Long-term abscisic acid promotes golden2-like1 degradation through constitutive photomorphogenic 1 in a light intensity-dependent manner to suppress chloroplast development
Funding information: a POSCO Science Fellowship from the POSCO TJ Park Foundation; National Research Foundation of Korea (NRF), Grant/Award Number: 2019R1A2B5B03099982
Abstract
Abiotic stress, a serious threat to plants, occurs for extended periods in nature. Abscisic acid (ABA) plays a critical role in abiotic stress responses in plants. Therefore, stress responses mediated by ABA have been studied extensively, especially in short-term responses. However, long-term stress responses mediated by ABA remain largely unknown. To elucidate the mechanism by which plants respond to prolonged abiotic stress, we used long-term ABA treatment that activates the signalling against abiotic stress such as dehydration and investigated mechanisms underlying the responses. Long-term ABA treatment activates constitutive photomorphogenic 1 (COP1). Active COP1 mediates the ubiquitination of golden2-like1 (GLK1) for degradation, contributing to lowering expression of photosynthesis-associated genes such as glutamyl-tRNA reductase (HEMA1) and protochlorophyllide oxidoreductase A (PORA), resulting in the suppression of chloroplast development. Moreover, COP1 activation and GLK1 degradation upon long-term ABA treatment depend on light intensity. Additionally, plants with COP1 mutation or exposed to higher light intensity were more sensitive to salt stress. Collectively, our results demonstrate that long-term treatment of ABA leads to activation of COP1 in a light intensity-dependent manner for GLK1 degradation to suppress chloroplast development, which we propose to constitute a mechanism of balancing normal growth and stress responses upon the long-term abiotic stress.
1 INTRODUCTION
Abiotic stress is one of most serious threats to plant survival. Thus, abiotic stress responses have been studied extensively (Zhu, 2016). The phytohormone abscisic acid (ABA) plays a crucial role in defending plants against abiotic stress (Vishwakarma et al., 2017). Under conditions of abiotic stress, cellular ABA levels increase either via de novo biosynthesis or the rapid hydrolysis of ABA-GE, ABA conjugated with glucose, by Arabidopsis thaliana β-glucosidase 1 (AtBG1) and Arabidopsis thaliana β-glucosidase 2 (AtBG2) (Lee et al., 2006; Tuteja, 2007; Xu et al., 2012). Subsequently, ABA binds to ABA receptors, pyrabactin resistance (PYR)/PYR1-LIKE (PYL)/regulatory components of ABA receptors (RCARs), which facilitates interaction between ABA-bound receptors and PP2C, thereby releasing sucrose non-fermenting (SNF1)-related protein kinase 2 (SnRK2) from protein phosphatase 2C (PP2C)/SnRK2 (Park et al., 2009). In Arabidopsis, freed SnRK2 phosphorylates downstream factors such as ABA-responsive element bindings, ABA insensitive 5 (ABI5) and respiratory burst oxidase homolog protein F to induce the abiotic stress responses (Raghavendra et al., 2010). As a result, ABA induces various responses such as stomatal closure and inhibition of primary root growth (Vishwakarma et al., 2017). In general, studies of molecular mechanisms of ABA-mediated responses to abiotic stress focussed on the processes that occur for short period of time (Lee & Seo, 2019; Zhu, 2016). However, abiotic stresses often persist for long periods in nature, thereby yielding completely different effect in plants compared to the short-term stress. For example, in the perspective of regulation of chloroplast activity, prolonged salinity decreases chlorophyll levels, often resulting in a leaf chlorosis phenotype. On the other hand, short-term exposure to salinity causes stomata closure and thereby results in decreased photosynthesis due to reductions in levels of carbon assimilation (Acosta-Motos et al., 2017). Intriguingly, ABA treatment, which could activate signalling against salinity stress, induced leaf yellowing with impairment in chloroplast development, accompanying reduction of chlorophyll levels and expression of photosynthesis-associated nuclear genes (PhANGs) such as HEMA1 and PORA if the treatment is prolonged (Wang et al., 2018). However, plants treated with ABA for short periods show green leaves, and the expression of PhANGs is sustained or even increased (Wang et al., 2018). These imply there are distinct differences between short-term and long-term stress responses induced by ABA in phenotypic and molecular level, especially in the regulation of chloroplast development. However, it remains largely elusive how chloroplast development which is mainly controlled by light signalling is suppressed by long-term ABA treatment or prolonged abiotic stress.
In plants, chloroplast development is a key process required for their autotrophic life (Jarvis & López-Juez, 2013). Chloroplast development is highly dependent on the activation of light signalling, which involves the suppression of constitutive photomorphogenic 1 (COP1) (Jarvis & López-Juez, 2013; Xu et al., 2015). In the dark, COP1 ubiquitinates important transcription factors involved in photomorphogenesis, such as elongated hypocotyl 5 (HY5) and long hypocotyl in far-red 1, leading to the 26S proteasome-dependent degradation to induce skotomorphogensis (Osterlund et al., 2000; Yang et al., 2005). Upon light exposure, COP1-mediated degradation of these transcription factors is inhibited (Xu et al., 2015). In addition, proplastids or etioplasts develop into chloroplasts via highly complex processes that involve induction of gene expression in both the nucleus and plastids, import of a large number of proteins from the cytosol, chlorophyll biosynthesis and the assembly of thylakoid membranes to build up various systems for chloroplast functions, such as photosynthesis (Jarvis & López-Juez, 2013; Xu et al., 2015). Golden2-like1 and 2 (GLK1 and GLK2) transcription factors play a crucial role in the expression of PhANGs required for chloroplast development, including chlorophyll biosynthetic enzymes such as HEMA1 and PORs (Apitz et al., 2014; Paddock et al., 2012; Sperling et al., 1997; Waters et al., 2009).
Despite extensive studies on the role of ABA in abiotic stress responses and on chloroplast development, the relationship between ABA and chloroplast development is not fully understood. In general, high levels of ABA suppress plant growth and development, whereas chloroplast development induced by light is crucial for plant growth and development. This suggests that there may be an antagonistic relationship between ABA signalling and light signalling (Jarvis & López-Juez, 2013; Tuteja, 2007; Wang et al., 2018; Xu et al., 2015). However, the relationship between ABA and light is more complex than a simple antagonistic relationship. In fact, ABA-insensitive 4, identified as an important transcription factor for ABA responses, and HY5, an essential transcription factor in light signalling, antagonistically regulate cell expansion-related genes during de-etiolation (Xu et al., 2016). However, HY5 positively regulates the expression of ABI5, another transcription factor involved in ABA responses, during seed maturation, seedling growth and under conditions of salt stress (Chen et al., 2008). Thus, it is possible that the interaction/relationship between ABA signalling and light signalling for chloroplast development is influenced by developmental programs of plants, as well as environmental factors including the severity and duration of abiotic stress, and the light intensity and wavelength.
Previously, we showed that long-term ABA treatment suppresses the expression of PhANGs, whereas short-term ABA treatment activates or maintains their expression, indicating the existence of interaction between ABA signalling and light signalling regarding the chloroplast development, and the mode of interaction changes according to the duration of ABA treatment (Wang et al., 2018). In this study, we investigated the mechanisms underlying the suppression of chloroplast development upon long-term ABA treatment and found that ABA and light intensity act jointly to activate the E3 ligase COP1 to mediate GLK1 degradation through ubiquitination and 26S proteasome, which in turn leads to suppression of the PhANGs expression, thereby resulting in the suppression of chloroplast development.
2 RESULTS
2.1 COP1 plays an essential role in the suppression of chloroplast development upon long-term ABA treatment
To elucidate molecular mechanism underlying the long-term ABA-induced leaf yellowing under the normal 16/8 h light/dark cycle, we first asked whether it was related to ABA-induced leaf senescence because it is well known that ABA promotes leaf senescence (Lim et al., 2007). We previously showed that transcript levels of senescence-related genes were not induced upon prolonged ABA treatment (Wang et al., 2018). However, to further test the possibility of ABA-induced yellow leaves resulting from leaf senescence, we hypothesized that if ABA-induced yellow leaves represent leaf senescence that accompanies cell death, a final step of leaf senescence, leaf colour would not change from yellow to green when plants are no longer treated with ABA (Lim et al., 2007). To test this, plants that had been treated with ABA for 11 days under a 16/8 h light/dark cycle, thus showing yellow colour, were transferred to half Murashige and Skoog medium (MS) plates containing dimethyl sulphoxide (DMSO) or 5 μM ABA, and their leaf colour was examined. Plants transferred to DMSO plates turned to green leaves but remained yellow onto ABA plates (Figure S1). Leaf greening began to appear 2 days after transfer, initiating at the centre of the plants where chloroplast development begins and gradually extended to older leaves (Charuvi et al., 2012). These results raise the possibility that the leaf yellowing induced by prolonged ABA treatment represents the suppression of chloroplast development but not leaf senescence.
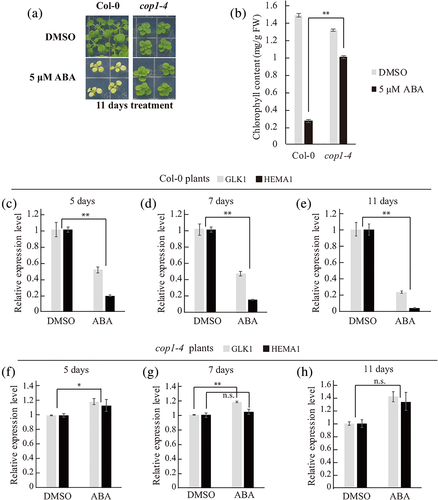
To investigate the signalling pathway that underlies suppression of chloroplast development by long-term ABA treatment, we examined the response of a quadruple ABA receptor mutant, pyr1/pyl1/pyl2/pyl4 mutant (QC3), after being treated with 5 μM ABA for 11 days. As a result, the QC3 mutant showed an ABA-insensitive phenotype (i.e., green leaves) (Figure S2a,b). Similarly, a previous study showed that the snrk2.2/snrk2.3/snrk2.6 triple mutant, which has mutations in core ABA signalling components, displayed a green leaf phenotype under the ABA treatment (Zhao et al., 2016). These results indicate that the core ABA signalling pathway is involved in long-term ABA-induced leaf yellowing. Next, we aimed to identify additional components of the pathway leading to leaf yellowing upon long-term ABA treatment. We put forward the idea that high level of ABA overrides light signalling pathway in plants. Indeed, expression patterns of genes important for chloroplast development such as HEMA1 and PORA upon prolonged ABA treatment under continuous white light resembled those in the dark, suggesting that ABA overrides light signalling (Barnes et al., 1996; McCormac et al., 2001). To further test this idea, we examined various mutant plants, phyA-211, phyB-9, cry1-304, cry2-1, cop1-4, pif1-2, pif3-3, pif4, pif1-2pif3-3, pifQ (pif1pif3pif4pif5) that had mutations in genes relevant to light perception or signalling. Of these mutants, only cop1-4 plants with a point mutation in COP1 showed green leaves in the presence of ABA, indicating that COP1 is an important component of long-term ABA-induced leaf yellowing (Figure 1a,b, Figure S3). To confirm the phenotype at the molecular level, we examined transcript levels of GLK1 and HEMA1 in wild-type (WT) (Col-0) and cop1-4 plants with or without ABA treatment in a time-dependent manner using quantitative real-time polymerase chain reaction (qRT-PCR). Upon long-term ABA treatment, transcripts levels of GLK1 and HEMA1 were reduced in WT plants but showed no difference or even an increase in cop1-4 plants (Figure 1c–h), confirming that COP1 is involved in long-term ABA-induced suppression of chloroplast development.
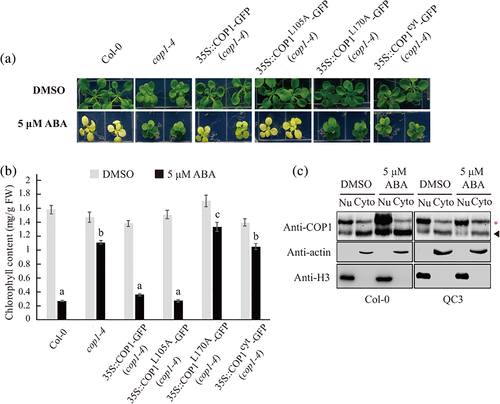
2.2 Long-term ABA-induced suppression of chloroplast development depends on COP1 activation via dimerization and nuclear localization
To elucidate the mechanism by which COP1 plays a role in the suppression of chloroplast development upon long-term ABA treatment, we examined transcript levels of COP1 in WT plants with or without ABA treatment. COP1 transcript levels did not significantly change after 1 day, 2 days or 11 days of ABA treatment (Figure S4a–c), indicating that ABA treatment does not affect the expression of COP1. In fact, COP1 activity is regulated by nucleocytoplasmic partitioning and dimerization (Lee et al., 2017; Subramanian et al., 2004; von Arnim & Deng, 1994). Therefore, we examined cop1-4 plants that express WT COP1, COP1L105A, COP1L170A or COP1cyt with or without ABA treatment. Two mutant constructs, COP1L105A and COP1L170A, which contained a mutation in the cytoplasmic localization signal of COP1, have been shown to accumulate in the nucleus at higher levels than WT COP1 (Subramanian et al., 2004). However, COP1L170A cannot form a dimer, resulting in loss of function, whereas COP1L105A is capable of dimerization (Subramanian et al., 2004, Lee and Kim et al., 2017). COP1cyt has a mutation within its nuclear localization signal and therefore localizes in the cytoplasm (Stacey et al., 1999). Consequently, WT COP1 and COP1L105A were active, whereas COP1L170A and COP1cyt were inactive. In the cop1-4 background, in the presence of ABA, the expression of WT COP1 and COP1L105A produced the leaf yellowing phenotype, whereas COP1L170A and COP1cyt plants had green leaves (Figure 2a, Figure S5). Chlorophyll levels closely correlated with the degree of leaf yellowing (Figure 2b). To support this finding, we examined the transcript levels of GLK1 and HEMA1 in DMSO- or ABA-treated plants expressing COP1L105A, COP1L170A or COP1cyt in the cop1-4 genetic background. Transcript levels of GLK1 and HEMA1 were decreased in plants harbouring COP1L105A upon ABA treatment, compared with DMSO control (Figure S6a). However, transcript levels of those genes were increased or sustained in plants harbouring COP1L170A or COP1cyt, upon ABA treatment, compared with DMSO control (Figure S6b,c). These results indicate that both nuclear localization and dimerization of COP1 are essential for COP1-mediaed suppression of chloroplast development after long-term ABA treatment. Next, we examined whether ABA treatment induced COP1 nuclear localization. Extracts from Col-0 or QC3 plants treated with ABA for 11 days were separated into nuclear and cytoplasmic fractions, and both fractions were analysed via Western blotting using an anti-COP1 antibody. Compared with the DMSO control, ABA treatment increased the amount of COP1 in the nuclear fraction in Col-0 but not QC3 plants (Figure 2c, Figure S7a). In addition, total amount of COP1 was also increased in Col-0 plants when plants were treated with ABA (Figure 2c, Figure S7b). In a previous study, we observed that short-term ABA treatment activated or sustained expression of PhANGs, whereas long-term ABA treatment suppressed their expression (Wang et al., 2018). Therefore, we examined the effect of short-term ABA treatment on the nuclear or cytoplasmic COP1 level. Consistent with previous observation, ABA treatment for 48 h (short-term ABA treatment) to Col-0 plants did not change nuclear or cytoplasmic COP1 level compared with DMSO control (Figure S8). These results indicate that long-term ABA treatment results in COP1 accumulation in the nucleus. Taken together, these results suggest that long-term ABA modulates COP1 activity to suppress chloroplast development.
2.3 COP1 promotes ubiquitination-dependent GLK1 degradation in response to long-term ABA treatment
COP1 can ubiquitinate a diverse set of proteins and thereby regulates multiple molecular pathways (Kim et al., 2017). Thus, one possibility of how ABA suppresses chloroplast development via COP1 is that long-term ABA treatment enables COP1 to control the level of a protein critical for chloroplast development. We presumed that GLK1 as a potential candidate since it plays a critical role in chloroplast development, functions in the nucleus and is subjected to ubiquitination (Chen et al., 2016; Tokumaru et al., 2017; Waters et al., 2009; Waters et al., 2008). Thus, we first tested the stability of GLK1 in response to ABA. We treated transgenic plants expressing Green fluorescent protein (GFP)-GLK1 in a glk1glk2 background (GFP-GLK1/glk1glk2) with 5 μM ABA for 7 and 11 days (Figure S9, Tokumaru et al., 2017). Afterward, GFP-GLK1 levels were measured by Western blot analysis. GFP-GLK1 levels were lower in ABA-treated plants than DMSO-treated plants (Figure 3a). However, in the presence of MG132, a 26S proteasome inhibitor, GFP-GLK1 levels did not decrease in response to ABA treatment, indicating that long-term ABA treatment reduces GFP-GLK1 levels in a 26S proteasome-dependent manner (Figure 3b). Next, we examined the effect of short-term ABA treatment on the level of GFP-GLK1. We treated GFP-GLK1/glk1glk2 plants with 5 μM ABA for 48 h, and the GFP-GLK1 level was analysed by western blotting using anti-GFP antibody. Consistent with previous observation (Wang et al., 2018), short-term ABA treatment did not change the GFP-GLK1 level (Figure S10). To examine COP1 involvement in GLK1 destabilization after long-term ABA treatment, we generated plants expressing GFP-GLK1 in a glk1glk2cop1-4 triple-mutant background (GFP-GLK1/glk1glk2cop1-4) by crossing GFP-GLK1/glk1glk2 with cop1-4 mutant plants (Figure S11a–f). GFP-GLK1/glk1glk2 and GFP-GLK1/glk1glk2cop1-4 plants were treated with DMSO or ABA for 11 days and protein extracts were examined to assess GFP-GLK1 levels by Western blot analysis using anti-GFP antibody. In contrast to GFP-GLK1/glk1glk2 plants, GFP-GLK1 levels were not reduced in GFP-GLK1/glk1glk2cop1-4 plants after long-term ABA treatment (Figure 3c, Figure S12), indicating that COP1 is involved in the degradation of GLK1 in response to ABA. However, in general, GFP-GLK1 levels were lower in the glk1glk2cop1-4 background than the glk1glk2 background (Figure 3c), and the underlying cause of this difference is not currently understood. To genetically confirm this result, we isolated glk1glk2cop1-4 triple mutants and treated them with DMSO or 5 μM ABA for 11 days. Both DMSO-treated and ABA-treated glk1glk2cop1-4 plants showed the yellow leaf phenotype in contrast to ABA-treated cop1-4 plants (Figure S13a,b). These results support the idea that GLK1 plays a role in the green leaf phenotype of cop1-4 plants upon the long-term ABA treatment.
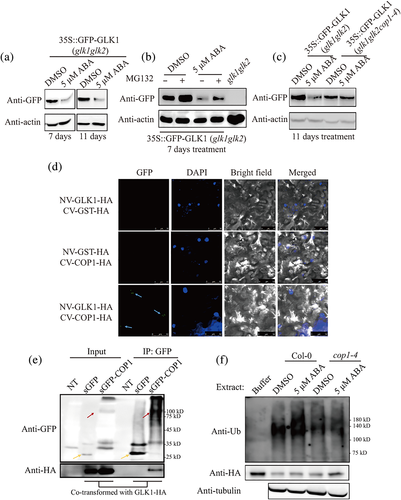
To further confirm this result, we examined GLK1 stability using a cell-free system. Total protein extracts from WT plants that had been grown in the presence of DMSO or ABA for 11 days were incubated with 6 × His-GLK1-hemagglutinin (HA) purified from E. coli in the presence of DMSO or MG132 for the indicated time. 6 × His-GLK1-HA levels were assessed by Western blot analysis using an anti-HA antibody. In agreement with in vivo results, 6 × His-GLK1-HA levels were lower in extracts derived from ABA-treated Col-0 plants than DMSO-treated Col-0 plants (Figure S14a), indicating that plant extracts from ABA-treated Col-0 plants have higher GLK1 degradation activity. Moreover, MG132 strongly inhibited degradation of GLK1, which indicated that ABA promoted the 26S proteasome-mediated degradation of 6 × His-GLK1-HA (Figure S14a). Next, we compared levels of 6 × His-GLK1-HA after incubation with total protein extracts from Col-0 or cop1-4 plants that had been grown in the presence of DMSO or ABA for 11 days. 6 × His-GLK1-HA levels did not significantly differ when it was incubated in total protein extracts from DMSO-treated and ABA-treated cop1-4 plants (Figure S14b), indicating that COP1 is involved in the degradation of GLK1 after long-term ABA treatment.
To elucidate the mechanism by which COP1 causes degradation of GLK1 after long-term ABA treatment, we examined whether COP1 directly interacts with GLK1. To test this, we performed a bimolecular fluorescence complementation (BiFC) assay using GLK1 tagged with N-terminal Venus (NV) and COP1 tagged with C-terminal Venus (CV) in Nicotiana benthamiana. Both of them were tagged with HA at their C-termini, which was used to examine their expression. p38, a gene silencing suppressor (Md Islam et al., 2019), was also cotransformed into N. benthamiana to increase the expression of the genes. We also generated glutathione S-transferase (GST)-fused NV or CV as negative control (Kudla & Bock, 2016; Lin et al., 2018). Fluorescent signal was detected with only combination of NV-GLK1-HA and CV-COP1-HA in a pattern of speckles but not the negative control sets, NV-GLK1-HA with CV-GST-HA or NV-GST-HA with CV-COP1-HA, indicating that GLK1 and COP1 directly interact with each other (Figure 3d). Expression of constructs was confirmed by Western blotting analysis (Figure S15). Next, we examined the localization of fluorescent speckles, which resembled photobody (Van Buskirk et al., 2012). To determine their location, we stained transformed cells with DAPI and found that GFP signals overlapped with DAPI, indicating that interaction between COP1 and GLK1 occurs in the nucleus in a punctate pattern (Figure 3d). To further confirm the interaction between COP1 and GLK1, we performed co-immunoprecipitation experiment between COP1 and GLK1. We transiently expressed GLK1-HA together with sGFP-COP1 or sGFP alone as control. Protein extracts from plant tissues were subjected to immunoprecipitation using anti-GFP, and resulting precipitates were analysed by Western blotting using the anti-HA antibody. GLK1-HA was detected in the immunoprecipitates of sGFP-COP1, but not in sGFP alone, indicating that COP1 physically interacts with GLK1 (Figure 3e).
Interaction between COP1 and GLK1 raises the possibility that COP1 mediates ubiquitination of GLK1 upon long-term ABA treatment. Thus, we analysed the degree of ubiquitination of GLK1 upon long-term ABA treatment. Purified 6 × His-GLK1-HA was incubated with total protein extracts from either Col-0 or cop1-4 plants that had been grown in the presence of DMSO or ABA for 11 days. After incubation for 40 min, 6 × His-GLK1-HA was captured from mixtures using Ni2+-NTA beads and analysed by Western blotting using the anti-ubiquitin antibody. 6 × His-GLK1-HA showed a higher degree of ubiquitination in the extracts from ABA-treated Col-0 plants than that from DMSO-treated Col-0 plants. However, the degree of ubiquitination in total protein extracts derived from cop1-4 plants did not significantly differ, regardless of ABA treatment (Figure 3f, Figures S16 and S17), indicating that COP1 is involved in the ubiquitination of GLK1 upon long-term ABA treatment.
2.4 Long-term ABA treatment causes suppression of chloroplast development in a light intensity-dependent manner
Next, we aimed to elucidate mechanism by which ABA regulates COP1 activity, which in turn leads to regulation of GLK1 stability. COP1 activity is down-regulated upon exposure to light, via the dissociation of the COP1-SPAs complex and a decrease in nuclear COP1 level (Subramanian et al., 2004; von Arnim & Deng, 1994; Xu et al., 2015). However, results of Figure 2 indicate that long-term ABA treatment activates COP1 and increases nuclear COP1 levels. These results strongly suggest that long-term ABA treatment overrides light signalling. In addition, long-term ABA treatment strongly suppressed expression of PhANGs such as GLK1, HEMA1 and PORA (Wang et al., 2018). In fact, transcript levels of these genes were highly coordinated with the situation in chloroplasts (Chan et al., 2016). When chloroplasts became dysfunctional by herbicide treatment, transcript levels of those genes were decreased (Kakizaki et al., 2009; McCormac & Terry, 2004; Tokumaru et al., 2017; Waters et al., 2009). Therefore, these observations raised one possibility that the mechanism regulating COP1 activity under long-term ABA highly correlates with chloroplast acclimation to environmental changes (Jarvis & López-Juez, 2013; Ruckle et al., 2012; Ruckle et al., 2007; Szechynska-Hebda & Karpinski, 2013). Light intensity is an important environmental factor that can affect chloroplast. Indeed, chloroplast metabolisms change according to the light intensity, which is required to acclimate to changing light environments (Szechynska-Hebda & Karpinski, 2013). In addition, long-term ABA treatment appeared to override light signalling required for chloroplast development (Figures 1 and 2). Thus, we reasoned that long-term ABA-induced suppression of chloroplast development is influenced by light intensity; low light intensity facilitates suppression of chloroplast development.
To test this idea, we examined the impact of light intensity on the impaired chloroplast development caused by long-term ABA treatment. Plants grown under the condition of 70 μmol m−2 s−1 for 8 days were transferred to half MS plates containing DMSO or ABA and were further grown under the light intensity of 10, 70 or 120 μmol m−2 s−1 (Figure 4a). However, in contrast to our expectation, changing to 10 μmol m−2 s−1 light intensity did not cause significant yellowing of leaves, whereas changing to 70 and 120 μmol m−2 s−1 caused yellow leaves phenotype at 7 and 11 days after ABA treatment (Figure 4b,d). To quantify the degree of leaf yellowing, we measured the chlorophyll contents of these plants. Under the light intensity of 10, 70 and 120 μmol m−2 s−1, the chlorophyll contents of plants treated with ABA for 7 days were reduced to 91%, 29% and 23% that of DMSO-treated plants, respectively, and for 11 days were 72%, 17% and 15%, respectively (Figure 4c,e). To further examine the effect of light intensity on ABA-induced leaf yellowing, plants grown under 10 μmol m−2 s−1 for 9 days were transferred to dimethyldioxirane (DMDO)- or 5 μM ABA-supplemented half MS plates and further grown under 10 or 100 μmol m−2 s−1 light for 11 days. Upon ABA treatment, 100 but not 10 μmol m−2 s−1 light caused yellow leaves (Figure S18). These results indicate that light intensity is a critical factor for suppression of chloroplast development by long-term ABA treatment.
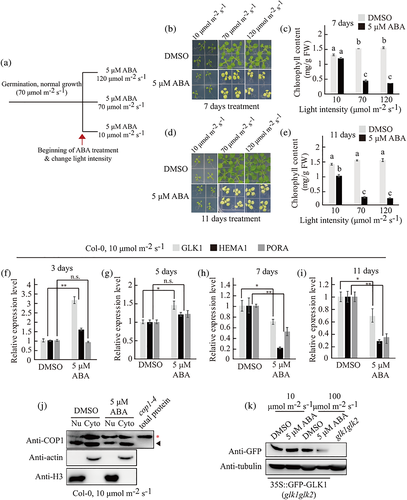
To elucidate the molecular process by which light intensity plays a role in suppression of chloroplast development by long-term ABA treatment, we measured transcript levels of GLK1, HEMA1 and PORA at various time points after treatment with ABA under the light intensity of 10 μmol m−2 s−1. Transcript levels of the genes were increased or sustained until 5 days after ABA treatment compared with DMSO control but decreased after 7 days ABA treatment, indicating that a transition in the expression mode occurs between 5 and 7 days after ABA treatment (Figure 4f–i). At a light intensity of 70 μmol m−2 s−1, the transitional expression of those genes occurs between 2 and 3 days after ABA treatment (Wang et al., 2018). These results suggest that under the low-intensity light, it takes longer for ABA to suppress the expression of PhANGs, and the degree of suppression was milder under low-intensity light compared to higher light intensities. Next, we asked whether this is a general phenomenon of the ABA effect on gene expression. We examined transcript levels of stress-responsive genes, RAB18 and RD29B, under the same conditions. In a previous study, these genes also showed a transition in the expression pattern during the period of ABA treatment, although the transition time point was earlier than PhANGs (Wang et al., 2018). However, transcript levels of these genes remained high until 11 days after ABA treatment under 10 μmol m−2 s−1 light intensity (Figure S19). These results indicate that light intensity is involved in determining the time point of the ABA-mediated transitional response of the PhANGs' expression and differentially affects the expression of PhANGs and stress-responsive genes under long-term ABA treatment.
Next, we asked whether light intensity plays a role in COP1 activity and COP1-mediated degradation of GLK1 induced by long-term ABA. We measured nuclear COP1 and GLK1 total protein levels after treating plants with ABA for 11 days under 10 μmol m−2 s−1 light intensity. In contrast to the plants exposed to a light intensity of 70 μmol m−2 s−1, those exposed to a light intensity of 10 μmol m−2 s−1 had decreased levels of nuclear-localized COP1 after ABA treatment for 11 days (Figure 4j). In addition, long-term ABA treatment did not significantly affect GFP-GLK1 levels in GFP-GLK1/glk1glk2 plants under the light intensity of 10 μmol m−2 s−1, compared with those treated with the DMSO control (Figure 4k). These results suggest that low light intensity (10 μmol m−2 s−1) was unable to accumulate COP1 in the nucleus, thereby unable to mediate degradation of GLK1 under the condition of long-term ABA treatment. Taken together, these results indicate that light intensity plays a crucial role in the process of suppression of chloroplast development by ABA.
To gain an insight into the physiological role of the interaction between ABA and light intensity, we examined the level of H2O2 as a common denominator. Both ABA and light intensity affect the cellular levels of H2O2 (Fahnenstich et al., 2008; Iwai et al., 2019; Szechynska-Hebda & Karpinski, 2013; Watkins et al., 2017). We treated Col-0 and cop1-4 plants with DMSO or ABA under the light intensity of 70 μmol m−2 s−1 or 10 μmol m−2 s−1 for 11 days. Under the 70 μmol m−2 s−1 light intensity, ABA-treated Col-0 plants showed higher level of H2O2 than DMSO-treated Col-0 plants, whereas ABA-treated cop1-4 plants showed lower levels of H2O2 than DMSO-treated cop1-4 plants. In addition, ABA treatment under 70 μmol m−2 s−1 light intensity led to higher levels of H2O2 than DMSO treatment in Col-0 plants, whereas ABA treatment under 10 μmol m−2 s−1 light intensity did not, suggesting the possibility that interaction between ABA and light intensity converged on H2O2 production (Figure S20).
2.5 COP1-mediated GLK1 degradation and light intensity are crucial for plant salt stress resistance
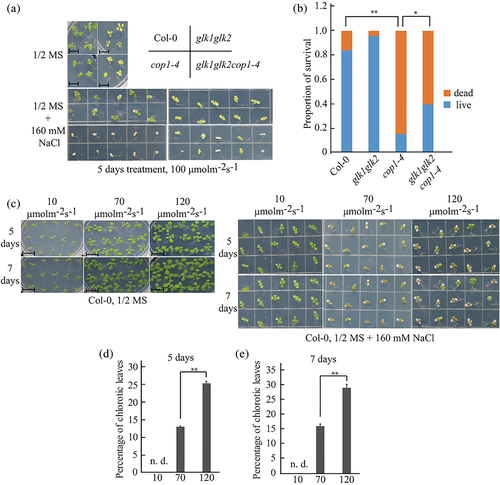
ABA plays a crucial role in various cellular processes including salt stress resistance. Therefore, we asked whether suppression of chloroplast development by long-term ABA treatment plays a role in salt stress resistance. To address this, we examined the phenotypes of Col-0, glk1glk2, cop1-4 and glk1glk2cop1-4 plants treated with 160 mM NaCl for 5 days. cop1-4 plants were more sensitive to salt stress than Col-0 and glk1glk2 plants in terms of survival. However, glk1glk2cop1-4 plants were more resistant to salt stress than cop1-4 plants, indicating functional GLKs render the plants to be more sensitive to salt stress (Figure 5a,b). In addition, we examined the response of Col-0 plants to salt stress under various light intensities. Sensitivity of Col-0 plants to 160 mM NaCl was higher with increase in the light intensity (Figure 5c–e). Collectively, these results indicate that COP1-mediated GLK1 degradation and light intensity play positive and negative roles, respectively, in resistance to salt stress in plants.
3 DISCUSSION
The chloroplast is the home for a large number of metabolisms, such as photosynthesis, biosynthesis of phytohormones, amino acid metabolism, etc. These chloroplast activities are dependent on the availability of many resources obtained from environment (Jarvis & López-Juez, 2013; Neuhaus & Emes, 2000). In fact, chloroplasts are one of main users of various cellular resources, in particular water. Thus, chloroplast activities should be closely regulated according to the environmental conditions. In this study, we showed that the chloroplast status is regulated by ABA, a hormone involved in dehydration stress signalling. In this regulation, long-term ABA treatment led to increase the activity of COP1, which mediates degradation of GLK1. Furthermore, light intensity contributed to the ABA-mediated regulation of the chloroplast activity.
The regulation of COP1 activity has been primarily studied in etiolation/de-etiolation process caused by dark/light. In the dark, COP1 is activated by increasing its nuclear levels and dimerization followed by tetramerization with SPAs (Subramanian et al., 2004; von Arnim & Deng, 1994; Xu et al., 2015). In the light, COP1 levels in the nucleus decrease, as does COP1 dimerization (Subramanian et al., 2004; von Arnim & Deng, 1994; Xu et al., 2015). Here, we elucidated a new mechanism by which COP1 is regulated by long-term ABA treatment. Even under the normal light/dark cycle, long-term ABA treatment causes COP1 to behave in the same as it does in the dark (Figure 2). These results suggest that under the long-term ABA treatment, ABA signalling overrides light-mediated inhibition of COP1 activity, which in turn leads to the reduction of expression of PhANGs, and produces a leaf yellowing phenotype (Figures 1 and 2). ABA is a key hormone for abiotic stress responses (Tuteja, 2007; Vishwakarma et al., 2017; Zhu, 2016). Thus, these results raise an intriguing possibility that COP1 plays a key role in long-term abiotic stress. Consistent with this idea, cop1-4 plants that stayed green under long-term ABA treatment were more sensitive to high salt than WT plants (Figure 5a,b). Plants have to respond to the constantly changing environmental conditions. Thus, one possible scenario is that COP1 functions as a toggle switch to balance between ABA-mediated growth suppression and light-induced growth promotion. In this balancing process, the key point is the regulation of chloroplast activity; ABA-mediated suppression of chloroplast activity vs. light-induced activation of chloroplast activity.
In balancing between two opposite processes, COP1 may suit well because it has E3 ligase activity; it can ubiquitinate target proteins, thereby leading to their degradation via the 26S proteasome (Kim et al., 2017; Xu et al., 2015). Thus, COP1 in long-term ABA-induced leaf yellowing likely ubiquitinates target proteins. Similar to ABA, ethylene also increases levels of COP1 in the nucleus (Yu et al., 2013). However, in contrast to ABA, ethylene treatment does not affect leaf colour. Instead, it promotes hypocotyl elongation, another feature of skotomorphogenesis, suggesting that COP1 controls multiple physiological responses to changing environmental conditions (Yu et al., 2013). One possibility of regulating multiple responses is that COP1 ubiquitinates different substrates depending on conditions. In fact, ethylene-activated COP1 ubiquitinates HY5 (Yu et al., 2013). We identified GLK1 that was ubiquitinated and degraded via 26S proteasome upon long-term ABA treatment. GLK1 is a key transcription factor for expression of PhANGs. Therefore, it is required for chloroplast development from the proplastid or etioplast in response to light exposure (Chen et al., 2016; Waters et al., 2008; Waters et al., 2009). Thus, upon long-term ABA treatment, COP1-mediated degradation of GLK1 is consistent with the leaf yellowing via suppression of chloroplast development. These results are also consistent with the idea that long-term ABA treatment overrides light signalling to induce suppression of chloroplast development. However, in contrast to this notion, under low light intensity such as 10 μmol m−2 s−1, the long-term ABA treatment rarely induce suppression of chloroplast development (Figure 4b–e). Consistent with this, light intensity is critical for COP1-mediated degradation of GLK1 upon long-term ABA treatment (Figure 4j,k). Thus taken together, these results showed that ABA signalling and light intensity function cooperatively to suppress chloroplast development under the long-term ABA-treated condition.
What underlies the cooperative interaction between ABA and light intensity in long-term ABA-induced suppression of chloroplast development? In general, ABA and light have opposite effects on plant physiology; ABA generally suppresses plant growth and development, whereas light promotes plant growth and development (Jarvis & López-Juez, 2013; Tuteja, 2007; Vishwakarma et al., 2017; Xu et al., 2015; Zhu, 2016). A clue to this question may be found by the fact that light intensity is critical for long-term ABA-induced leaf yellowing. Indeed, proper intensity of light is crucial for light-induced growth and development of plants and photosynthesis in chloroplasts (Jarvis & López-Juez, 2013). At the same time, in chloroplasts, excess light energy is used to produce reactive oxygen species (ROS), which are toxic compounds that can damage cellular components (Chan et al., 2016; Szechynska-Hebda & Karpinski, 2013; Xu et al., 2015). However, ROS also plays a crucial role in coordination of nuclear gene expression with chloroplast situations (Chan et al., 2016; McCormac & Terry, 2004; Szechynska-Hebda & Karpinski, 2013; Tokumaru et al., 2017; Xu et al., 2015). ABA treatment also causes ROS production in chloroplasts (Iwai et al., 2019; Watkins et al., 2017). Indeed, H2O2, one of the types of ROS, generated in chloroplast by short-term ABA treatment induces stomatal closure, indicating that ABA-induced H2O2 produced in chloroplasts plays a critical role in physiology (Watkins et al., 2017). Thus, one possible scenario is that upon the long-term ABA treatment, light intensity and ABA cooperate to control the activity of COP1, thereby reducing GLK1 levels, contributing to suppression of chloroplast development. The outcome of the cooperation would be production of ROS such as H2O2 in chloroplasts. Consistent with this notion, long-term ABA treatment increased the level of H2O2. However, the same treatment did not increase the H2O2 level in cop1-4 plants. Also, the long-term ABA treatment did not increase H2O2 level in Col-0 plants when plants were treated with DMSO or ABA under the low light intensity (Figure S20). In fact, H2O2 is one of triggers inducing retrograde signals from chloroplasts to nucleus, thus being well positioned to play a role in regulation of COP1 activity in the nucleus (Chan et al., 2016). Indeed, a previous study showed that chloroplast-specific H2O2 production by overexpression of glycolate oxidase (GO) resulted in growth retardation, yellow rosette leaves and reduced rosette diameter when plants were grown at a light intensity above 75 μmol m−2 s−1 (Fahnenstich et al., 2008). However, these phenotypes and H2O2 accumulation were alleviated when the light intensity was reduced from 75 to 30 μmol m−2 s−1 (Fahnenstich et al., 2008). This is similar to our data showing that long-term ABA treatment does not cause significant leaf yellowing under 10 μmol m−2 s−1, compared with that under 70 or 120 μmol m−2 s−1. Thus, ABA and light intensity cooperatively act together to produce H2O2 above a certain level so as to suppress chloroplast development under the long-term ABA-treated condition. In this cooperation, light intensity should be higher than a certain intensity. In the future study, it would be worthwhile to investigate the effect of ABA-induced H2O2 in chloroplasts on COP1 activity and GLK1 level during long-term ABA treatments.
What are other regulators involved in suppression of chloroplast development by long-term ABA treatment? In fact, regulation of gene expression is one of main mechanisms in ABA responses (Vishwakarma et al., 2017). glk1glk2cop1-4 triple-mutant plants displayed yellow leaf phenotype under the normal growth condition or DMSO-treated condition. These triple mutant plants displayed more severe yellow leaf phenotype upon ABA treatment (Figure S13). However, under the low light-intensity condition, long-term ABA treatment did not cause GLK1 degradation but still caused a reduction in the expression of PhANGs compared to DMSO control although severity of the reduction was milder than that under the high light intensity condition (Figure 4d–k). These results suggest that a COP1-GLK1-independent pathway may play a role in the ABA-induced suppression of PhANGs under the low light-intensity condition. Therefore, it would be interesting to figure out a transcriptional regulator suppressing PhANGs in response to long-term ABA treatment under the low light-intensity condition.
4 EXPERIMENTAL PROCEDURES
4.1 Plant growth, ABA and NaCl treatments
Arabidopsis (Arabidopsis thaliana) seeds were sown on half-strength MS media (1/2 MS) plates containing 2 mM MES (pH 5.7) and 0.8% agar without sucrose. These plates were incubated at 4°C in the dark, transferred to a growth chamber and grown for additional 8 to 9 days. The growth chamber was set up with 70–80 μmol m−2 s−1 white light under a 16/8 h light/dark cycle at 22 ± 1°C during the day and 16 ± 4°C during the night.
To treat plants with ABA or high salt, plants were transferred to the same medium as described but supplemented with either 5 μM ABA or 160 mM NaCl and grown for additional periods under the same growth condition in a chamber. ABA treatment was started at 2:00 p.m., and plant samples were collected at 2:00 p.m. Unless indicated otherwise, plants were grown using 70–80 μmol m−2 s−1 white light. One square shown in pictures indicated 18 mm × 18 mm.
To treat plants with MG132, 35S::GFP-GLK1 (glk1glk2) plants that had been treated with DMSO or 5 μM ABA for 7 days were sprayed with DMSO or 100 μM MG132. Plant tissues were collected 9 h after incubation. One square shown in pictures indicated 18 mm x 18 mm, and a scale bar shown in Figure 5a,c indicated 10 mm.
4.2 RNA isolation and quantitative real-time PCR
Total RNA was isolated from plant tissues using the Qiagen RNeasy mini kit according to the manufacturer's instructions. Eluted RNA was treated with TURBO DNase (Invitrogen). 2 μg of total RNA were converted into single-stranded cDNA using the high-capacity cDNA reverse transcription kit (Applied Biosystems) (Wang et al., 2018). To perform qRT-PCR reactions, PowerUP SYBR Green Master Mix (Applied biosystems), cDNA and gene specific primers (Table S1) were mixed, and PCR was performed by applying a cycling protocol that included 15 s at 95°C and 60 s at 60°C for 40 cycles. ACT2 was used as an internal qRT-PCR control.
4.3 Measurement of chlorophyll contents
Chlorophyll contents were measured as described previously (Wang et al., 2018). To measure chlorophyll contents from plant shoots, chlorophyll was extracted using 95% ethanol (v/v) from plant shoots at 4°C overnight in the dark. Extracted chlorophyll was measured by optical density (OD) at 664 and 648 nm to determine contents of chlorophyll a and b, respectively. The formula 5.24 × OD664/20 was used to calculate chlorophyll a contents and 22.24 × OD648/20 for chlorophyll b contents.
4.4 Fractionation of nuclear and cytoplasmic proteins
Nuclear and cytoplasmic protein fractionation was carried out using CelLytic PN (Sigma) according to the manufacturer's instructions with some modifications, as previously described (Yu et al., 2013). In brief, 1× NIB was prepared by adding a final concentration of 50 μM MG132 and EDTA-free protease inhibitor cocktail (Roche). DMSO- or ABA-treated plants were ground in liquid nitrogen and mixed with 3 ml 1× NIB/g tissue. The mixture was filtered using a mesh, and the sample solution collected to 50 ml tubes was centrifuged at 1,260 × g in swing rotor for 10 min. The supernatant was collected as a cytoplasmic fraction. The pellet was resuspended in 500 μl 1× NIB, and 10% Triton X-100 was added to the solution to a final concentration of 0.3%. This sample was loaded onto 1.5 M sucrose cushion and centrifuged at 12,000 × g for 10 min. After discarding the supernatant, the pellet was washed twice with 1 ml of 1× NIB and spun down via centrifugation at 12,000 × g for 5 min. After discarding the supernatant, the pellet containing the nuclear portion of the extract was resuspended in protein extraction buffer. All procedures were performed at 4°C. Protein level of each fraction was analysed by Western blotting using anti-COP1 (Lee et al., 2017), anti-H3 (abcam, 1:1000) for nucleus fraction and anti-actin (1:1000, MP Biomedicals) for the cytoplasmic fraction.
4.5 Transient expression in Nicotiana benthamiana
Agrobacterium tumefaciens transformed with sGFP, sGFP-COP1, GLK1-HA, nVenus-GLK1-HA, cVenus-COP1-HA, nVenus- GST-HA, cVenus-GST-HA or p38 driven by the CsV promoter were cultured in the LB medium at 28°C and harvested via centrifugation at 6,000 × g at 4°C for 15 min (Md Islam et al., 2019). After discarding the supernatant, harvested cells were resuspended in tobacco infiltration buffer (10 mM MES-KOH, pH 5.6, 10 mM MgSO4) at a concentration that corresponded to an OD value of 0.8 at 600 nm. Resuspended cells were mixed with 200 μM acetosyringone and incubated for 2–3 h at room temperature. The cell suspension was infiltrated into N. benthamiana leaves using a 1 ml syringe without a needle. After infiltration, plants were further grown for appropriate period of time.
4.6 Co-immunoprecipitation
Nicotiana benthamiana leaves transformed with Agrobacterium expressing sGFP together with GLK1-HA or sGFP-COP1 together with GLK1-HA were harvested and frozen using liquid nitrogen. As control, untransformed N. benthamiana leaves were included. The harvested leaves were ground in liquid nitrogen, and ground powder was mixed with protein extraction buffer (50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 10% glycerol, 1.5% Triton X-100, EDTA-free protease inhibitor cocktail (Roche), 2 mM Na3OV4, 10 mM NaF, 50 μM MG132) (Lee et al., 2017). After the powder was homogenized with protein extraction buffer, the extract was centrifuged at 18,000 × g at 4°C for 10 min. The supernatant was collected as total protein extract. The total protein extract was incubated with GFP-trap agarose beads (Chromotek) at 4°C using shaking rotator (Keicher et al., 2017). The mixture was centrifuged at 18,000 × g at 4°C for 1 min, and the supernatant was discarded. Beads were washed with 1 ml of washing buffer (50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 10% glycerol, 0.1% Triton X-100, EDTA-free protease inhibitor cocktail (Roche), 2 mM Na3OV4, 10 mM NaF, 50 μM MG132) for three times. Captured proteins were eluted in 2.5× Laemmli sample buffer at room temperature for 20 min. Eluate was analysed by Western blotting using anti-GFP (Takara, 1:1000) or anti-HA (Roche, 1:1000) antibodies.
4.7 Cell-free protein ubiquitination assay
A cell-free protein ubiquitination assay was performed as described previously with some modifications (Xu et al., 2016). Arabidopsis plants that had been treated with DMSO or ABA were harvested and ground in liquid nitrogen, and the resultant powder was mixed with buffer (25 mM Tris–HCl, pH 7.5, 10 mM NaCl, 10 mM MgCl2, EDTA-free protease inhibitor cocktail (Roche), 10 mM ATP). The mixture was centrifuged at 18,000 × g at 4°C for 10 min (Xu et al., 2016). The supernatant was collected as total extract. Protein concentration of extracts was determined using the Bradford assay (Bio-rad Protein Assay Dye Reagent Concentrate). To examine ubiquitination, 6 × His-GLK1-HA was incubated with total extract in the presence of 250 μM MG132 at 22°C for 40 min, and 6 × His-GLK1-HA was captured using Ni2+-NTA agarose beads (Qiagen) at 4°C. The beads were washed with washing buffer, and proteins were eluted using Laemmli sample buffer by boiling for 3 min. The eluted proteins were analysed by Western blotting using the anti-ubiquitin antibody (Calbiochem, 1:1000) or anti-HA antibody (Roche, 1:1000). anti-tubulin (1:1000, Agrisera) antibodies were used as an internal control for total protein extract incubated with 6 × His-GLK1-HA.
4.8 Preparation of the total protein extract and Western blotting
Total protein extract was prepared as described previously with some modifications (Lee et al., 2017). Arabidopsis plant samples were ground in liquid nitrogen and homogenized using extraction buffer (50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 10% glycerol, 0.5% Triton X-100, EDTA-free protease inhibitor cocktail (Roche), 2 mM Na3OV4, 10 mM NaF, 50 μM MG132). The mixtures were centrifuged at 18,000 × g in 4°C for 10 min, and supernatants were collected as total soluble proteins. Concentration of total soluble proteins was measured using a Bradford assay (Protein Assay Dye Reagent Concentrate, Bio-rad). Equal amount of total soluble proteins was loaded on an sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) gel, and separated proteins were transferred to polyvinylidene difluoride (PVDF) membranes using semi-dry transfer (Hoefer). To detect GFP-tagged proteins, anti-GFP antibody (1:2000, Bioapp, Korea) was used, and anti-actin (1:1000, MP Biomedicals) or anti-tubulin (1:1000, Agrisera) antibodies were used as control for loading. Chemifluorescence was detected using an Amersham Imager 680 systems (GE healthcare).
4.9 Confocal microscopy for bimolecular fluorescence complementation and DAPI staining
BiFc experiment was performed as described previously with some modifications (Kudla & Bock, 2016; Lin et al., 2018). The N-terminal, 173 amino acid residue fragment (NV) and C-terminal, 83-residue fragment (CV) of Venus were inserted into a 326-sGFP-3G-2 vector by replacing sGFP to give NV and CV, respectively. COP1-HA, GLK1-HA or GST-HA were amplified by PCR and ligated into the 326-NV-3G-2 or 326-CV-3G-2 (by replacing sGFP into NV or CV as described above) vectors to produce NV-GLK1-HA, CV-COP1-HA, NV-GST-HA, and CV-GST-HA. Finally, these constructs from NV or CV to HA were amplified via PCR again and ligated into a binary vector pCsV1300 vector (Kudla & Bock, 2016; Lin et al., 2018). The resulting constructs were transformed into Agrobacterium for the transient expression in N. benthamiana via Agrobacterium-mediated transformation. Leaves infiltrated with Agrobacteria were harvested and soaked in PBS buffer containing 5 μg/μl DAPI for 10 min in the dark. The fluorescent images were taken using confocal laser scanning microscopy (Leica). After samples were observed, leaves were rapidly frozen in liquid nitrogen. Total proteins extracts were prepared using extraction buffer (50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 10% glycerol, 1.5% Triton X-100, EDTA-free protease inhibitor cocktail (Roche), 2 mM Na3OV4, 10 mM NaF, 50 μM MG132) and analysed by Western blotting using the anti-HA antibody.
ACKNOWLEDGEMENTS
We thank Nam-Chon Paek and Byong-Doo Lee at Seoul National University for providing cop1-4 seeds, Giltsu Choi at the Korea Advanced Institute of Science and Technology for providing pif1-2, pif3-3, pif4, pif1-2pif3-3, and pifQ seeds, Takehito Inaba at the University of Miyazaki for 35::GFP-GLK1/glk1glk2 seeds, and Jorge José Casal at Universidad de Buenos Aires for providing 35S::YFP-COP1/cop1-4. In addition, we thank Young-Hee Shin of the lab for taking care of plants used in experiments. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2019R1A2B5B03099982). Lee, J. was supported by a POSCO Science Fellowship from the POSCO TJ Park Foundation.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




