Changes in graft outcomes in recipients <10 kg over 25 years of pediatric kidney transplantation in the United States
Abstract
Background
Kidney transplant (KT) was initially associated with poor outcomes, especially in smaller recipients. However, pediatric transplantation has evolved considerably over time. We investigated the impact of weight at the time of transplant and whether outcomes changed over 25 years for <10 kg recipients.
Methods
Using the UNOS database, pediatric recipient outcomes were analyzed between 1/1/99 and 12/31/14. KT weight was stratified: <8.6 kg (mean weight of recipients <10 kg), 8.6–9.9 kg, 10–14.9 kg, 15–29.9 kg, and ≥30 kg. Outcomes in recipients <10 kg were then compared between 1990–1999 and 2000–2014.
Results
17 314 pediatric KT recipients were included; 518 (3%) had a transplant weight <10 kg. The highest rates of allograft loss and death were in recipients <8.6 kg and ≥30 kg. Recipients <8.6 kg also had higher rates of delayed graft function, rejection, and longer hospital length of stay. In the multivariable Cox regression model, transplant weight was not a predictor of allograft loss. When compared with recipients <8.6 kg, patient survival hazard ratios associated with recipient weight of 10–14.9 kg, 15–29.9 kg, and ≥30 kg were 0.61 (95%CI: 0.4, 1), 0.42 (95%CI: 0.3, 0.7) and 0.32 (95%CI: 0.2, 0.6), respectively. In the later era of transplant, recipients <10 kg had improved outcomes on univariate analysis; however, the era of transplantation was not an independent predictor of allograft loss or patient survival in Cox regression models.
Conclusions
Outcomes in children weighing 8.6–9.9 kg at the time of KT were similar to higher weight groups and improved over time; however, special precautions should be taken for recipients <8.6 kg at the time of transplant.
Abbreviations
-
- BMI
-
- body mass index
-
- CAKUT
-
- congenital anomalies of the kidney and urinary tract
-
- CI
-
- confidence interval
-
- DCGF
-
- death-censored graft survival
-
- DGF
-
- delayed graft function
-
- ESKD
-
- end-stage kidney disease
-
- HLA
-
- human leukocyte antigens
-
- HR
-
- hazard ratio
-
- KT
-
- kidney transplantation
-
- PRA
-
- peak panel reactive antibody
-
- UNOS
-
- United Network for Organ Sharing
1 INTRODUCTION
Kidney transplantation is the treatment of choice in children with kidney failure as it has been shown to significantly improve long-term outcomes when compared to any other renal replacement therapy.1 However, the advent of pediatric kidney transplants was initially associated with poor outcomes, especially in smaller recipients.2 This was primarily due to challenges in donor-recipient size matching, a higher risk of surgical and infectious complications, and peri-transplant difficulties with fluid management and hemodynamics.3-5 Pediatric transplantation has evolved considerably when compared to the earlier era of kidney transplantation, including changes in organ allocation policy, rates of living donor transplantation, surgical technique, and immunosuppression.2, 4, 6
In more recent years, potential kidney recipients have been considered transplantable from weights as small as 7–8 kg depending on the surgical experience of the transplant surgeon.6, 7 While outcomes are reported to be favorable,8 most previous studies focused on body mass index (BMI) percentiles at the time of transplant and not absolute weight.9-12 BMI norms are typically assessed using percentiles and are designed to compare weight adjusted for length. While they are important, they may not fully capture the impact of weight at the time of transplantation on allograft outcomes which in most centers is more commonly used criterion for determining eligibility for transplantation. No study to date has focused on long-term outcomes in small kidney transplant recipients weighing <10 kg in the United States. Furthermore, no study has investigated differences in allograft and patient survival between children weighing 10 kg at the time of transplant and lower weight groups of <8–9 kg.
The objective of our study was to (1) compare the impact of weight at the time of transplant on kidney transplant outcomes in pediatric kidney transplant recipients weighing <10 kg to higher weight groups and (2) investigate whether outcomes (patient and graft survival) changed over time for kidney transplant recipients who weighed <10 kg at the time of transplant using a national database over a 25-year period. We hypothesized that children undergoing kidney transplant who were <10 kg at the time of transplant had similar patient and graft survival when compared other children and that outcomes of kidney transplant in children weighing <10 kg improved over time.
2 PATIENTS AND METHODS
2.1 Study population
We conducted a retrospective study of all pediatric transplant recipients in the United States. Using the United Network for Organ Sharing (UNOS) database, we sought to estimate differences in both patient and graft survival in children who were <10 kg at the time of primary kidney alone transplant compared to those greater than or equal to 10 kg. We also sought to compare how patient and allograft outcomes have changed over time in recipients weighing <10 kg at the time of transplant by comparing two eras of transplantation: 1990–1999 to 2000–2014. All children (age ≤ 18 years) who underwent primary kidney alone transplants from 1990 through 2014 were eligible for this study.
The UNOS maintains a national database of all patients awaiting and those who have received organ transplants. The database provides detailed deidentified demographic and clinical data for both recipients and donors. Recipient data extracted included age at the time of transplant, gender as a biological variable, race/ethnicity [black, white, Hispanic, Asian, and other (Native American, Pacific Islander, and multiracial)], weight at the time of transplant, cause of end-stage kidney disease (ESKD) (available for 7628 transplant recipients), dialysis history (yes/no variable), and peak panel reactive antibody (PRA). Donor data extracted included age, gender, and race/ethnicity. The ratio of donor/recipient weight was calculated using dividing donor weight by recipient weight in kilograms. The cause of ESKD was categorized into five groups: congenital anomalies of the kidney and urinary tract (CAKUT), glomerular, hereditary nephritis, cystic kidney disease, and others. Clinical variables included the type of transplant (living vs. deceased donor), the number of HLA-ABDR mismatches (categorized as 0, 1–3, 4–6 mismatches), cold ischemia time (hours), delayed graft function (DGF, yes/no variable), the hospital length of stay (days), history of biopsy-proven rejection, patient survival, and graft survival.
Weight at the time of transplant was stratified into 5 groups: <8.6 kg (mean weight of recipients weighing <10 kg), 8.6–9.9 kg, 10–14.9 kg, 15–29.9 kg, and ≥30 kg. Patients with missing weight data were excluded from our study.
2.2 Outcome measurements
The primary outcomes of interest were patient survival and graft survival. Secondary recipient outcomes of interest included DGF defined as the need for dialysis within the first week after kidney transplantation (KT), the hospital length of stay (days), and history of biopsy-proven rejection. The exposure variables independently examined were weight at the time of KT and era of transplantation (comparing transplants performed from 1990–1999 to 2000–2014). Death-censored graft survival (DCGS) was defined as the time between date of KT and either the date of graft failure (marked by re-transplantation or return to dialysis) or last date of follow-up with a functioning graft, censoring for death at the administrative end of study (September 27, 2019). Patient survival was defined as the time from KT to death or last follow-up, censoring for administrative end of study. Death and graft loss ascertainment were supplemented by linkage to data from the Centers for Medicare and Medicaid Services. Death ascertainment was also supplemented by linkage to the Social Security Death Master File.
2.3 Statistical analysis
Recipient outcomes of the groups were compared using the ANOVA or Mann–Whitney tests for continuous variables (if parametric or non-parametric, respectively) and the chi-squared test for categorical variables.
Variables were included apriori in the multivariate models if they were known to be associated with the outcome of interest or if the p-value was <.2 in the bivariate analyses. DCGS and patient survival were estimated using Kaplan–Meier methods, log-rank tests, and multivariable Cox proportional hazards. All tests were 2-sided, with statistical significance set at p < .05. Analyses were performed by using Stata 16/SE (Stata Corp, College Station, TX).
3 RESULTS
3.1 Study population stratified by weight at the time of transplant.
A total of 17 314 pediatric kidney transplant recipients were included in our study. Weight at the time of transplant was missing for 480 (2.7%) recipients and was excluded from the study. 518 (3%) of recipients were <10 kg at the time of transplant with a mean weight of 8.6 kg and the mean age was 1.3 ± 0.9 years. When stratified according to weight, there were 205 (1.2%) recipients who were <8.6 kg, 313 (1.8%) between 8.6 and 9.9 kg, 1865 (10.8%) between 10 and 14.9 kg, 3930 (22.7%) between 15 and 29.9 kg, and 11 001 (63.5%) ≥30 kg (Table 1).
| <8.6 kg (n = 205) | 8.6–9.9 kg (n = 313) | 10–14.9 kg (n = 1865) | 15–29.9 kg (n = 3930) | ≥30 kg (n = 11 001) | p | |
|---|---|---|---|---|---|---|
| Recipient factors | ||||||
| Age at transplant (years) | 1 (1, 1) | 1 (1, 2) | 2 (1, 3) | 8 (5, 10) | 15 (13, 17) | <.001 |
| Weight at transplant (kg) | 7.3 ± 1 | 9.4 ± 0.4 | 12.3 ± 1.4 | 21.9 ± 4.3 | 54.7 ± 17.4 | <.001 |
| Height at transplant (cm) | 67.8 ± 6 (n = 172) | 74.4 ± 6.2 (n = 293) | 84.1 ± 8.3 (n = 1799) | 113.9 ± 13.9 (n = 3808) | 156.7 ± 14.6 (n = 10 779) | <.001 |
| Male Sex, % | 64.4 | 68.4 | 66.5 | 59.5 | 57 | <.001 |
| Recipient Ethnicity, % | ||||||
| Caucasian | 64.4 | 69.7 | 61.2 | 57.2 | 52 | <.001 |
| African American | 9.3 | 11.5 | 15.5 | 15.9 | 21 | |
| Hispanic | 22.5 | 14.1 | 18 | 21.8 | 21.6 | |
| Other | 3.9 | 4.8 | 5.3 | 5.1 | 5.4 | |
| Cause of ESKD, % | ||||||
| CAKUT | 70.8 | 65.5 | 63.2 | 47.7 | 31 | <.001 |
| Glomerular | 2.1 | 4.8 | 15.3 | 34 | 48.9 | |
| Hereditary nephritis | 6.3 | 1.2 | 0.5 | 4.5 | 7.8 | |
| Cystic kidney disease | 18.8 | 17.9 | 12.3 | 7.4 | 5.5 | |
| Other | 2.1 (n = 48) | 10.7 (n = 84) | 8.8 (n = 636) | 6.4 (n = 1641) | 6.8 (n = 5219) | |
| Pre-transplant dialysis, % | 74.3 (n = 187) | 75.8 (n = 297) | 77.4 (n = 1793) | 73.4 (n = 3720) | 75.5 (n = 10 595) | .021 |
| Transplant type, % | <.001 | |||||
| Living | 71.7 | 66.8 | 59.1 | 51 | 46.3 | |
| HLA-ABDR mismatch, % | ||||||
| 0 | 4.1 | 2.3 | 2.6 | 3.2 | 3.8 | <.001 |
| 1–3 | 70.9 | 61.2 | 56.2 | 51.4 | 46.7 | |
| 4–6 | 25 (n = 196) | 36.6 (n = 309) | 41.2 (n = 1841) | 45.4 (n = 3880) | 49.5 (n = 10 897) | |
| Peak panel reactive antibody (PRA) | 0 (0, 6) (n = 70) | 0 (0, 4) (n = 108) | 0 (0, 4) (n = 680) | 0 (0, 3) (n = 1686) | 0 (0, 3) (n = 5203) | .04 |
| Donor characteristics | ||||||
| Donor age (years), % | ||||||
| <35 | 74.6 | 74.7 | 77.2 | 66.5 | 57.2 | <.001 |
| 35–49 | 22.4 | 22 | 20.4 | 30.4 | 36.7 | |
| ≥50 | 2.9 | 3.2 | 2.4 | 3.1 | 6.1 | |
| Donor ethnicity, % | ||||||
| Caucasian | 72.2 | 76.4 | 69.2 | 66.4 | 65.3 | <.001 |
| African American | 9.3 | 9.3 | 13.2 | 11.7 | 13.2 | |
| Hispanic | 16.6 | 13.4 | 14.4 | 18.9 | 18.3 | |
| Other | 2 | 1 | 3.2 (n = 1862) | 3 (n = 3921) | 3.2 (n = 10 987) | |
| Donor weight (kg) | 64.7 ± 26.1 (n = 122) | 70.9 ± 23.4 (n = 235) | 70 ± 21.4 (n = 1480) | 70.6 ± 21.1 (n = 3036) | 73.1 ± 21.2 (n = 8896) | .002 |
| Donor/recipient weight ratio | 8.7 ± 3.6 (n = 122) | 7.6 ± 2.5 (n = 235) | 5.7 ± 1.9 (n = 1480) | 3.4 ± 1.2 (n = 3036) | 1.4 ± 0.6 (n = 8896) | <.001 |
| Cold ischemia time in hours | 2.3 (1, 14) (n = 148) | 3 (1, 13) (n = 231) | 3.6 (1, 13.1) (n = 1501) | 6.8 (1, 15) (n = 3248) | 8 (1, 16) (n = 9257) | <.001 |
- Note: p values below .05 were considered statistically significant and marked bold.
The mean age at the time of KT for the study population was 11.5 ± 5.4 years. The mean weight of the entire study population was 41.3 ± 21.8 kg. In the 205 recipients who were <8.6 kg at the time of transplant, their mean weight was 7.3 ± 1 kg, while their mean age was 1 ± 0.8 years. The mean weight for recipients 8.6–9.9 kg at the time of transplant was 9.4 ± 0.4 kg, and their mean age was 1.5 ± 0.9 years.
3.2 Donor and recipient characteristics stratified by weight at the time of transplant
Demographic, recipient, and donor characteristics are summarized in Table 1. Pediatric transplant recipients were predominantly male across all age categories. Most transplant recipients were on dialysis at the time of transplant regardless of their weight. The most common reason for requiring a kidney transplant in smaller children was CAKUT, but the frequency decreased as weight increased with then glomerular disease becoming more prevalent. Pediatric kidney transplant recipients <15 kg were more likely to receive a living donor, while the other weight groups were more likely to receive a deceased donor. The majority of the study population had at least 1 HLA mismatch, and the median PRA was 0% at the time of transplantation for all groups. Analysis of the donor characteristics showed that most donors were <35 years of age. The donor weight was lowest in recipients <8.6 kg, and the donor/recipient weight ratio was the highest when compared to remaining patient weight groups. Cold ischemia time was shortest in recipients <15 kg, presumably due to the higher proportion of living transplant recipients in these lower weight groups.
3.3 Recipient outcomes stratified by weight at the time of transplant
Table 2 shows the clinical outcomes stratified by weight at the time of KT. The frequency of delayed graft function was highest in children transplanted at <8.6 kg (10.3%) p = .007. The median hospital length of stay (LOS) was also significantly longer in children transplanted at <8.6 kg [15.5 (9.5, 28) days] and decreased as weight increased [≥30 kg group: 86, 10 days; p < .001]. The frequency of documented biopsy-proven rejection was highest in the <8.6 kg group at 8% compared to the other weight groups where the rates of rejection ranged from 4.1% to 6.6%; however, this finding was not statistically significant (p = .06).
| <8.6 kg (n = 205) | 8.6–9.9 kg (n = 313) | 10–14.9 kg (n = 1865) | 15–29.9 kg (n = 3930) | ≥30 kg (n = 11 001) | p | |
|---|---|---|---|---|---|---|
| Delayed graft function, % | 10.3 (n = 204) | 9 (n = 312) | 6.1 (n = 1860) | 7 (n = 3920) | 8.1 (n = 10 958) | .007 |
| Hospital length of stay (days) | 15.5 (9.5, 28) (n = 108) | 14 (9, 21) (n = 213) | 11 (8, 16) (n = 1338) | 9 (7, 14) (n = 2642) | 8 (6, 10) (n = 7756) | <.001 |
| Biopsy-proven rejection, % | 8 (n = 151) | 6.6 (n = 167) | 5.1 (n = 886) | 4.8 (n = 2144) | 4.1 (n = 5676) | .06 |
| Graft loss, % | 39.5 | 31.3 | 31.1 | 35.1 (n = 3929) | 41.3 (n = 10 990) | <.001 |
| Death, % | 9.8 | 6.1 | 6.2 | 7.1 | 9.2 | <.001 |
- Note: p values below .05 were considered statistically significant and marked bold.
The highest proportion of transplant recipients who experienced graft loss was in the ≥30 kg group weight group (41.3%) and in the <8.6 kg (39.5%) compared to between 31.1% and 35.1% in other weight groups. The difference in graft loss between the 4 groups was statistically significant; p < .001. Death rates were significantly higher in children <8.6 kg at 9.8% followed by the >30 kg group at 9.2% compared to ranges of 6.1%–7.1% in the 10–29.9 kg weight groups; p < .001. A comparison of the study population and outcomes stratified by <10 kg and ≥10 kg is demonstrated in Tables S1 and S2, respectively.
3.4 Donor and recipient characteristics for recipients < 10 kg stratified by era of transplantation
As recipients <8.6 kg and those 8.6–9.9 kg did not significantly differ by era of transplantation (p = .4), recipient demographic and pre-transplant characteristics were compared in all children <10 kg stratified by era of transplantation [(1990–1999) vs. (2000–2014)] as shown in Table 3. Although the age at transplant was statistically higher in the 2nd era, this difference was not clinically significant. Interestingly, the median weight at transplant was significantly lower in the earlier era of transplant when compared to the later era [8.4 (7.1, 9.3) kg vs. 9.1 (8.4, 9.7) kg; p < .001]. There were no significant differences in gender, race/ethnicity, the frequency of pre-transplant dialysis, or type of transplant (deceased vs. living). However, the proportion of African American patients transplanted in the later era nearly doubled (6.7 vs. 13.8%). The number of HLA-ABDR mismatches increased significantly in the second era with 4–6 mismatches increasing from 25.9% to 36.5%; p = .04. The only donor characteristic which changed significantly over time was the donor weight which increased from a median of 59.4 (25, 77) kg to 73 (62.6, 84.8) kg; p = .001. The donor/recipient weight mismatch was significantly higher in the later era of transplantation (6.7 vs. 8.2; p < .001).
| Transplantation period | p | ||
|---|---|---|---|
| 1990–1999 (n = 220) | 2000–2014 (n = 298) | ||
| Age at transplant (years) | 1.2 ± 0.9 | 1.4 ± 0.9 | .03 |
| Weight at transplant (kg) | 8.4 (7.1, 9.3) | 9.1 (8.4, 9.7) | <.001 |
| Male sex | 65.9% | 67.4% | .71 |
| Recipient race/ethnicity | |||
| Caucasian | 69% | 65.4% | .052 |
| African American | 6.7% | 13.8% | |
| Hispanic | 20% | 16.1% | |
| Other | 4.3% | 4.7% | |
| Pre-transplant dialysis | 70.5% | 70.1% | .06 |
| Transplant type | |||
| Deceased | 31.8% | 30.9% | .82 |
| Living | 68.2% | 69.1% | |
| HLA-ABDR mismatch | |||
| 0 | 3.8% | 2.4% | .04 |
| 1–3 | 70.3% | 61.1% | |
| 4–6 | 25.9% | 36.5% | |
| Donor characteristics | |||
| Donor age (years) | |||
| <35 | 72.7% | 76.2% | .24 |
| 35–49 | 22.7% | 21.8% | |
| ≥50 | 4.6% | 2% | |
| Donor ethnicity | |||
| Caucasian | 79.1% | 71.5% | .12 |
| African American | 5.9% | 11.7% | |
| Hispanic | 13.6% | 15.5% | |
| Other | 1.4% | 1.3% | |
| Donor weight (kg) | 59.4 (25, 77) | 73 (62.6, 84.8) | .001 |
| Donor/recipient weight ratio | 6.7 (3.6, 9.5) (n = 75) | 8.2 (6.9, 9.7) (n = 282) | <.001 |
| Cold ischemia time | 2 (0, 17) | 3 (1, 10.1) | .11 |
- Note: p values below .05 were considered statistically significant and marked bold.
3.5 Outcomes in recipients ≤ 10 kg stratified by era of transplantation
The clinical outcomes according to era of transplantation [(1990–1999) vs. (2000 vs 2014)] in children who were transplanted at <10 kg are demonstrated in Table 4. The frequency of delayed graft function did not differ significantly over time. The hospital length of stay decreased significantly from a median of 18 days (IQR: 13.30) days to 14 (IQR; 9.22); p = .02. In addition, the frequency of biopsy-proven rejection decreased significantly (from 9.5% to 2%; p = .02). Graft loss also decreased significantly over time in this group of children from 49.1% to 23.8%; p = .0001. Finally, we observed that children were less likely to die after kidney transplantation over time. The frequency of death decreased from 11.4% between 1990 and 1999 to 4.7% between 2000 and 2014; p = .004.
| Transplantation period | p | ||
|---|---|---|---|
| 1990–1999 (n = 220) | 2000–2014 (n = 298) | ||
| Delayed graft function | 9.5% | 9.4% | .26 |
| Hospital length of stay (days) | 18 (13, 30) | 14 (9, 22) | .02 |
| Biopsy-proven rejection | 9.5% | 2% | .02 |
| Graft loss | 49.1% | 23.8% | .0001 |
| Death | 11.4% | 4.7% | .004 |
- Note: p values below .05 were considered statistically significant and marked bold.
3.6 The effect of weight on death-censored allograft and patient survival
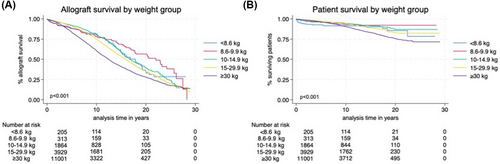
Kaplan–Meier survival curves stratified by weight at the time of transplant over the 25-year follow-up period are demonstrated for allograft survival and patient survival in Figure 1A,B, respectively. Allograft survival significantly differed across weight groups (p < .001) and in the immediate post-transplant period appeared to be lower in the <8.6 and 8.6–9.9 kg weight groups then over follow-up the ≥30 kg weight group had the lowest survival. Early patient survival was lowest in the <8.6 kg weight group however was then comparable to the 8.6–29.9 kg weight groups until about 20 years follow-up duration, whereas patient survival steadily declined over time in recipients ≥30 kg (p < .001).
Using multivariable Cox proportional hazards (Table 5), we sought to determine independent risk factors for allograft loss and patient survival adjusting for weight at the time of transplant in addition to recipient age, recipient biological sex, recipient race/ethnicity, cause of ESKD, living vs deceased donor transplant, number of HLA mismatches, and delayed graft function. Both models included 17 061 recipients. Weight at the time of transplant was not an independent predictor of allograft loss. For every 1-year increase in age at the time of transplant, there was a 3% increased hazard of graft loss (95% CI: 1.02, 1.04; p < .0001). Male sex was protective for allograft loss (HR 0.84, 95% CI: 0.8, 0.9; p < .0001). In addition, African American and ‘other’ race, glomerular disease as the cause of ESKD, deceased donor transplant, any HLA mismatches, and delayed graft function were significant predictors of allograft loss in the multivariate model. In the multivariable model with patient survival as the outcome of interest, recipient weight of ≥10 kg was associated with a graded association of improved patient survival with increasing weight group compared to recipients weighing <8.6 kg at the time of transplant. Hazard ratios associated with recipient weight of 10–14.9 kg, 15–29.9 kg and ≥ 30 kg were 0.61 (95% CI: 0.4, 1), 0.42 (95% CI: 0.3, 0.7), and 0.32 (95% CI: 0.2, 0.6), respectively. Older age at transplant, African American race, unknown cause of ESKD, deceased donor transplant, and delayed graft function were significant predictors of patient death, whereas male sex, Hispanic, and ‘other’ race/ethnicity (compared to Caucasian reference group) were protective for patient survival.
| Allograft loss | Patient survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | 95% Confidence Limits | p | Hazard Ratio | 95% Confidence Limits | p | |||
| Weight at transplant (ref = <8.6 kg) | ||||||||
| 8.6–9.9 kg | 0.78 | 0.6 | 1.1 | .1 | 0.61 | 0.3 | 1.2 | .13 |
| 10–14.9 kg | 0.82 | 0.6 | 1.0 | .12 | 0.61 | 0.4 | 1.0 | .05 |
| 15–29.9 kg | 0.79 | 0.6 | 1.0 | .6 | 0.42 | 0.3 | 0.7 | .001 |
| ≥30 kg | 0.87 | 0.7 | 1.1 | .3 | 0.32 | 0.2 | 0.6 | <.001 |
| Age at transplant (years) | 1.03 | 1.02 | 1.04 | <.001 | 1.1 | 1.1 | 1.1 | <.001 |
| Male Sex (ref = female) | 0.84 | 0.8 | 0.9 | <.001 | 0.8 | 0.7 | 0.9 | <.001 |
| Recipient race/ethnicity (ref = Caucasian) | ||||||||
| African American | 1.65 | 1.6 | 1.8 | <.001 | 1.5 | 1.3 | 1.7 | <.001 |
| Hispanic | 1.04 | 1.0 | 1.1 | .18 | 0.67 | 0.6 | 0.8 | <.001 |
| Other | 0.88 | 0.8 | 1.0 | .03 | 0.72 | 0.5 | 0.9 | .021 |
| Cause of ESKD (ref = CAKUT) | ||||||||
| Glomerular | 1.21 | 1.1 | 1.3 | <.001 | 1.0 | 0.8 | 1.2 | .96 |
| Cystic kidney disease | 1.0 | 0.8 | 1.2 | .98 | 0.87 | 0.6 | 1.3 | .51 |
| Hereditary nephritis | 0.87 | 0.7 | 1.0 | .13 | 1.1 | 0.8 | 1.6 | .6 |
| Other | 0.95 | 0.8 | 1.1 | .51 | 0.73 | 0.5 | 1.1 | .12 |
| Unknown/Missing | 0.96 | 0.9 | 1.0 | .34 | 1.3 | 1.1 | 1.5 | .007 |
| Deceased donor transplant (ref = living donor) | 1.11 | 1.0 | 1.2 | .002 | 1.35 | 1.2 | 1.6 | <.001 |
| HLA mismatch (ref = 0) | ||||||||
| 1–3 | 1.44 | 1.2 | 1.7 | <.001 | 1.1 | 0.8 | 1.5 | .53 |
| 4–6 | 1.62 | 1.4 | 1.9 | <.001 | 0.94 | 0.7 | 1.3 | .66 |
| Delayed graft function | 2.09 | 1.9 | 2.3 | <.001 | 2.5 | 2.1 | 2.9 | <.001 |
- Note: p values below .05 were considered statistically significant and marked bold.
- Abbreviations: CAKUT, congenital anomalies of kidney and urinary tract, HLA, human leukocyte antigens.
3.7 The effect of era of transplantation in recipients <10 kg on death-censored allograft and patient survival.
Figure 2A,B show the Kaplan–Meier survival curves stratified by the two eras of transplantation: 1990–1999 and 2000–2014 in recipients weighing <10 kg at the time of transplant. On univariate analysis, there was no significant difference in allograft survival between both eras (p = .88); however, patient survival significantly improved for recipients weighing <10 kg in the later era of transplantation (p < .001).
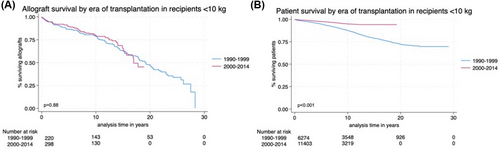
Table 6 demonstrates the multivariable Cox proportional hazards model used to determine independent-risk factors for allograft loss and patient survival in recipients weighing <10 kg at time of transplantation adjusting for era at the time of transplantation (1990–1999 vs. 2000–2014) in addition to recipient age, recipient biological sex, recipient race/ethnicity, cause of ESKD, living vs deceased donor transplant, number of HLA mismatches, and delayed graft function. Both models included 503 recipients. The era of transplantation was not an independent predictor of graft loss (HR 0.96, 95% CI: 0.7, 1.4; p = .83). African American race was associated with 2.2 times increased hazard of allograft loss (95% CI: 1.3, 3.6; p = .002). Delayed graft function was also an independent predictor of allograft loss (HR 4.7, 95% CI: 3, 7.2; p < .001). In the multivariable model with patient survival as the outcome of interest, the era of transplantation was not an independent predictor in recipients weighing <10 kg at the time of transplant (HR: 0.52, 95% CI: 0.3, 1.1; p = .08). In contrast, having 1–3 HLA mismatches and delayed graft function was associated with a significantly increased hazard for patient death.
| Allograft Loss | Patient Survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | 95% Confidence Limits | p | Hazard Ratio | 95% Confidence Limits | p | |||
| Era of transplantation 2000–2014 (ref = 1990–1999) | 0.96 | 0.7 | 1.4 | .83 | 0.52 | 0.3 | 1.1 | .08 |
| Age at transplant (years) | 0.9 | 0.7 | 1.1 | .28 | 1.15 | 0.8 | 1.7 | .46 |
| Male Sex (ref = female) | 0.9 | 0.6 | 1.2 | .47 | 0.76 | 0.4 | 1.5 | .44 |
| Recipient race/ethnicity (ref = Caucasian) | ||||||||
| African American | 2.2 | 1.3 | 3.6 | .002 | 2.17 | 0.8 | 6.2 | .14 |
| Hispanic | 1.5 | 0.99 | 2.2 | .59 | 1.17 | 0.5 | 2.8 | .71 |
| Other | 1.2 | 0.5 | 2.4 | .71 | 0.46 | 0.1 | 3.5 | .46 |
| Cause of ESKD (ref = CAKUT) | ||||||||
| Glomerular | 0.33 | 0.04 | 2.3 | .28 | 6.1 × 10−20 | – | – | – |
| Cystic kidney disease | 1.2 | 0.6 | 2.7 | .58 | 2.1 | 0.4 | 10.9 | .39 |
| Hereditary nephritis | 1.2 | 0.3 | 5.3 | .82 | 2.8 × 10−19 | – | – | – |
| Other | 1.7 | 0.5 | 5.5 | .41 | 1.5 × 10−19 | – | – | – |
| Unknown/Missing | 0.76 | 0.5 | 1.2 | .23 | 1.23 | 0.4 | 3.5 | .69 |
| Deceased donor transplant (ref = living donor) | 1.2 | 0.7 | 2.0 | .59 | 1.04 | 0.4 | 2.9 | .94 |
| HLA mismatch (ref = 0) | ||||||||
| 1–3 | 3.7 | 0.9 | 15.7 | .08 | 4.3 × 108 | 1.5 × 108 | 1.2 × 109 | <.001 |
| 4–6 | 4.1 | 0.9 | 18.8 | .07 | 4.9 × 108 | – | – | – |
| Delayed graft function | 4.7 | 3.0 | 7.2 | <.001 | 8.1 | 3.8 | 17.1 | <.001 |
- Note: p values below .05 were considered statistically significant and marked bold.
- Abbreviations: CAKUT, congenital anomalies of kidney and urinary tract, HLA, human leukocyte antigens.
4 DISCUSSION
This national study shows that a weight of 8.6–9.9 kg at the time of transplant was associated with similar outcomes to children with a higher weight at the time of transplant. Our study is the first to examine outcomes more granularly in recipients weighing <10 kg at the time of transplantation by comparing the mean lower weight group of <8.6 kg to those weighing 8.6–9.9 kg. The main findings of our study are that, when stratified by weight at the time of transplant, children <8.6 kg and >30 kg had the highest rates of both allograft loss and death; however, recipients 8.6–9.9 kg had comparable outcomes to weight groups between 10 and 29.9 kg. We also found that the later (vs. the 1990s) era of transplantation was associated with improved outcomes in small (<10 kg) pediatric kidney transplant recipients on univariate analysis. Over time, they also had a shorter hospital length of stay at the time of transplant, less biopsy-proven rejection, and were less likely to experience graft loss or death. Finally, we found that the weight at the time of transplant and era of transplantation were not significant predictors of allograft loss in the adjusted multivariable Cox proportional hazards models. However, when compared to recipients who weighed <8.6 kg at the time of transplant, having a transplant weight of ≥10 kg was increasingly protective for patient survival with each higher weight group, whereas the era of transplantation had no impact on patient survival when adjusted in our model.
When deciding on the optimal timing of a pediatric kidney transplant in an infant/toddler, the goal is to perform the transplant early enough to prevent the long-term complications of advanced chronic kidney disease or ESKD but at an age/size where the kidney will fit in the abdomen and the likelihood of surgical and peri-operative complications is decreased.13
The optimal weight at which an infant toddler may be transplanted varies considerably, with some specialized larger transplant centers performing more kidney transplants in smaller children. Once kidney transplantation became more common in young children, technical factors that have enabled improved results were careful intraoperative fluid management, use of adult kidneys with arterial anastomoses to the common iliac artery or aorta, and sequential immunosuppression.14, 15
Infants account for a minor fraction of the number of transplants performed at kidney transplant programs given the low incidence of ESKD in children <2 years (7–8 per million). In our cohort, we found an increase in the minimum weight at which surgeons were willing to perform kidney transplant in the later era of transplantation. This is presumably in the setting of increased surgical complications such as renal vein thrombosis which have led to increased caution with transplanting very small infants. Thus, the incidence of renal vascular thrombosis has declined over time.16 These improvements are also due to improved surgical skill and the understanding that utilizing kidneys from young pediatric donors for small children actually leads to inferior outcomes as the small size in both donor and recipient vessels leads to more vascular complications.4
In European registries, this age group represents 2.8% of pediatric ESKD while US registries have found that they account for 5.8%.17, 18 The CERTAIN registry investigated the outcomes of children who received a kidney transplant at <10 kg.19 They followed 38 children (<10 kg) to 76 children (10–15 kg) for 2 years after transplant and showed that both groups had excellent patient and graft outcomes and that there were no significant differences between the 2 groups. Boehm et al. compared outcomes in European children between 2000 and 2016 and identified a significant lower 1-year graft survival of 90% in children <10 kg compared to 95% in children ≥10 kg.20 However, 5-year graft survival was comparable. Our study identified the similar findings of early graft loss in children weighing <8.6 and 8.6–9.9 kg in the immediate post-transplant period that over time became comparable to 10–29.9 kg weight groups. Similarly, patient survival appeared to rapidly decline particularly in the <8.6 kg weight group in the immediate post-transplant period. Several other single-center studies have examined outcomes in children <15 kg.21-24 Two studies comparing children <15 kg and those ≥15 kg showed that there was no difference in allograft outcomes based on weight at the time of transplant.22, 24 One study showed that recipients with a weight <15 kg at the time of transplant were associated with an increased occurrence of venous thrombosis.21 A more recent larger study used the UK Transplant Registry and found significantly improved allograft survival over a 10-year follow-up period in children <15 kg at the time of transplant when compared to children ≥15 kg (85.4% vs. 73.5%; p = .002).25 Our univariate analysis identified comparable outcomes in recipients weighing 8.6–9.9 kg at the time of transplant to higher weight groups, however, lower allograft and patient survival in recipients <8.6 kg at the time of transplant. The etiology again is likely related to the increased risk of surgical complications in these very small infants.
Our findings indicate that over time, there was a reduction in the rate of biopsy-proven rejection. However, although not statistically significant, children <8.6 kg had the highest frequency of biopsy-proven rejection when compared to other weight groups; the etiology of this difference is unclear and warrants further examination of potential differences in induction and maintenance immunosuppression. These findings differ from a previous study using the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) transplant registry that demonstrated lower risk of rejection in recipients 0–1 years old and the highest risk in recipients >12 years of age.6, 26 It is important to note, however, that using creatinine as a trigger for biopsy to identify acute allograft rejection is especially flawed in this population because an adult-sized kidney in a small pediatric recipient can undergo substantial damage before a change in creatinine occurs.1 When comparing small recipients by the era of transplantation, the improvement in biopsy-proven rejection and graft loss rates may be accounted for by improvements in immunosuppression.4 Tacrolimus and mycophenolate both became the first-line calcineurin inhibitors and anti-metabolite drugs in the second era of transplantation, replacing cyclosporine, and azathioprine,27 leading to decreased biopsy-proven rejection and ultimately improved overall long-term graft survival for kidney transplant recipients. The inferior graft survival seen in the ≥30 kg weight group (predominantly adolescents) is likely due to poor adherence.28 The lower graft survival rates seen in the 11–17-year age group are well described.17 The lower graft survival and death rates in the <8.6 kg weight group are presumably secondary to perioperative complications.
There are several limitations to this study. The data were obtained using a national database and there was limited and missing data which may lead to unintentional bias as we were not able to adjust for all factors which affect patient and graft survival. Due to missing rejection data, the variable was excluded from our models; however, all multivariate models yielded similar results. The data on causes of death and graft loss were also limited. Across the entire cohort, there was 69.3% missing graft loss data and 75% missing in recipients <10 kg; thus, we were unable to examine potential differences across weight groups and potential improvements in surgical complications between eras of transplantation. There was also no available data regarding differences in surgical approach including intraperitoneal and extraperitoneal techniques. In addition, while this paper focused on graft outcomes, we were not able to capture data regarding infectious complications, a significant cause of morbidity in small kidney transplant recipients. Infectious complications are of increased concern in younger transplant recipients who may be naïve to viral infections such as Epstein–Barr virus (EBV) and Cytomegalovirus (CMV) and who also may be unvaccinated or under vaccinated at the time of transplant. It is important to note that emerging data suggest safety of live vaccines in pediatric solid organ transplant recipients. While further studies are needed, specifically in kidney transplant recipients, special considerations could be taken for vaccination post-transplant in this population who may be under vaccinated pre-transplan.29-31 Furthermore, we were unable to adjust for outcomes based on transplant center; however, we speculate that the majority of transplants performed in small recipients (<10 kg) would have been performed in larger centers with low weight recipient experience. Lastly, the generalizability of our data is limited to primary alone kidney transplant recipients; thus, future studies are needed to assess how outcomes may differ among repeat and multi-organ transplant recipients.
In conclusion, children weighing between 8.6 and 9.9 kg at the time of transplant had allograft outcomes similar to higher weight groups and had improved clinical outcomes over time. Very small recipients weighing <8.6 kg at the time of transplantation, had increased rates of biopsy-proven rejection, allograft loss, and death when compared to children who were 8.6–9.9 kg. Weight at the time of transplantation and the era of transplant were not independent predictors of allograft loss; however, a weight of ≥10 kg at the time of transplant was associated with improved patient survival. Pediatric kidney transplant programs may proceed with transplantation in recipients <10 kg if the surgical expertise is present; however, special precautions should be taken for smaller recipients weighing <8.6 kg at the time of transplant.
ACKNOWLEDGMENTS
The author S.K. would like to thank Dr. Frederick Kaskel and Dr. Michal Melamed as the research described was supported by the TL1DK136048 New York Consortium for Interdisciplinary Training on Kidney, Urological and Hematological Research (NYC Train KUHR) as well as by the NIH/National Center for Advancing Translational Science (NCATS) Einstein-Montefiore CTSA Grant Number 1UM1TR004400.
CONFLICT OF INTEREST STATEMENT
Dr. Kilduff, Dr. Steinman, and Dr. Hayde have nothing to disclose.
ETHICS STATEMENT
The study was approved by the institutional review board for Montefiore Medical Center/Albert Einstein College of Medicine.
INFORMED CONSENT
The need for informed consent was waived due to the use of de-identified data.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in United Network for Organ Sharing at https://unos.org.