Let's get physical: Aerobic capacity, muscle strength, and muscle endurance after pediatric heart transplantation
Abstract
Background
Pediatric heart (HTx) and kidney transplant (KTx) recipients may have lower physical fitness than healthy children. This study sought to quantify fitness levels in transplant recipients, investigate associations to clinical factors and quality of life, and identify whether a quick, simple wall-sit test is feasible as a surrogate for overall fitness for longitudinal assessment.
Methods
Aerobic capacity (6-min walk test, 6MWT), normalized muscle strength, muscle endurance, physical activity questionnaire (PAQ), and quality of life (PedsQL™) were prospectively assessed in transplanted children and matched healthy controls.
Results
Twenty-two HTx were compared to 20 controls and 6 KTx. 6MWT %predicted was shorter in HTx (87.2 [69.9–118.6] %) than controls (99.9 [80.4–120] %), but similar to KTx (90.3 [78.6–115] %). Muscle strength was lower in HTx deltoids (6.15 [4.35–11.3] kg/m2) and KTx quadriceps (9.27 [8.65–19.1] kg/m2) versus controls. Similarly, muscle endurance was lower in HTx push-ups (28.6 [0–250] %predicted), KTx push-ups (8.35 [0–150] %predicted), HTx curl-ups (115 [0–450] %predicted), and KTx wall-sit time (18.5 [10.0–54.0] s) than controls. In contrast to HTx with only 9%, all KTx were receiving steroid therapy. The wall-sit test significantly correlated with other fitness parameters (normalized quadriceps strength R = .31, #push-ups R = .39, and #curl-ups R = .43) and PedsQL™ (R = .36).
Conclusions
Compared to controls, pediatric HTx and KTx have similarly lower aerobic capacity, but different deficits in muscle strength, likely related to steroid therapy in KTx. The convenient wall-sit test correlates with fitness and reported quality of life, and thus could be a useful easy routine for longitudinal assessment.
Abbreviations
-
- 6MWT
-
- 6-min walk test
-
- HC
-
- healthy children
-
- HFZ
-
- healthy fitness zone
-
- HICCUP
-
- Healthy Infants and Children Clinical Research Program
-
- HTx
-
- heart transplant
-
- KTx
-
- kidney transplant
-
- QoL
-
- quality of life
1 INTRODUCTION
Pediatric solid organ transplantation has evolved from a high-risk, last-resort procedure to a highly successful intervention with the majority of transplanted children surviving well into adulthood.1 Pediatric transplant recipients are reported to have reduced physical activity, known herein as fitness.2, 3 The extent of their fitness deficits and resulting effects on quality of life may vary depending on the transplanted organ, and pre- and post-transplant history. However, the underlying cause(s) of impairment in fitness level have not been clearly identified. A better understanding of fitness level, potential underlying causes, and the relationship between fitness level on quality of life is needed to identify specific targets for potential interventions, such as activity recommendations and rehabilitation programs.
Fitness level can be assessed using different parameters, including aerobic capacity, muscle strength, muscle endurance, and flexibility.4 Aerobic capacity reflects how well the body uses oxygen to perform movement.4 Muscle strength is defined as the maximum force that a muscle group can exert.4 Muscle endurance is defined as the number of times a movement can be repeated, or the amount of time a position can be held, before exhaustion.4 Performing a detailed fitness assessment is time consuming and burdensome for the patients and the transplant program, accordingly simple surrogate tests with a good reflection of the overall fitness would be useful for routine clinical practice and longitudinal follow-up. The wall-sit test appeared suitable for this purpose as it allows supervision by any clinical staff in a short time frame and does not require any technical devices.
In the setting of pediatric transplantation, many factors may affect fitness levels. These include medications,5, 6 graft function,7, 8 hemoglobin level,9 physical activity level,10, 11 pre- and post-transplant course,12 and natural ability and genetics.
This study prospectively investigated aerobic capacity and muscle strength and endurance in pediatric heart transplant (HTx) recipients in comparison to matched healthy children (HC) and kidney transplant (KTx) recipients. Further, possible relationships between fitness level and various transplant-related factors as well as standardized quality of life assessment were investigated. We further aimed to assess whether the quick and simple “timed wall-sit test” reflects overall fitness, in order to evaluate its potential use as a longitudinal assessment tool in the clinical setting.
2 METHODS
Research ethics board approval was obtained at the main conducting institution (Pro00067592) and each participating center. Families of patients being followed at the Stollery Children's Hospital, Alberta Children's Hospital, and British Columbia Children's Hospital were called and/or approached in clinic for recruitment. Healthy children were recruited from the Healthy Infants and Children Clinical Research Program (HICCUP) database at the University of Alberta. All eligible patient families were contacted, and those who volunteered for the study provided written, informed consent before participating. These included 32 participants from the University of Alberta, including all kidney transplant families, 15 from the Alberta Children's Hospital, and one from the British Columbia Children's Hospital.
Eligibility criteria included patients who received a heart or kidney transplant between the ages of 0–17 years, were a minimum of 5 years of age and 1-year post-transplant at the time of inclusion, and enrolled in a structured classroom or virtual elementary or secondary school program. Candidates were excluded from participation if their parents or guardians believed they would not be able to complete two or more fitness tests for any reason. All eligible, consented patients participated in the study.
To minimize variability due to lifestyle and genetic factors, patient siblings were also recruited into the control group of healthy children if they had suitable matching criteria. In this study, nine of the health controls were patient siblings. The remainder of the control group was populated with healthy, height-matched children recruited from the HICCUP database. Height, weight, and BMI z-scores and percentiles were calculated from age-matched CDC growth charts (PediTools).13
2.1 Physical fitness, movement behavior, and quality of life testing
Tests were performed in a single session lasting 75–90 min in the following order: anthropometrics, physical activity and quality of life (QoL) questionnaires (PedsQL™ generic questionnaire), trial 1 of the 6-min walk test (6MWT), muscle strength tests (deltoid, abdominal, then quadriceps strength, rotating through the set for three trials per muscle group), muscle endurance tests (push-ups, curl-ups, then wall-sit), and then trial 2 of the 6MWT. Testing was repeated and the average was reported as there is a learning effect in the first attempt. We are aware this comes with a small tiring effect, however, as this was consistent for all participants, the effect should be negligible. Encouragement during the activity was provided in a consistent manner to all participants using standardized scripts.
Movement behavior was quantified using the Physical Activity Questionnaire (PAQ) for children up to grade 8 or equivalent (PAQ-C) and adolescents from grades 9–12 or equivalent (PAQ-A).14 Children aged 5–7 years, though younger than the validated group, were given a modified PAQ-C. For this age group, the questionnaire was read aloud to children and guardians. Though children were able to provide input to their guardian, ultimately, the guardian's response was recorded.
The 6MWT was used to assess functional exercise capacity using standard procedures.15 Distances (meters) were compared to age-reference values, used clinically at the University of Alberta, to generate a 6MWT percent of predicted distance.16, 17 Heart rate was measured while standing at the starting line immediately before starting the 6MWT and at the spot of test completion immediately after the test.
Muscle strength was measured using a hand-held dynamometer (Lafayette Instruments). The quadriceps, deltoid, and abdominal muscle groups were tested using manual muscle testing positions.18 All tests were performed by the same researcher to avoid inter-observer variability. Body surface area (BSA) was calculated using the Mosteller equation,19 and it was used to control for muscle mass in all strength calculations.
The FITNESSGRAM protocol20 for push-ups and curl-ups was used to measure upper body and core muscle endurance, respectively. For each test, there is a sex and age-specific standard range termed the “Healthy Fitness Zone” (HFZ).20 We calculated how many participants reached the HFZ as well as what percentage of the lower range was achieved, as previously reported in assessments of pediatric transplant patients.21, 22 The wall-sit test was used to measure lower body muscle endurance.23 For this test, participants were asked to stand against a wall with their legs extended slightly beyond their comfort zone and squat until their knees made a 90-degree angle, holding as long as possible.
Clinical data were extracted from patient charts retrospectively. Data included type of diagnosis leading to transplant, age at transplant, time since transplant, most recent hemoglobin level, most recent eGFR (calculated using the Schwartz equation24), previous cardiac or kidney surgeries, number of days on dialysis, number of days on prednisone, medication list at the time of assessment, and other significant medical events (stroke and presence of resulting motor deficits as per the clinical neurologist's assessment, use of ventricular assist device [VAD] or extracorporeal membrane oxygenation [ECMO] before transplant, and episodes of graft rejection or post-transplant lymphoproliferative disease [PTLD]).
2.2 Statistical analysis
Participants who attempted the study but were unable to complete two or more fitness tests due to physical or behavioral limitations were excluded from the analysis. Data was analyzed by two-tailed and non-parametric tests, including Kruskal–Wallis with Dunn's post hoc test and Mann–Whitney. Data are reported as medians and range or 25–75th percentiles, and figures show individual patient data with medians. The number of participants that reached the HFZ for each group was analyzed by the Fischer exact test. Correlations were analyzed by Spearman correlation. The correlation coefficients used are as follows: >.70 = very strong relationship, .40–.69 = strong relationship, .30–.39 = moderate relationship, .20–.29 = weak relationship, .01–.19 = no or negligible relationship.25 The significance level was p < .05. Statistical analyses were performed using GraphPad Prism.
3 RESULTS
Twenty-two HTx, 20 healthy controls, and six KTx recipients were included in the study. One eligible HTx patient was excluded because they were deemed unable to complete the fitness testing by their guardians. Three HTx recipients attempted to participate in the study but were excluded from analysis due to incomplete fitness testing (one for behavioral issues, two for physical limitations). One participant completed the study but was unable to perform the push-up test due to neuromuscular deficits, so they were included in all analyses except the push-up test. Table 1 shows participant demographics (Table 1A) and clinical characteristics (Table 1B) for the final study population. None of the HTx patients had graft vasculopathy. Study groups were similar, except for the following significant differences: KTx patients were shorter and had lower eGFR than the other groups, transplant recipients weighed less than controls with HTx patients having significantly lower Z-scores and height percentiles in the HTx group.
| HC | HTx | KTx | p-Value | |
|---|---|---|---|---|
| No. subjects | 20 | 22 | 6 | |
| Female, n (%) | 7 (35) | 11 (50) | 2 (33) | ns (.56) |
| Age, years | 11.1 (9.2 to 12.4) | 10.7 (7.7 to 13.2) | 8.8 (7.8 to 9.6) | ns (.33) |
| Height, cm | 150.6 (138.9 to 160.2) | 137.9 (125.3 to 155.3) | 125.9* (124.2 to 126.7) | .04 |
| Height %ile | 85 (49 to 93) | 46 (19 to 73) | 21** (4 to 36) | .007 |
| Height Z-score | 1.0 (0.0 to 1.5) | -0.1 (−0.9 to 0.6) | −0.9** (−1.7 to −0.4) | .007 |
| Weight, kg | 40.4 (31.7 to 52.5) | 29.7 (22.9 to 42.7) | 25.9 (23.4 to 29.5) | .04 |
| Weight %ile | 56 (38 to 88) | 28** (7 to 46) | 36 (30 to 41) | .01 |
| Weight Z-score | 0.2 (−0.3 to 1.2) | −0.6** (−1.5 to 0.1) | −0.4 (−0.5 to −0.2) | .009 |
| BMI for age, kg/m2 | 17.6 (15.3 to 20.0) | 15.6 (14.0 to 17.3) | 17.5 (15.4 to 18.1) | ns (.07) |
| BMI %ile | 55 (19 to 82) | 9* (3 to 45) | 55 (26 to 83) | .01 |
| BMI Z-score | 0.1 (−0.9 to 0.9) | −1.4* (−1.9 to −0.1) | 0.1 (−0.6 to 10) | .01 |
- Note: Z-scores and percentiles (%ile) were calculated based on age-matched CDC growth charts (PediTools). All data are expressed as median (25–75th percentile). Analyzed by Kruskal–Wallis non-parametric test with Dunn's post hoc test for multiple comparisons: ns = nonsignificant, *p < .05, **p < .01, comparing HTx or KTx to HC. The p-value column represents the outcome of the Kruskal–Wallis non-parametric test. Bold values indicate p < .05 considered as statistically significant.
| HTx | KTx | |
|---|---|---|
| Type of diagnosis | CHD 11 (50%) | Urinary tract malformations 3 (50%) |
| CMP 9 (41%) | Renal dysplasia 2 (33%) | |
| Other 2 (9%) | Unknown 1 (17%) | |
| Age at tx, years | 2.5 (0.2–8.7) | 3.3 (1.5–8.6) |
| Time post–tx, years | 7.0 (1.9–15) | 3.3 (0.9–12.0) |
| Blood pressure, measured, mmHg | 108 (90–148)/68 (52–86) | 111 (92–129)/66 (44–82) |
| Hg level, most recent, mmol/L | 129 (116–152) | 112 (103–129)** |
| eGFR (mL/min/1.73 m2) | 142 (76–225) | 76 (40–154)* |
| Other significant medical events | Stroke 7 (32%) | Stroke 0 (0%) |
| Motor deficits 4 (18%) | Motor deficits 0 (0%) | |
| VAD 9 (41%) | VAD 0 (0%) | |
| ECMO 7 (32%) | ECMO 0 (0%) | |
| Rejection 5 (23%) | Rejection 2 (33%) | |
| PTLD 4 (18%) | PTLD 0 (0%) | |
| Previous cardiac or kidney surgeries | Single ventricle staged palliation 6 (27%) | Nephrectomy 2 (33%) |
| Other 5 (23%) | ||
| No previous surgery 11 (50%) | No previous surgery 4 (67%) | |
| Dialysis, no. days | n/a | 761 (220–1543) |
| Peritoneal 3 (50%) | ||
| Hemodialysis 0 (0%) | ||
| Prednisone, no. days | 217 (31–2436) | 983 (1–2852) |
| Medications at time of assessment | Tacrolimus 21 (95%) | Tacrolimus 6 (100%) |
| Prednisone 2 (9%) | Prednisone 6 (100%) | |
| MMF 16 (73%) | MMF 6 (100%) | |
| Statin 13 (59%) | Statin 0 (0%) | |
| Blood pressure 18 (82%) | Blood pressure 6 (100%) | |
| Beta-blocker 1 (5%) | Beta-blocker 1(17%) | |
| ACEi 10 (45%) | ACEi 3 (50%) | |
| CCB 9 (41%) | CCB 3 (50%) | |
| Vitamin D 19 (86%) | Vitamin D 5 (83%) | |
| Iron 8 (36%) | Iron 3 (50%) |
- Note: Data were analyzed by Mann–Whitney test and are reported as median (range) or n (%) *p < .05, **p < .01. Bold values indicate p < .05 considered as statistically significant.
- Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; CCB, calcium channel blocker; CHD, congenital heart disease; CMP, cardiomyopathy complex; ECMO, extracorporeal membrane oxygenation; MMF, mycophenolate mofetil; PTLD, post-transplant lymphoproliferative disorder; tx, transplant; VAD, ventricular assist device.
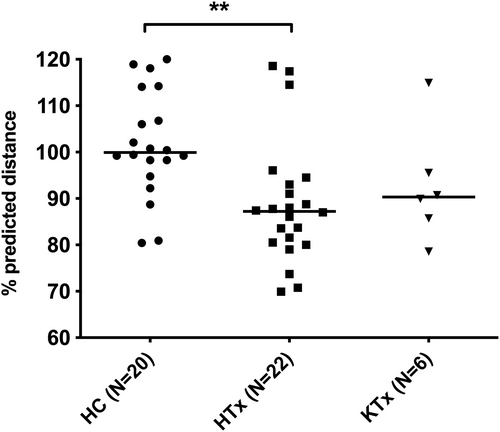
The median 6MWT distance was 87.2% predicted in HTx, which was significantly lower than controls at 99.9% predicted (Figure 1, Table 2). The KTx 6MWT distance was 90.3% predicted, which was not significantly different from the controls. The %increase in HR from the beginning to the end of the 6MWT was significantly lower in HTx (23 [−15 to 72] %) than in controls (62 [9–143] %, p < .01), while not significantly different between KTx (32 [3–55] %) and the other groups (p > .05). The %change in HR was not correlated with 6MWT in HTx (R = −.03, p = .88). The 6MWT was significantly lower in HTx with stroke, which had occurred in 32% of HTx, as compared to those without stroke (80.0 vs. 88.8%, p < .01). When considering only those with persisting neuromotor deficits from stroke (14% of HTx), this relationship was not maintained (Table 3). In HTx, 6MWT showed a borderline significant correlation with age at transplant (R = −.42, p = .05) and in trend with eGFR (R = .37, p = .09), but no other assessed factors. These correlations were not detected in the KTx group (age at transplant: R = −.14, p = .80; eGFR: R = −.43, p = .42).
| HC | HTx | KTx | p-Value | |
|---|---|---|---|---|
| Aerobic capacity | ||||
| 6MWT (% pred distance) | 99.9 (80.4–120.0) | 87.2** (69.9–119.0) | 90.3 (78.6–115.0) | .003 |
| Muscle strength | ||||
| Deltoid force/BSA (kg/m2) | 8.5 (4.8–10.8) | 6.2* (4.4–11.3) | 6.1 (4.3–8.3) | .012 |
| Abdominal force/BSA (kg/m2) | 5.0 (1.3–17.5) | 4.3 (0.0–14.0) | 4.3 (0.0–10.3) | .357 |
| Quadriceps force/BSA (kg/m2) | 15.4 (11.7–21.3) | 13.1 (8.9–24.8) | 9.3** (8.7–19.1) | .003 |
| Muscle endurance | ||||
| Push-ups | ||||
| Participants in HFZ | 11 (55%) | 6 (27%) | 1 (17%) | |
| % HFZ attained | 112 (0–400) | 29* (0–250) | 8* (0–150) | .005 |
| Curl-ups | ||||
| Participants in HFZ | 16 (80%) | 14 (64%) | 4 (67%) | |
| % HFZ attained | 167 (47–500) | 115* (0–450) | 121 (0–217) | .035 |
| Wall–sit time (s) | 62 (11–203) | 37 (11–132) | 19* (10–54) | .025 |
- Note: Data were analyzed by the Kruskal–Wallis non-parametric test with Dunn's post hoc analysis and reported as median (range). *p < .05, **p < .01, comparing HTx or KTx to HC. Push-up and curl-up participants in Healthy Fitness Zone (HFZ) were analyzed by the Fischer exact test and reported as n (%). The p-value column represents the outcome of the Kruskal–Wallis non-parametric test. Bold values indicate p < .05 considered as statistically significant.
| Wall-sit vs. | All (N = 48) | HC (N = 20) | HTx (N = 22) | |||
|---|---|---|---|---|---|---|
| Spearman R | p-Value | Spearman R | p-Value | Spearman R | p-Value | |
| QOL (PedsQL score) | .36* | .014 | .52* | .027 | .25 | .27 |
| BSA (m2) | .32 | .079 | .56* | .010 | .14 | .63 |
| Quad force/BSA (kg/m2) | .31* | .035 | .28 | .23 | .15 | .50 |
| Push-ups (no.) | .39** | .007 | .42 | .067 | .20 | .37 |
| Curl-ups (no.) | .43** | .002 | .62** | .004 | .31 | .17 |
| 6MWT (%pred distance) | −.088 | .55 | .20 | .41 | −.48* | .023 |
- Note: Analyzed by Spearman correlation of all participants (HC, HTx, KTx, N = 48), HC (N = 20), and HTx (N = 22) *p < .05, **p < .01. Bold values indicate p < .05 considered as statistically significant.
Deltoid normalized strength was lower in HTx than in controls (Figure 2, Table 2; p < .05). Abdominal normalized strength did not significantly differ between groups. Quadriceps normalized strength was significantly lower in KTx but not HTx as compared to controls.
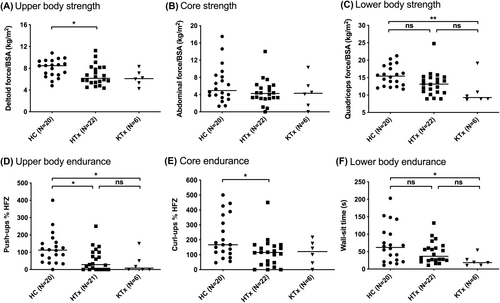
Push-ups (% of HFZ) were lower in both HTx and KTx as compared to controls (Figure 2, Table 2). Curl-ups (% of HFZ) were significantly lower in HTx than controls. Curl-ups in the KTx group were similar to HTx. Additionally, there was no significant difference between the amount of participants in the HFZ compared to controls for either group. Wall-sit time was lower in KTx than controls (p = .025) but not significantly different for HTx from either group, with the median ranging between the medians of the KTx and control groups.
The wall-sit test had weak to moderate, significant correlations with several fitness variables in an analysis of all study participants (Table 3): Lower body muscle strength, upper body muscle endurance, core muscle endurance, and QoL. Wall-sit time was negatively correlated with 6MWT in HTx (R = −.48, p = .02). No clinical variables correlated with the wall-sit test (Table 4).
| 6MWT (%pred distance) | Wall-sit time (s) | |||
|---|---|---|---|---|
| R-value | p-Value | R-value | p-Value | |
| Continuous variables | ||||
| Age at tx (years) | −.42 | .05* | .33 | .14 |
| Time post-tx (years) | −.18 | .43 | .17 | .44 |
| Time from diagnosis to tx (days) | −.09 | .69 | .19 | .39 |
| Hg level (most recent mmol/L) | −.08 | .74 | −.28 | .21 |
| eGFR (mL/min/1.73 m2) | .37 | .09 | −.21 | .36 |
| Pre-transplant surgeries (no.) | −.22 | .34 | −.05 | .83 |
| Prednisone (days) | −.25 | .25 | −.19 | .39 |
| Difference of medians (N-Y) | p-Value | Difference of medians (N-Y) | p-Value | |
|---|---|---|---|---|
| Dichotomous variables (Y/N) | ||||
| Prednisone | 0.70 | .88 | −21.0 | .41 |
| Statin | −3.60 | .20 | 29.0 | .26 |
| ACE inhibitor | −1.95 | .64 | −26.5 | .43 |
| Calcium channel blocker | −0.60 | .76 | 27.0 | .17 |
| ECMO | −0.80 | .88 | −28.0 | .29 |
| VAD | −4.30 | .32 | −25.0 | .26 |
| PTLD | 1.40 | >.99 | 24.0 | .20 |
| Stroke | −8.80 | .01** | 11.0 | .72 |
| Persistent neuromotor deficits | −11.1 | .08 | 32.0 | .31 |
| Rejection episode(s) | −1.40 | .64 | −27.0 | .29 |
- Note: Continuous variables were analyzed by Spearman's correlation, and dichotomous variables were analyzed by Mann–Whitney test * p < .05, **p < .01. Difference in medians presented for dichotomous variables. Bold values indicate p < .05 considered as statistically significant.
- Abbreviations: ECMO, extracorporeal membrane oxygenation; N, variable not present; PTLD, post-transplant lymphoproliferative disorder; tx, transplant; VAD, ventricular assist device; Y, variable present.
The QoL score of controls was 78 (46–100), HTx was 64 (33–97), and KTx was 71 (50–91). In the combined group of all participants (HTx, KTx, and controls), there was a moderate correlation between QoL score and wall-sit time (R = .36, p < .05). When looking at controls only, this correlation was strong (R = .52, p < .05). There were no significant correlations between QoL score and other fitness variables.
The difference in movement behavior (PAQ score) between groups was not significant (p = .17). Median PAQ score was highest in HTx at 2.96 (1.70–4.17), followed by controls at 2.74 (1.51–4.27), and then KTx at 2.36 (2.08–2.86).
4 DISCUSSION
In this study, we assessed the physical fitness of pediatric HTx and KTx recipients and compared it to a control group of healthy children using 6MWT %predicted distance, heart rate response, and muscle strength and endurance. 6MWT was 13% lower in HTx than controls, which is consistent with previous studies that show lower VO2max in HTx as compared to healthy children.26-28 This study included patients who were relatively young at the time of transplant (median 2.5 years), which may have resulted in higher aerobic capacity than other pediatric HTx cohorts. The relationship between younger age at the time of transplant and greater aerobic capacity has been shown in prior studies.7, 28 Although the aerobic capacity of the KTx group appeared more similar to the HTx group than controls, it was not significantly different from controls, however, this is likely related to the small number of participants in the KTx group.
The %change in HR during the 6MWT of HTx was significantly lower than that of controls. This finding may reflect heart denervation during the transplant procedure and subsequently altered response to autonomous regulation.29 While a reduced change in HR has the potential to affect aerobic capacity, it has been shown that pediatric HTx patients compensate for lower HR by increasing their stroke volume to maintain cardiac output during exercise.26 In our study, there was no correlation between %change in HR and 6MWT in HTx, which supports the idea that HR is not the determining factor for aerobic capacity in these children. However, with the 6MWT being a submaximal test, this study is limited in its ability to provide insight into the role that reinnervation might play in aerobic capacity closer to its threshold and during intense exercise.
We found some interesting novel aspects when looking at muscle strength and endurance: Both upper body muscle strength and endurance were lower in HTx as compared to controls. However, the difference in upper body muscle endurance (75%) was much greater than the difference in strength (27%) in HTx versus controls. Similarly, the difference in upper body muscle endurance (93%) was much greater than the difference in strength (28%) in KTx versus controls. These findings may reflect a difference in muscle fiber type (type I > type IIb) affected by transplantation or be associated with long-term impact of the thoracic injury at heart transplantation or previous cardiac surgeries or may relate to testing methods. Further, performance on the push-up test may have been limited by the greater proportional resistance in the push-up versus deltoid test (participant body weight versus examiner resistance), as well as the coordination with other muscle groups required to perform a push-up. Therefore, we suggest that future studies utilize alternative testing modalities that do not as quickly reach an expected maximum in children, such as modified, supported push-ups, to better analyze upper body strength and endurance separately.
Core muscle endurance, but not strength, was lower in HTx as compared to controls. In contrast, there was no difference in core muscle strength or endurance in KTx as compared to controls. It is unclear whether HTx core endurance is more affected than that of KTx, or if this reflects a type II error due to the small sample size of KTx.
Lower body muscle strength and endurance were not different between HTx and controls, which differs from a previous study in adult HTx showing reduced lower body muscle strength in HTx versus controls.30 While the HTx group did have a numerically lower wall-sit test than controls, we cannot comment on the clinical relevance of this. The difference in lower body strength between pediatric and adult transplant recipients may be due to peripheral muscle deconditioning, which is common in adults before and after transplantation,30 but not in children. In contrast, both lower body muscle strength and endurance were lower in KTx than controls. We speculate, that this difference in lower limb function is associated with continuous glucocorticoid therapy, which was maintained only in the KTx group in this cohort. This finding may be explained by the direct effects of glucocorticoids on skeletal muscle,5 or perhaps by glucocorticoid-impaired regeneration of skeletal muscle after muscle wasting related to pre-transplant illness.12
Quality of life was not significantly different between controls, HTx, and KTx in this study. However, QoL ranged lower in both transplant groups compared to controls to a clinically meaningful extent. In the literature, QoL is reported as lower in both KTx and HTx, the latter being measured at about one standard deviation divergence lower than their healthy peers.31, 32 This inconsistency may reflect a difference in study cohorts, with our patients being at least one-year post-transplant at assessment with possibly recovered QoL due to being longer after transplant, an advancement in care for more recent transplant recipients, recruitment bias, or insufficient power due to comparably small cohort in this study.
The relationship between QoL score and fitness parameters was investigated in this study. There was a moderate significant correlation between QoL score and wall-sit time in the control group, but not in the HTx or KTx groups alone. Given the non-normal distribution of data points in the HTx and KTx groups, it is difficult to conclude whether or not QoL is directly reflected in the wall-sit time in pediatric transplant recipients and further longitudinal evaluation is required. The wall-sit test correlated with muscle mass (BSA used as a proxy), quadriceps strength, and core and upper body muscle endurance (#curl-ups and #push-ups, respectively). These relationships support that the simple wall-sit test (no tools required, takes <5 min) could be helpful to assess longitudinal changes in fitness at routine clinic visits. Intra-individual changes may be reflective of changing overall fitness and indicate patients at need for a more thorough assessment of these components of well-being. Given the correlation with QoL assessment, it could also be used as an indicator for further assessment of QoL during or after a clinic visit. This important application needs to be tested in prospective, longitudinal studies.
In contrast to previous reports that movement behavior level is lower in HTx and KTx than controls,10 we found no difference in reported movement behavior levels between HTx, KTx, and controls in this study. This may be explained by participants self-reporting more movement behavior than their objective level, as previously observed10, 33 since the deficit in fitness suggests lower fitness levels. Future studies would benefit from the inclusion of an objective assessment of movement behaviors for example by using accelerometers.
We did not identify many clinical and lifestyle factors with a relationship with the 6MWT or wall-sit test. However, 6MWT was significantly lower in HTx with stroke compared to those without it. Interestingly, this was not primarily driven by stroke patients with persisting neuromotor deficits and may hint towards residual subclinical deficits or altered lifestyle habits in children who experienced a stroke. This would also explain the reduced aerobic capacity in HTx with stroke. Interestingly, the history of stroke was not related to wall-sit time, which suggests that the wall-sit test may be more accessible than the 6MWT in HTx with subtle physical disabilities.
Limitations of this study include the overall small sample size but particularly the small KTx group, which was likely underpowered to statistically confirm some visually overt differences, which is also represented in some of the correlations only being significant when looking at the whole group but not the sub-groups. The difference in the size of participants was accounted for with internal or external controls in each fitness test. The KTx group had a lower mean hemoglobin, which is associated with 6MWT distance in pediatric KTx,9 but there was no correlation between those variables in this study. The HTx group included seven patients' post-stroke, which affected the 6MWT, however, thereby reflects a typical cohort after pediatric heart transplant with stroke being a common adverse event while on mechanical circulatory support or in the course of pre-transplant cardiac surgeries.
In summary, this study provides a comprehensive assessment of muscle strength and endurance in the pediatric HTx population as compared to KTx and healthy children, confirming that many fitness parameters are reduced in pediatric HTx recipients especially those with a history of stroke. KTx shows specific weakness in the lower limb not present after HTx, likely secondary to prolonged steroid use. In contrast to healthy children, no clear correlation was identified between fitness level and QoL in transplanted children, suggesting that physical fitness may be less important for QoL in this population. We found the wall-sit test to be a quick and easy tool well reflecting overall fitness thereby representing a convenient way to perform longitudinal assessments of muscle function in both the clinical setting and research studies and could be integrated into the routine clinical follow-ups to identify children in need of further assessment, however, further confirmation of this correlation in larger sample sizes is needed. Future studies should focus on the impact of structured interventions such as rehabilitation and fitness programs on the fitness and well-being of transplanted children.
AUTHOR CONTRIBUTIONS
The authors confirm participation in the study as follows. Research design: CJA, SM, LJW, SU. Data curation: CJA, IL, SU. Data analysis: ARK, CJA, SM, LJW, SU. Funding acquisition: CJA, IL, SM, LJW, SU. Investigation: CJA, IL. Methodology: CJA, SM, SCG, TB-H, CM, LJW, SU. Project administration: CJA, IL. Resources: SM, SCG, TB-H, CM, LJW, SU. Supervision: LJW, SU. Validation: SM, SCG, TB-H, LJW, SU. Visualization: CJA, SU. Writing – original draft: ARK, CJA. Writing, review & editing: ARK, CJA, SM, SCG, TB-H, CM, MK, LJW, SU.
FUNDING INFORMATION
This research was supported by the Astellas ATI CNTRP Research Innovation Grant funded by Astellas Canada.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.