and posttransplant populations, the complement inhibitor specific to the C5a receptor was not an affordable option.
C5 inhibitors eculizumab and ravulizumab have been FDA approved for use in children for atypical hemolytic uremic syndrome to inhibit complement-mediated thrombotic microangiopathy and paroxysmal nocturnal hemoglobinuria with other off-label indications including treatment of C3 glomerulopathy, refractory myasthenia gravis, antibody-mediated rejection, and for desensitization of solid organ transplant recipients. In addition to their actions for prevention of C5a formation, eculizumab and ravulizumab block the formation of C5b, which is a crucial component for formation of the terminal complement complex (C5b-9), or the complement membrane attack complex (MAC). Prevention of generation of MAC is the primary mechanism by which C5 inhibitors succeed in the treatment of many non-ANCA-related indications, however, this inhibition prevents MAC-induced osmolysis of bacteria, predisposing the patient to increased risk of infection with encapsulated bacteria including meningococcal infections, even in the setting of full vaccination.12-14 Our patient continues to receive prophylaxis with penicillin VK for the duration of complement inhibitor use and has had to take on this infection risk in the setting of barriers to access to newer therapies including selective complement inhibitors. The number of available complement inhibitors for kidney diseases continues to expand at a rapid pace.15 However, they are not accessible to all. Inclusivity and diversity are important to clinical trials as stated by The National Institute of Health “to account for the diverse lived experiences and exposures of various populations.”16
5 CONCLUSIONS
C5 inhibition appears to have been effective for induction and maintenance of remission in combination with other therapies including apheresis and rituximab in this pediatric patient with immediate AAV recurrence postkidney transplant. Utilizing a one-time dose of rituximab, apheresis, and eculizumab followed by conversion to ravulizumab for long-term maintenance therapy, we were able to salvage the graft and normalize function with no infectious complications. It should be a priority to work toward the removal of barriers for underrepresented populations to allow everyone a fair and just opportunity to attain their highest level of health.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.