Let’s talk about sex: A biological variable in immune response against melanoma
Panshak P. Dakup and Adam J. Greer equally contributed to this work.
Funding information
This work was supported by the National Institutes of Health grant R01ES030113 and in part by the CDMRP Peer Reviewed Cancer Research Program Award W81XWH-18-1-0061 (S.G.).
Abstract
As science culture gravitates toward a more holistic inclusion of both males and females in research design, the outlining of sex differences and their respective intersections with disease physiology and pathophysiology should see reciprocal expansion. Melanoma skin cancer, for example, has observed a female advantage in incidence, mortality, and overall survival since the early 1970s. The exact biological mechanism of this trend, however, is unclear and further complicated by a layering of clinical variables such as skin phototype, age, and body mass index. In this perspective, we highlight epidemiological evidence of sex differences in melanoma and summarize the landscape of their potential origin. Among several biological hallmarks, we make a note of sex-specific immune profiles—along with divergent hormonal regulation, social practices, DNA damage and oxidative stress responses, body composition, genetic variants, and X-chromosome expression—as probable drivers of disparity in melanoma initiation and progression. This review further focuses the conversation of sex as an influencing factor in melanoma development and its potential implication for disease management and treatment strategies.
Melanoma is the deadliest form of skin cancer, accounting for over 80% of skin cancer-related deaths but contributing to only 1% of cases (American Cancer Society, 2021). Exposure to ultraviolet radiation (UVR) remains a major risk factor and is implicated in about 86% of melanoma cases (Parkin et al., 2011). While UVR is mostly associated with initiation, biological sex is another less understood risk factor in melanomagenesis where males are at greater risk than females. The extent to which biological sex impacts melanoma disease initiation, progression, and patient survival/mortality is not clear and warrants further empirical research. Current limiting factors include a historical dearth of study frameworks evaluating both sexes, a lack of reliable models for investigating melanoma, and a prevalence of confounding clinical variables between biological sexes. A recent push by the National Institute of Health (in January 2016) to factor sex as a biological variable (SABV) into experimental designs is an appropriate first step toward alleviating these unknowns and will be discussed in greater detail toward the end of this review.
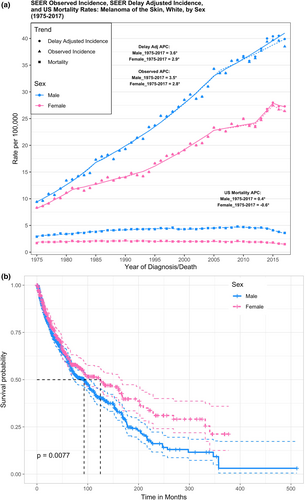
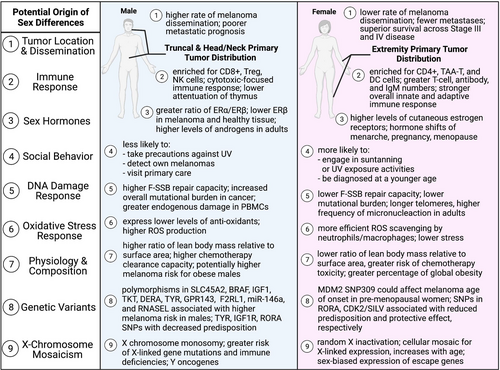
Over the past four decades, epidemiological studies and cancer registries have reported distinct sex differences in melanoma incidence, progression, and survival. According to the Surveillance, Epidemiology, and End Results (SEER) program database from the National Cancer Institute, both males and females of Caucasian ethnicity showed a striking positive trend in melanoma incidence since 1975 (Siegel et al., 2021). Male individuals experienced more intense rates of recorded incidence and mortality compared with females over the same 40-year timeframe (Figure 1A). Upon stratification for age, the trends in mortality did not change; however, females tended to have more frequent diagnosis of melanoma than males for ages less than 50. This might be due to increased engagement in tanning activities in women compared with men (Lazovich et al., 2016). Data from 2017 showed males had an incidence rate of 38.5 and mortality rate of 3.6 per 100,000 individuals, while females recorded lower rates of 26.4 and 1.5, respectively (Siegel et al., 2021). Importantly, these trends persist into the present day, and males are projected to account for almost two-thirds of melanoma incidences and mortality in 2021 (American Cancer Society, 2021; Siegel et al., 2021). Cutaneous melanoma data extracted from cBioportal also showed significantly reduced survival probability (p = 0.0077) in males compared with females over a 500-month period (Figure 1b) (Cerami et al., 2012; Gao et al., 2013). The median survival was 92.8 months in males versus 124.9 in females. Stratifying for Age at Diagnosis, the trend of superior female survival maintained significance only for ages less than 50. Although few studies included in this analysis recorded tumor stage, it too is an important clinical variable to account for. SEER 5-year Relative Survival rates, for example, note that females tend to have greater survival in the context of regional or distant initial tumor diagnoses. These observations are consistent with Joosse et al. who report a continuous advantage for both pre- and post-menopausal women in overall survival, disease-specific survival (DSS), and progression-free survival across both stage III and stage IV melanomas (Joosse et al., 2013). Additionally, a study of melanoma cases from the Munich Cancer Registry (Germany) reported that localized melanomas in females had a lower propensity to metastasize and led to better survival (Joosse et al., 2011). Thus, females fare better both before and after distant metastases—although significance dissipates rapidly with increasing metastatic tumor load (Figure 2, Point 1). Explaining why these trends exist presents a daunting task. This overwhelming epidemiological evidence of sex differences is balanced by a strikingly inconclusive landscape of potential variables driving the disparity. Regarding various stages of melanoma progression, the mechanisms underlying the sex disparity are yet to be clearly defined.
In our recent study (Dakup et al., 2020), we sought to understand sex differences in tumor growth using a syngeneic B16-F10/BL6 melanoma mouse model. The B16 murine melanoma cell line is derived from a spontaneous tumor identified in 1954 on the ear of a male C57BL/6 mouse (https://web.expasy.org/cellosaurus/CVCL_F936). We implanted B16-F10 tumor cells subcutaneously in male and female mice and observed tumor growth rate over fourteen days. By the end of the study, females had significantly less tumor volume (~threefold) and growth rate compared with male counterparts, providing phenotypic results consistent with human findings (Liu et al., 2006). We used blood and tumor tissues to further investigate the link of our tumor phenotype to adaptive immune response in our models. We observed significantly higher CD4+ helper and CD8+ cytotoxic T lymphocyte counts in female mice compared with male mice. Furthermore, there was a higher tumor-infiltrating CD8 lymphocyte count per mm3 volume in female mice, which correlated inversely with tumor volumes. As a potential drawback of our experimental design, various factors that put humans at higher risk of melanoma—such as UVR exposure, sunbathing/tanning, weakened immune systems, and living at higher elevation or equatorial regions—were not considered in our protocol. It is possible these variables can modify the degree of tumor growth by sex and require further investigation. Additionally, given that male tumors tend to exhibit greater antigenicity, there exists concern of B16 implantation eliciting a more severe immunogenic response in female mice. Neoantigen-specific CD4+ and CD8+ T-cell reactivity is a well-accepted property of melanoma (Linnemann et al., 2015). Male tumor cells may, furthermore, present Y-chromosome neoantigens (Y-chromosomal histocompatibility; H-Y) capable of triggering a female-specific cytotoxic response (Goldberg et al., 1973). Early in vivo work with B16 implantation, however, failed to identify any significant differences in tumor growth patterns between male and female mice (Simon & Ershler, 1985). It is thus plausible that higher female T lymphocyte counts observed in our experiment might be derived from properties of the B16-F10 subtype. The B16 lineage has numerous sublines—each with varying degrees of tumor aggressiveness and immune protection—which may restrict generalizations about how host physiology responds to the cell line. Such factors should be considered when reviewing the above results and when selecting a melanoma mouse model for experimental design. Nevertheless, our study purely investigated the events of tumor growth, independent from initiation, and highlights a mechanistic link between sex-specific immunity in melanoma growth. We also provide an immunocompetent model that can be used to characterize sex differences in immune response with respect to melanoma tumor and metastasis in future investigations.
Immunological response against melanoma generally occurs in two phases: early innate immune activation driven by macrophages, granulocytes, natural killer (NK) cells, and dendritic cells (DCs), followed by late adaptive immune response of effector CD4+ and CD8+ T cells, which have been primed against melanoma antigens by interferon-gamma (IFN-γ) (Passarelli et al., 2017). Naïve CD4+ T cells exhibit plasticity and can differentiate into Th1, Th2, Th17, Treg, and TFH cells that target specific classes of pathogens and differ functionally (Zhu et al., 2010). Among these components of the immune system, females have a more robust presence of immune response players against tumor cell growth (Figure 2, Point 2) (Klein & Flanagan, 2016). In human blood, female immune systems are enriched for tumor-associated antigen-specific T cells (TAA-T), B cells, and DCs (antigen-presenting), whereas male immune systems are enriched for NK and regulatory T cells (Abdullah et al., 2012; Klein & Flanagan, 2016). Lymphocyte culture supernatants from human serum further show sex disparities in cytokine levels consistent with the above immune players. Males, for example, present a TH1 cytokine profile—higher levels of IFN-γ and IL-2, lower levels of IL-4 and IL-10—while women present a TH2 profile (Giron-Gonzalez et al., 2000). The characteristics of TH1 are consistent with cytotoxic immune responses through CD8+ T or NK cells. TH2, in contrast, emphasizes the secretion of antibodies and could explain the comparatively higher levels of immunoglobulin and increased prevalence of auto-immune disorders observed in females (Schuurs & Verheul, 1990). Additionally, the sex hormone estrogen can notably influence immune profiles (Figure 2, Point 3). Estrogen, for example, transcriptionally enhances IFN-γ secretion thereby promoting and expanding T-cell populations and responses (Klein & Flanagan, 2016; Kovats, 2015). This topic will be reviewed more holistically in later sections. The observed changes to the immune pool, in part due to hormonal factors, are also largely driven by age. Naïve T-cell populations in human plasma decline at a faster rate in males, while B cells remain constant in females but decrease in males over time (Marquez et al., 2020; Takahashi & Iwasaki, 2021). The differing immune pools and shifting immune landscapes between males and females might predispose males to poorer adaptive immune responses. A more recent case in point is the COVID-19 pandemic with recorded male bias in disease severity and mortality (about two times higher in males) in nearly all countries (Scully et al., 2020). In line with these observations, single-cell level transcriptomic analyses of human peripheral blood mononuclear cells (PBMCs) further substantiate sex differences in immunity, with males having elevated pro-inflammatory (X-linked TLR7 and BTK) responses to COVID-19 (Hou et al., 2021). Immune response is undoubtedly a key factor driving the sex disparity in melanoma risk.
A recent report investigated sex differences in melanoma development with a frequently utilized BrafCA; Tyr-CreERT2; Ptenf/f transgenic mouse model. Opposite to our B16-F10/BL6 observations, it found faster initiation and progression of melanoma in female mice compared with male mice (Zhai et al., 2020). The precise reason for these inverted observations is not immediately clear. The mechanism inducing tumor development primarily differentiates this experiment from our own and is a likely source of difference. Lang et al. applied topical 4-hydroxy-tamoxifen (4-OHT) for three consecutive days to induce the Tyr-CreERT2 transgene that targets Braf/Pten alleles, whereas we utilized a syngeneic model with C57BL/6 wild-type mice and subcutaneously injected tumor cells. This transgene insertion specifically induces Cre expression in cutaneous melanocytes. Our dissemination model, on the contrary, operates orthotopically. Lang et al. cite initial observations of pigmented lesions to a range of 13–21 days in females and 17–28 days in males. There were also age differences between mice in the two experiments, with a slightly younger cohort used by Lang and colleagues (6–7 weeks versus 8–10 weeks). The concept of sex hormones and sexual maturity introduces an intriguing variable (Figure 2, Point 3). For one, neither study accounted for estrous cycle stage in female mice, which could lead to differing endogenous levels of hormones such as follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol, and progesterone. Chakraborty et al. identified the Estrogen/ERɑ axis as responsible for driving macrophages toward an immune-suppressive state in the melanoma tumor microenvironment (Chakraborty et al., 2021). Therefore, if female mice were in a proliferative phase upon tumor induction, the higher levels of endogenous estrogens could potentially favor tumorigenesis (Freeman, 2006). This theory, however, does not necessarily translate for human females, as pregnancy, contraceptives, and hormone replacement do not appear to influence melanoma risk or prognosis (MacKie et al., 2009). Another possibility involves the action of tamoxifen as an inducer. 4-OHT is a selective estrogen receptor modulator capable of both agonistic and antagonistic interactions with the receptor. Tamoxifen, in the context of modern breast cancer treatment, competitively binds to estrogen receptors (ER) to block the action of estrogen. It has conversely been shown to act as an estrogen-like agonist in the bone of female rats (Turner et al., 1987) and uterus of female mice (Terenius, 1971)—although these studies did not administer topically. Dorsal implantation of estrogen has further been found to promote expansion of TReg populations in vivo for female C57BL/6 mice (Polanczyk et al., 2004). Tamoxifen, more precisely, operates as a partial estrogen agonist (AF1)/antagonist (AF2) through ERɑ and full antagonist via ERβ (Berry et al., 1990; Paech et al., 1997). ERɑ plays a role in estrogen-mediated proliferation, whereas ERβ has anti-proliferative and pro-apoptotic effects. Thus, a profile featuring upregulation of ERɑ and downregulation of ERβ favors malignant transformation. Human males tend to have lower levels of ERβ than females, regardless of healthy tissue or melanoma (de Giorgi et al., 2013), although this would theoretically point toward a more proliferative phenotype in males. Furthermore, another study on thyroid cancer identified hierarchical expression in estrogen receptors favoring ERɑ > GPER1 > ERβ in males and GPER1 > ERβ > ERɑ in females (Zane et al., 2017). Treatment with estrogen, however, saw a downregulation of both receptors in males but an overexpression of ERɑ in females. Schmidt et al. identified not only strong immunoreactivity of ERβ (weak ERɑ) in melanocytic lesions of the epidermis (KAMs), papillary dermis, and periphery of dermal aggregates (SAMs) but also a sharp decrease in immunoreactivity for nodules deeper in the dermis (MAMs) (Schmidt et al., 2006). Taken together with the results from Chakraborty et al, this might suggest disparate mechanistic roles for ERɑ and ERβ in melanoma initiation and progression (Chakraborty et al., 2021). Exogenous treatment of estrogen may facilitate faster tumor initiation in females through elevated expression of ERɑ, whereas lower levels of ERβ inherent to males cultivate more aggressive lesions in later stages of disease. Overall, the report by the Lang group (Zhai et al., 2020) indicates that a deeper understanding of mutant oncogenes, hormonal activity, and genetic variants in sex-specific signaling mechanisms/pathways might be necessary for molecular explanations that drive sex differences in melanomagenesis.
There are other possible factors driving sex differences in melanoma, as summarized in Figure 2. One common theory that seeks to explain the male disadvantage in susceptibility to diseases and health outcomes is the construction of masculinity (Courtenay, 2000). In this theory, the structuring and behaviors of gender by social practices undermine the health of men and can inform decisions to seek medical attention when needed (Figure 2, Point 4). Men, for example, are less likely than females to engage in sun-protective options such as sunscreen or shade, visit physicians on a yearly basis, or self-detect melanomas (Brady et al., 2000; Burnside et al., 2018; Holman et al., 2018). While this theory may contribute partly to sex differences in melanoma incidences, it does not necessarily account for differences in progression and survival after disease initiation (Figure 1B). Our progression-based mouse protocol (Dakup et al., 2020) controlled for incidence (no UVR exposure) in a laboratory environment, as well as for social practices and behaviors in melanoma growth. UVR, the major instigator of melanoma, induces DNA damage and further causes genomic instability in cutaneous melanocytes to promote malignant transformation. Subsequent activation of DNA damage response programs could therefore also vary by sex and gender (Figure 2, Point 5) (Dakup & Gaddameedhi, 2017). Trzeciak et al. observed not only a lower fast single-strand break repair capacity (F-SSB-RC) in white females but also an increase in the rate with age (Trzeciak et al., 2008). This study, which sampled a healthy, age-matched human population, examined γ-radiation-induced DNA damage via alkaline comet assay in human PBMCs. These findings, however, appear to be in the minority. Adopting a similar focus, Garm et al. collected a random cross-sectional sampling of a Danish population and assessed both γ-irradiation-induced and endogenous DNA damage in PBMCs through a more reproducible fluorometric analysis of DNA-unwinding (FADU) assay (Garm et al., 2013). They found no significant associations between age-adjusted sex for either endogenous SSB, SSB repair, DSB repair, or γ-H2AX response. Slyskova et al. also failed to identify a sex-specific effect in nucleotide excision repair (NER) activity in PBMCs of healthy individuals (Slyskova et al., 2011). The same can be said for investigations of repair capacities in T lymphocytes from basal-cell carcinoma (BCC) or squamous-cell carcinoma (SCC) patients, although a combination of low repair and history of severe sunburn dramatically increases the risk of BCC in females (Hall et al., 1994; Wei et al., 1993). With regards to actual amounts of DNA damage in PBMCs, males have been found to have higher levels of endogenous SSBs and alkali labile sites than females (Hofer et al., 2006; Slyskova et al., 2011). This endorses observations of male bias toward larger mutational burdens for metastatic melanoma, greater oxidative stress, and poorer overall survival in melanoma (Figure 2, Points 5 & 6) (Gupta et al., 2015; Ide et al., 2002; Joosse et al., 2010). On the contrary, these levels of genetic damage seem to conflict with observations of a 19% higher micronucleated binucleate cell (MNC) frequency in females (Bonassi et al., 2001; Wojda et al., 2007). Micronucleus expression in PBMCs functions as a quantification of chromosome damage—increased micronuclei correspond with more chromosomal aberrations and damaged cells. Thus, although the literature remains largely inconclusive as to who fares better, it is still highly probable that biological sex plays a significant role in melanoma onset through a combination of genetic factors such as DNA damage response, mutational burden, and genomic instability (Gupta et al., 2015).
Differences in the physiological composition of males and females may also drive a wedge in disease progression and response to treatment (Figure 2, Point 7). Men, for example, have a higher ratio of lean body mass (LBM) to body surface area (BSA), which facilitates more rapid clearance of chemotherapeutics and less drug toxicity (Dobbs et al., 1995; Joerger et al., 2006; Prado et al., 2007). Obesity, which can be evaluated by body mass index (BMI), is further classified as a risk factor for mortality and morbidity in chronic disease. On a global scale, age-standardized prevalence of obesity in 2008 was 9.8% in males and 13.8% in females—BMI trends, however, are strongly stratified by country, age, gender norms, and socioeconomic status (Finucane et al., 2011; Garawi et al., 2014). There also exists an “obesity paradox” in which high BMI individuals (mainly men, BMI ≥ 30) tend to have lower mortality risk than healthy-weight individuals in the context of severe chronic illness (Greenberg, 2013). It is worth noting that BMI indicates weight with respect to height and does not discern between LBM and fat mass. For example, the paradox phenomenon might be explained by partitioning low BMI individuals into normal (healthy) and low (unhealthy) LBM. LBM (e.g., skeletal muscle) and fat mass (e.g., adipose tissue) furthermore are believed to have inverted effects on human health. In one longitudinal study evaluating 38,006 men, both LBM and fat mass were predicted to increase with higher BMI (Lee et al., 2018). Fat mass, however, showed a positive linear trend for mortality from all causes, including cardiovascular disease and cancer, whereas LBM had a U-shaped association. Applying these features to malignant melanoma, a recent meta-analysis summarized the landscape of cohort and case control studies reviewing obesity and melanoma risk (Sergentanis et al., 2013). The pooled mean effect size estimate for a combination of overweight and obese males (BMI ≥ 25) was 1.31 and 0.97 for females. Furthermore, a pooling of individuals with a BSA ≥ 2 m2 had a pooled estimate of 1.84 for males and 1.37 for females. There also appears to be a mutual confounding between obesity (either in terms of BMI or BSA) and sunlight exposure that alters directionality of the association between obesity and melanoma risk in female case control versus cohort studies (positive versus negative, respectively). Overall, the meta-analysis highlights an increased risk of melanoma for overweight and obese males, as well as for females after correction for sunlight exposure. Obesity is generally classified as a state of low-grade chronic inflammation and connected to dysregulation of cell-mediated immune responses (Marti et al., 2001). As such, excess adipose tissue could be partially responsible for the sex-specific trends.
Genetic variants add additional complications to the landscape of sex differences in melanoma (Figure 2, Point 8). The functional single-nucleotide polymorphism (SNP) at position 309 in the P2 promoter of MDM2 potentially affects the age of initiation of melanoma in females. Comprising one of the more well-studied variants, the MDM2 oncogene is in a tight autoregulatory feedback loop with the tumor suppressor p53, and its overexpression elicits a variety of favorable and unfavorable phenotypes depending on the tumor of study. Despite its role in proteolytic degradation of p53, MDM2, when overexpressed, demonstrates a surprising extension of both overall and disease-free survival in primary melanoma patients (Polsky et al., 2002). As a general overview for selected cancers, MDM2 overexpression was associated with poorer survival in gliomas and acute lymphocytic leukemia but improved survival in melanoma, non-small-cell-lung cancer, and ERɑ+ breast cancer (Onel & Cordon-Cardo, 2004). There also exists a related p53 Arg72Pro polymorphism, although associations between its genotypes and melanoma risk—either alone or in concert with MDM2 SNP309—remain controversial. In terms of sex biases, the literature is once again inconclusive with regards to favorable SNP309 genotypes (GG, TG, or TT). In one study, for example, GG women less than 50 years old were nearly four times (OR: 3.89) more likely to be diagnosed with melanoma (Firoz et al., 2009). Conversely, another study found that GG women in premenopausal age brackets (<50–60 years of age) were half (OR: 0.52) as likely to be diagnosed at a younger age, compared with TG or TT individuals (Cotignola et al., 2012). The complexity of this MDM2-p53 axis and prevalence of contradictory clinicopathologic evidence ultimately warrant more extensive future investigation. The residual landscape of polymorphisms in melanoma is ultimately weighted against the male sex. Other genetic variants identified to have stronger association with melanoma risk in males include the SLC45A2 SNP rs16891982 (Kocarnik et al., 2014), BRAF SNPs rs1639679, rs1267601, rs1267609, rs1267636, rs1267649, and rs1639675 (Meyer et al., 2003), recessive IGF1 SNP rs1520220 (Yuan et al., 2020), genotype combination of TKT SNP rs9864057-GA + AA and DERA SNP rs12297652-AG + GG (Gu et al., 2020), allelic combination of miR-146a SNP rs2910164-C and RNASEL SNP rs486907-A (Sangalli et al., 2017), TPCN2/MYEOV SNP rs12418451 (Kocarnik et al., 2015), F2RL1 SNP rs2242991, GPR143 SNPs rs2521667 and rs2732872, and TYR SNP rs5021654 (Hernando et al., 2016). Polymorphisms associated with a protective effect or reduced predisposition toward melanoma include the TYR SNP rs1042602 (Hernando et al., 2016), IGF1R SNP rs2229765 (Yuan et al., 2020), and RORA SNP rs10519097-T allele (Benna et al., 2021) in males and CDK2/SILV SNP rs206939 (Hernando et al., 2016), and RORA SNP rs339972-C allele (Benna et al., 2021) in females. The sex-disproportionate excess of risk variants is consistent with previously outlined epidemiological data of inferior survival in males with melanoma.
Sex chromosomes are widely implicated in sex-specific biases toward disease susceptibility, immunity, and progression (Figure 2, Point 9). Whereas males (XY) possess a maternal X-chromosome and paternal Y chromosome, females (XX) carry an X chromosome from each parent. X chromosome inactivation (XCI) or lyonization occurs as a form of dosage compensation to silence gene expression on one of the X chromosomes and strive for equal expression between males and females. The random nature of inactivation cultivates cellular mosaicism in females, with approximately half of the cells expressing paternally inherited X genes and half maternally inherited X genes. Thus, deleterious mutations that target X-linked genes will result in systemic loss of function of the protein in males but only a 50% reduction in heterozygous females. Haupt et al. even suggest females have a capacity to restrict expression of X-linked somatic gene mutations to allow for selective protection of key protein interaction networks (Haupt et al., 2019). This falls in line with observations of tissue or cell-type-specific skewing of XCI, which occurs both at random and in response to X-linked gene mutations that negatively target proliferation or immune cell functional integrity (Migeon, 1998; Orstavik, 2009). Males lacking these buffering pathways are therefore almost exclusively susceptible to severe clinical manifestations of X-linked primary immunodeficiencies (Libert et al., 2010). On the inactive chromosome, it is further believed that around 15% of X genes escape permanent inactivation in humans (3.3% for mice), which could lead to a dosage effect driving higher (or more variable) expression of X-linked genes in females (Carrel & Willard, 2005; Yang et al., 2010). Consider a scenario in which several of these un-silenced X genes are immune related—females might then possess elevated functional levels of immune proteins that amount to a superior disease response. These “escape genes” have also been confirmed to have sex-biased expression dependent upon their location on the X chromosome. Genes within distal pseudoautosomal region PAR1, for example, are more highly expressed in males, whereas non-PAR regions show female-biased expression (Tukiainen et al., 2017). The protein phosphatase 2A regulatory subunit PR70 was recently identified as a gonosomal tumor suppressor and could demonstrate implications of the above findings in melanoma. PR70 is located on PAR1 in females (with a functional copy on the Y chromosome in males) and has been shown to reduce melanoma growth in vitro and in vivo through interference with replication origin firing. Increased protein expression was associated with improved survival, and males were shown to have lower expression than females (van Kempen et al., 2016). To further explain the varied nature of expression in XX and XY, Golden et al. introduced another possible epigenetic mechanism in T lymphocytes—accumulation of DNA methylation at CpG islands (ideally on the paternally donated X chromosome) to silence specific genes in males (Golden et al., 2019). TSPY, for example, is a Y-linked proto-oncogene characterized by hypermethylation of a CpG island and subsequent downregulation during progression of metastatic melanoma in male patients (Gallagher et al., 2005). Golden and colleagues postulate that sex differences in immune functionality are ultimately driven by a tug-of-war between expression of parentally imprinted X-linked genes that ignore targeting by XCI and those sex-linked genes that naturally escape XCI to incite dosage effects. Sex hormones (e.g., estrogen and testosterone) may also influence expression of these genes and, in turn, the immune capacities of each sex (Libert et al., 2010; Palaszynski et al., 2005).
The above-mentioned factors driving sex differences in melanoma may further translate into efficacy of therapeutic interventions or immunotherapy. Anti-PD-1/PD-L1 and anti-CTLA-4 antibodies are promising immune checkpoint inhibitors (ICI) that bolster the host's anti-tumoral immune response by interfering with immune-inhibitory signals secreted by tumor cells (Wang et al., 2019). Since women already exhibit a stronger immune environment, therapeutic strategies to boost immune response are generally expected to be more effective in men. Although pan-cancer meta-analyses appear to support this prediction, statistical significance has yet to affirm any clear trends in melanoma. Conforti et al. for example, carried out a meta-analysis of 20 randomized controlled trials of ICIs—CTLA-4 (Ipilimumab, Tremelimumab) or PD-1/PD-L1 (Pembrolizumab, Nivolumab) (Conforti et al., 2018). Across multiple cancer types, there was a significant difference in efficacy between male and female patients when compared to controls for each sex (HR: 0.72, 95% CI 0.65–0.79 versus HR: 0.86, 95% CI 0.79–0.93; p < 0.0019). By cancer type, 7 of the 20 trials investigated stage II to IV melanoma patients. This analysis included 2173 males and 1459 females treated with inhibitors, either alone or in combination with other drugs (Gp100 peptide vaccine or dacarbazine chemotherapy). The experimental groups showed greater benefit for males versus females, although not by a statistically significant margin (HR: 0.66, 95% CI 0.55–0.79 versus HR: 0.79, 95% CI 0.70–0.90; p < 0.72). A closer examination of tumor-infiltrating lymphocytes (TILs) in a retrospective study with over 14,000 patients further failed to identify sex differences in TILs (Yang et al., 2021). In this case, however, it would be interesting to know the “treatment state” of these patients—that is, whether immunotherapy was factored into the analysis. Results have generally shown an increased efficacy of ICI in men compared with women, but again, there lacks a clearly defined mechanism (Grassadonia et al., 2018; Wang et al., 2019). Regulatory T cells represent one therapeutic target of ICI, as they highly express immune-inhibitory receptors such as CTLA-4 and PD-1. The minor benefits of immune checkpoint treatments in females could, therefore, be due to their lower populations of TReg cells. Additionally, the higher number of CD8+T lymphocytes at baseline conditions in males could suggest a higher impact of ICI in relieving T-cell exhaustion. Although these conclusions require further support, the contribution of sex-specific approaches to melanoma treatment options and prognoses comprise a promising future for the improvement of treatment efficacy in a personalized sense.
The National Institutes of Health (NIH) has recently engaged in developing policies and resources that factor sex as a biological variable (SABV) in research designs, analyses, and reporting (Arnegard et al., 2020). It is important to note, however, that while many designs may begin to incorporate both sexes, evaluation of sex-specific responses or, at the very least, disaggregation of data by sex is equally as vital for the broader research community. The SABV guidelines do not explicitly require male-to-female comparisons, so while the frequency of data across both sexes increass, the proportion of those studies that stratify analysis by sex may not follow suit (Woitowich et al., 2020). Furthermore, Garcia-Sifuentes and Maney suggest that NIH’s lack of specificity regarding experimental design, data analysis, or statistical approaches to properly test for sex differences could be counter-productive (Garcia-Sifuentes & Maney, 2021). In an analysis of factorial studies across eight disciplines, the authors found that, for those articles claiming a sex-specific effect, 71% failed to support the conclusion with adequate statistical evidence. The benefits of understanding sex differences across diverse disease contexts are far-reaching beyond melanoma—but only if reported correctly. As a recent case in point, the highly cited article by Takahashi et al., (2020) claiming sex disparities in the immune landscape of COVID-19 patients has since been disputed as having overstated its findings (Shattuck-Heidorn et al., 2021). In particular, the “Matters arising” response cites the presentation of raw data without correction for possible covariates (e.g., age and BMI), loss of significance following adjustment, and the necessity of both between- and within-sex differences to classify a sex-specific effect. Overall, this accentuates the need for a standardized analysis pipeline to evaluate sex differences in disease. Such an effort should further be extrapolated to both in vivo work in model organisms and clinical human study. A recent report mapped sex-biased gene expression across multiple mammalian species—from humans to mice—to assess the conservation of phenotypic sex differences (Naqvi et al., 2019). The authors note, for example, that sex-biased expression could contribute to similar sex-specific trends observed in height or body size among mammalian taxa. This approach promises a viable infrastructure for translating physiological-level findings in animal models into human counterparts. Historically, expectations set by the “estrus-mediated variability hypothesis” have been used as justification to exclude one sex from experimental designs. A large-scale study assessing murine sex bias in trait variance, however, concluded that neither males nor females exhibited universally higher trait variability (Zajitschek et al., 2020). Rather, sex differences were dependent upon specific functional trait groups, with higher female bias in immunological traits but greater male variability in morphological features like size. Indeed, both sexes should be similarly incorporated into animal or human-focused investigations. Sex-specific effects integrate more broadly into biological mechanisms of carcinogenesis such as genomic instability, immunity, metabolism, senescence, oxidative stress, and angiogenesis (Rubin et al., 2020), so before sex-dependent approaches to prevention can be adopted in clinical care, the research community must prioritize lexical and analytical consistency. The trajectory of modern science inevitably prioritizes a future of personalized medicine and, as the landscape of disease treatment evolves, one size does not necessarily fit all.
CONFLICTS OF INTEREST
The authors declare no potential conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The melanoma incidence and mortality rate data presented in this review were derived from the following resources available in the public domain: SEER Program Database: https://seer.cancer.gov/cBioPortal: https://www.cbioportal.org/.