Venous vascular reconstruction of a robotically procured right kidney with two renal veins transplanted into a pediatric recipient
Abstract
Background
Right versus left kidney donor nephrectomy remains a controversial topic in renal transplantation given the increased incidence of right kidney vascular anomalies and associated venous thrombosis. We present the case of a 3-year-old pediatric recipient with urethral atresia and end-stage kidney disease who received a robotically procured living donor right pelvic kidney with two short same-size renal veins and a short ureter.
Methods
We utilized a completely deceased iliac vein system (common iliac vein with both external and internal veins) to extend the two renal veins. Due to the distance between both renal veins, the external iliac vein was anastomosed to the upper hilum renal vein, and the internal iliac vein was anastomosed to the lower hilum renal vein. The donor's short ureter was anastomosed to the recipient's ureter end-to-side.
Results
The patient had immediate graft function and there were no post-operative complications. Renal ultrasound was unremarkable at 48 hours post-transplant. Serum creatinine was 0.5 mg/dL at 3 months post-transplant.
Conclusion
We demonstrate the successful transplantation of a robotically procured right pelvic donor kidney with two short renal veins using a deceased donor iliac vein system for venous reconstruction without increasing technical complications. This technique of venous reconstruction can be used in right kidneys with similar anatomical variations without affecting graft function.
Abbreviations
-
- ESKD
-
- End-Stage Kidney Disease
-
- UA
-
- Urethral Athresia
-
- RALDN
-
- Robotic-Assisted Living Donor Nephrectomy
-
- VCUG
-
- Voiding Cystourethrogram
-
- IVC
-
- Inferior Cava Vein
-
- EKG
-
- Electrocardiogram
-
- PDS
-
- Polydioxanone
1 INTRODUCTION
The first ever robotic-assisted living donor nephrectomy (RALDN) was reported in 2001.1 Since then, it has been demonstrated to be a feasible and safe tool for performing living donor nephrectomy and living donor kidney transplantation.1 Left donor kidneys are preferred due to the usually longer length of its renal vein and therefore fewer vascular complications.2 Nevertheless, right-living donor nephrectomies are another option for transplantation and represent 20%–30% of the cases reported in the literature.2 The main concern in utilizing right donor kidneys for transplantation is the likely short length of its renal vein, the existence of multiple vessels, and ultimately an association with a greater risk of venous thrombosis occurring in the transplanted recipient.2-4
We present our experience of a 3-year-old male with end-stage kidney disease (ESKD) due to urethral atresia (UA) who received a robotically procured living donor right pelvic kidney with two short same-size renal veins and a short ureter. Given the vascular anomaly, complex vascular reconstruction with deceased donor iliac vein extension grafts was key in the utilization of this living donor kidney. The uniqueness of this case comes from the combination of performing a right pelvic robotic nephrectomy with a complex renal vein vascular reconstruction. Moreover, this transplant was performed in a small pediatric patient. This complex venous reconstruction resulted in immediate graft function with no observed vascular complications.
2 CASE PRESENTATION
This is a 3-year-old male with a history of ESKD due to UA diagnosed at birth. A fetal vesico-amniotic shunt was placed as early treatment for the lower urinary tract obstruction. He underwent several urethral dilatations since birth and remained on peritoneal dialysis until the day of transplantation. Prior to transplantation, voiding cystourethrogram (VCUG) showed grade 4 to 5 right-sided reflux. The right ureter was found to be dilated and tortuous. There was no opacification of the pelvicalyceal system. There was no dilatation of the posterior urethra. This pediatric patient had small, cystic kidneys bilaterally and a small 40-50 cc bladder with a tubular aspect and a large diverticulum. The patient was evaluated by a multidisciplinary team which included urologists, transplant nephrologists, and transplant surgeons. Despite his small bladder capacity and dysfunctionality, the consensus was to perform bladder augmentation only if the patient's bladder did not recover after the transplant. For that reason, it was decided to keep his right dysfunctional kidney intact to preserve the ureter for possible bladder augmentation in the future.
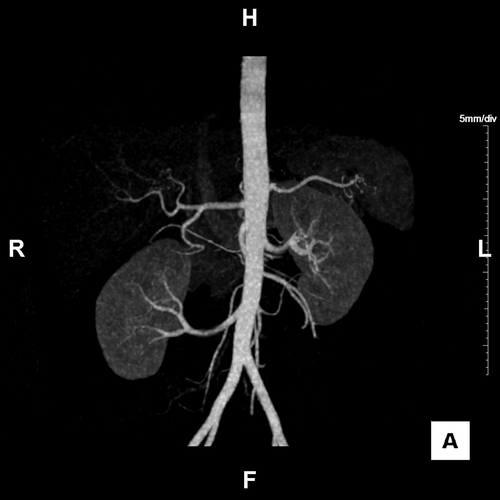
The patient's weight and height were 14.1 kg and 87.5 cm at the time of surgery. He underwent extensive laboratory work-up including complete blood count, comprehensive metabolic panel, serologies, echocardiogram, electrocardiogram (EKG), chest X-ray, and abdominal ultrasound. He showed no human leukocyte antibodies of either class I or II. With informed consent from his parents, the patient underwent an unrelated living donor kidney transplant from a 28-year-old female. Computed tomography angiography of the living donor showed that the volumes of the right and left kidneys were 148 and 182 mL, respectively. The donor's right kidney was shown to be located low in the abdomen almost down to the pelvis (Figure 1). Nuclear medicine studies showed the right kidney with 39.1% function and the left kidney with 60.9% function. Although the living kidney donor had extensive pre-transplant workup by the transplant nephrology team, the exact reason for the discrepancy is unknown. As a possible cause of the discrepancy, function of the pelvic kidney is often less than that of an orthotopic kidney.5
Our induction immunosuppression protocol includes antithymocyte globulin 1 mg/kg (1 dose), basiliximab 10 mg (2 doses), and methylprednisolone starting at 10 mg and tapered to 0.3 mg/kg/day of prednisone.6, 7 Oral mycophenolate mofetil was introduced on the second post-operative day at a dose of 600 mg/m2 twice daily. Dose adjustment was performed according to white blood cell count and gastrointestinal tolerance. Patient has remained on steroids in order to avoid rejection. Tacrolimus trough levels were targeted to 6–8 ng/mL.
The patient had immediate graft function and normal post-operative renal ultrasound without any immediate post-operative complications. Renal ultrasound was also normal 48 hours after the transplant. No anticoagulation was administered to the patient intra- or post-operatively. Serum creatinine of 0.5 mg/dL at 3 months after transplant with no vascular or urological complications. As of the last follow-up (3 months post-transplantation), the patient is voiding with no issues, and there has been no decision for bladder reconstruction at this time. He is closely followed by pediatric transplant, nephrology, and urology teams.
3 PROCEDURE
A modified Gibson incision was made on the right lower quadrant and retroperitoneal space was approached. Peritoneum was displaced medially, and blunt dissection was performed posteriorly to the right renal fossa in order to expose the inferior vena cava (IVC) and right iliac vessels. The surgeon's hand was utilized to separate the posterior abdominal wall and Gerota's fascia. Hepatic medial and upward mobilization was also performed in order to obtain a better exposure of the renal pedicle and the IVC. The right common iliac artery and the IVC were identified and dissected free. This also created more space for the graft.8
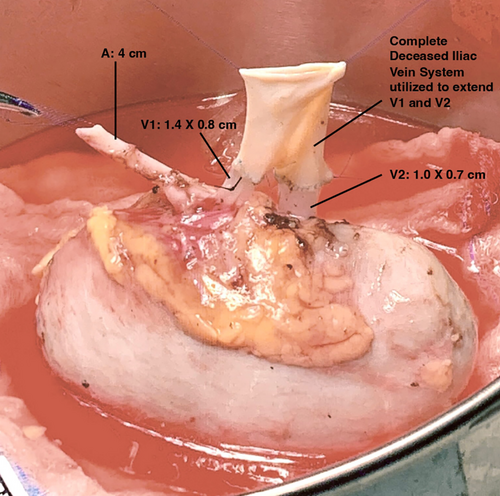
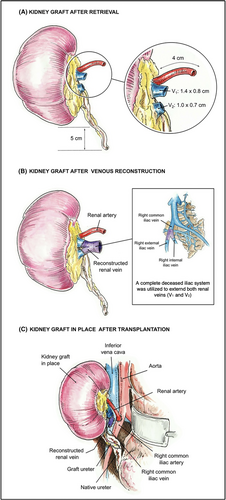
The robotic-assisted procured right kidney of the donor was prepared on the back table. Perinephric fat was trimmed, and the renal artery and two renal veins were identified and dissected. The short ureter was identified and minimally dissected. The single renal artery was 4 cm in length. The upper hilum vein (V1) had a width of 0.8 cm and a length of 1.4 cm. The lower hilum vein (V2) had a width of 0.7 cm and a length of 1 cm. The ureter was 5 cm long from the lower pole.
We utilized a complete deceased iliac vein system graft (common iliac vein with both external and internal veins) to extend the two renal veins. Both reconstructions were performed with 6–0 Prolene in a running fashion (Figure 2). Due to the distance between the two renal veins, the external iliac vein was anastomosed to the upper hilum renal vein (V1) (longer and wider compared with lower hilum vein), and the internal iliac vein was anastomosed to the lower hilum renal vein (V2) (Figure 3).
The vessels are derived from other multiorgan procedures and transplantations (liver, pancreas, intestine) and stored in our hospital for 2 weeks. The surgery discussed in our case report was scheduled once we were notified that the iliac vessels were available. The deceased donor iliac vein system was chosen based on blood group match, which was group O. The cross match between recipient and deceased donor was negative.
The allograft was then brought to the field. The first anastomosis was between the deceased donor's common iliac vein graft (with the two right renal veins already reconstructed) and the recipient IVC using running 6–0 polypropylene sutures. The allograft renal artery was anastomosed to the recipient's right common iliac artery with 7–0 Prolene in a running fashion. Reperfusion was obtained maintaining the patient's mean arterial pressure > 90 mmHg at the time of blood flow restoration. The renal allograft was then positioned in the retroperitoneum posterior to the right native kidney and the liver. Due to the donor kidney having a natural pelvic position, the ureter was short and did not reach the recipient's bladder. A decision was then made to anastomose the donor's ureter to the recipient's ureter (Figure 3). The allograft ureter was spatulated and anastomosed to the recipient's ureter with 6–0 PDS in a running fashion. No ureteral stents or Jackson–Pratt drains were used.
4 DISCUSSION
Kidney transplantation is the best treatment of choice for pediatric patients with ESKD.9 Novel surgical techniques, immunotherapy regimens, and antimicrobial agents have enhanced pediatric transplant outcomes.10-12 Living donor kidney transplantation has helped shorten waiting times on the deceased donor kidney transplant waitlist and has also been shown to provide better graft survival outcomes when compared to deceased donor kidney transplantation.13 Many potential living donor kidneys are rejected due to anatomical or surgical reasons.13 Currently, there is no uniform criteria on how different transplant centers accept/utilize organs with vascular abnormalities. The use of complex vascular reconstruction techniques in anatomically abnormal kidneys can be used to expand the donor pool for pediatric recipients.
Right donor kidneys are procured in living donors when left kidney function predominates or if the left kidney has multiple vessels, a duplicated collecting system, anatomical variances or calcified renal arteries.14, 15 The possibility of multiple renal vessels and shorter renal veins are greater when transplanting right donor kidneys compared to left donor kidneys.16 These anomalies present greater technical difficulties that can increase warm ischemia time, posing a potential risk for early graft dysfunction.14 Therefore, left kidneys are preferred as right extracted kidneys present a greater risk of IVC lesions occurring in the donor and renal vein thrombosis occurring in the recipient.16
Although multiple vessel kidneys have historically been a limitation for renal transplantation, recent studies show that augmented anastomosis surgery time or ischemia time in multiple renal vessel kidney procedures has no considerable effect on graft function and graft survival in the long term.10 Supernumerary renal veins are relatively common anatomical abnormalities defined as the existence of more than one renal vein that appear due to variations in normal embryological development.17, 18 Studies indicate that 1% of left kidneys and 32.5% of right kidneys present supernumerary renal veins (dual right veins on 30.5% and triple right veins on 2.5%).17 Due to this high prevalence, transplant surgeons must be technically prepared to deal with these anatomically challenging abnormalities. Moreover, compared to renal arteries, renal vein walls are considerably thinner and more challenging to anastomose and reconstruct.17
Ghidini F et al19 demonstrated that complex bench surgery in pediatric renal transplantation does not increase vascular complications or alter graft functionality or overall graft survival. In order to prevent prolongation of warm ischemia time in the donor kidney, substantial vessel grafts such as iliac vessels are utilized, facilitating the vascular reconstruction procedure.18, 20 Renal vein extension using allogenic gonadal veins or cadaveric iliac veins is an important option as it can reduce vascular dissection, therefore preventing transplant-related bleeding or lymphatic complications.14 Riella et al12 demonstrate no increased risk of complications in transplantation of multiple vessel kidneys which required back table vascular reconstruction to achieve a single orifice graft. On the other hand, reconstructions of multiple vessel kidneys have been shown to be associated with higher incidence of vascular complications and tubular necrosis caused by an increase in warm ischemia time.20
O'Kelly et al21 present a pediatric study that compares multiple vessel and single vessel kidney transplants. They show no differences in the incidence of perioperative complications or graft function in patients receiving multiple vessel kidneys. They noted an increased risk of lymphocele due to more aggressive hilar allograft and recipient vessel dissection.21 Modi et al22 demonstrate that right donor nephrectomy with multiple renal veins is a safe and viable procedure.
5 CONCLUSION
In efforts to mitigate the widening gap between donor supply and demand in kidney transplantation, transplant surgeons are utilizing grafts with vascular-anatomical abnormalities by implementing innovative reconstruction techniques prior to transplantation. We demonstrate the successful pediatric transplantation of a robotically procured right donor kidney with two short renal veins using a deceased donor iliac vein system in order to perform vascular reconstruction, showing no increased risk of the occurrence of venous thrombosis or graft dysfunction. This technique can be implemented by other surgeons when faced with vascular anomalies of right donor kidneys.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.