Contrasting anther glucose-6-phosphate dehydrogenase activities between two bean varieties suggest an important role in reproductive heat tolerance
Funding information: MSU Plant Resilience Institute
Abstract
Common beans (Phaseolus vulgaris) are highly sensitive to elevated temperatures, and rising global temperatures threaten bean production. Plants at the reproductive stage are especially susceptible to heat stress due to damage to male (anthers) and female (ovary) reproductive tissues, with anthers being more sensitive to heat. Heat damage promotes early tapetal cell degradation, and in beans this was shown to cause male infertility. In this study, we focus on understanding how changes in leaf carbon export in response to elevated temperature stress contribute to heat-induced infertility. We hypothesize that anther glucose-6-phosphate dehydrogenase (G6PDH) activity plays an important role at elevated temperature and promotes thermotolerance. To test this hypothesis, we compared heat-tolerant and susceptible common bean genotypes using a combination of phenotypic, biochemical, and physiological approaches. Our results identified changes in leaf sucrose export, anther sugar accumulation and G6PDH activity and anther H2O2 levels and antioxidant-related enzymes between genotypes at elevated temperature. Further, anther respiration rate was found to be lower at high temperature in both bean varieties. Overall, our results support the hypothesis that enhanced male reproductive heat tolerance involves changes in the anther oxidative pentose phosphate pathway, which supplies reductants to critical H2O2 scavenging enzymes.
1 INTRODUCTION
Carbohydrates are essential for plant development because they are used for various processes including energy generation, amino acid synthesis, production of plant defensive compounds and signalling. Plants produce carbohydrates through photosynthesis, where atmospheric CO2 is assimilated by leaves to produce carbon compounds such as starch and sucrose. During the day, starch is transiently stored in leaf chloroplasts for break down during the night into maltose, glucose and maltodextrins to support leaf metabolism, partitioning and plant growth (Schleucher, Vanderveer, & Sharkey, 1998; Weise, Weber, & Sharkey, 2004; Zeeman, Smith, & Smith, 2007).
During the light period, sucrose is allocated from photosynthetically active source leaves to sink tissues (i.e., anthers, pollen, fruits, seeds and roots) to sustain metabolism and development. During this process, sucrose moves symplasmically from the mesophyll cells towards the vasculature and, depending on plant species, moves to the phloem companion cell via a passive (symplastic), or active (apoplastic or symplastic, polymer trap mechanism), or some mixture of these phloem loading mechanisms (Ayre & Turgeon, 2018; Giaquinta, 1976; Rennie & Turgeon, 2009; Zhang & Turgeon, 2018). From the companion cell, sucrose finally diffuses to the adjacent phloem sieve element for transport to the sink tissue/organs. Sucrose phloem loading is an essential precursor for sink development and is highly regulated by sucrose levels, temperature and leaf nitrogen status and partitioning (Santiago & Tegeder, 2017; Vaughn, Harrington, & Bush, 2002; Xu, Chen, Yunjuan, Chen, & Liesche, 2018; Zhang et al., 2010). Consequently, reduced transport of metabolites from the leaves to sinks decreases the formation of fruits and seeds, thereby negatively affecting overall plant productivity (Hackel et al., 2006; Kühn et al., 1996; Slewinski, Meeley, & Braun, 2009; Santiago & Tegeder, 2017).
Within flowers, the anthers and pollen grains are the strongest sinks for carbon and nitrogen metabolites (Castro & Clément, 2007; Lee & Tegeder, 2004; Schneidereit, Scholz-Stark, & Buttner, 2003; Yuan et al., 2008). Sucrose arriving in the anthers via the phloem is cleaved into glucose and fructose that are either stored in the vacuole or used for cytosol and plastid-localized metabolism (De Storme & Geelen, 2014). Anther cells adjacent to the vascular bundle (connective tissue, endothecium, middle layer) also accumulate and store carbohydrates, most likely as a reserve to support anther and pollen metabolism (Clément & Audran, 1995; Clément, Chavant, Burrus, & Audran, 1994; Saini, 1997; Saini & Lalonde, 1998). From the vasculature, sugars and other nutrients move to and from the anther connective tissue towards the tapetum cells, which represents the last symplastic step for movement of assimilates to developing pollen grains. The tapetum, therefore, performs a critical function in supplying the developing microspores with sugars and other nutrients (De Storme & Geelen, 2014; Pacini, Franchi, & Hesse, 1985).
Heat stress causes a wide range of damage to plants, especially to reproductive tissues, with the male gametogenesis being more sensitive than the female gametogenesis (Djanaguiraman et al., 2018; Jiang et al., 2019; Sage et al., 2015). In anthers, elevated temperature causes reduced accumulation of soluble carbohydrates and is associated with the decline in pollen development and viability (Firon et al., 2006; Oshino et al., 2007; Pressman, Peet, & Pharr, 2002; Saini, Sedgley, & Aspinall, 1984; Sakata, Takahashi, Nishiyama, & Higashitani, 2000; Zinn, Tunc-Ozdemir, & Harper, 2010). Common bean is particularly sensitive to elevated temperature, exhibiting early tapetum degradation, pollen deformity, anther indehiscence, and reduced yield (Porch & Jahn, 2001; Suzuki, Takeda, Tsukaguchi, & Egawa, 2001). These phenotypes are also seen in situations where there is (a) reduced allocation of photoassimilates from the leaves to the sink tissues at elevated temperature (Aloni, Pashkar, & Karni, 1991; Basu & Minhas, 1991; Dinar & Rudich, 1985; Soltani et al., 2019), (b) altered carbon metabolism in anthers (Firon et al., 2006; Kaushal et al., 2013; Pressman et al., 2002) and (c) changes in anther reactive oxygen species (ROS) levels and quenching (Du et al., 2019; Yi et al., 2016; Zhao et al., 2018). Although these three changes could explain the reduced fertility at elevated temperature, it is not entirely clear how these mechanisms are related to each other.
In this study, we hypothesized that carbon metabolism in anthers via the oxidative pentose phosphate pathway (OPPP) helps maintain the redox balance in anthers at elevated temperature. Through the OPPP, phosphorylated glucose is converted to 6-phosphoglucunolactone mediated by the enzyme glucose-6-phosphate dehydrogenase (G6PDH) and produces reductant (NADPH) as a byproduct. A few studies have shown the importance of G6PDH in plant cells during biotic and abiotic stress conditions (Gong et al., 2012; Nemoto & Sasakuma, 2000; Scharte, Schön, Tjaden, Weis, & von Schaewen, 2009). However, there is limited information on the involvement of G6PDH in heat tolerance specifically in anthers. Further, knowledge on the regulation of enzyme activity by elevated temperature is limited. We address this hypothesis through a comparative analysis between a heat-tolerant and heat-sensitive common bean genotype. Through a combination of physiological, biochemical and molecular analyses, we found that increased G6PDH activity and continued sucrose delivery to anthers are important contributing factors to heat tolerance in common beans. Further, our data suggest a shift in carbon partitioning between glycolysis and the OPPP in heat-tolerant bean genotype which could support H2O2 removal in anther cells.
2 MATERIALS AND METHODS
2.1 Plant material, growth conditions and tissue collection
Two Phaseolus vulgaris L. cultivars, Red Hawk and Sacramento, were used in this study. Our previous research on these cultivars found that Red Hawk is heat-sensitive, while Sacramento is heat-tolerant (Soltani et al., 2019) but are otherwise very similar. Both cultivars belong to the kidney bean market class from the Andean gene pool indicating relative genetic similarity. For a comparative analysis of reproductive function and metabolism between Red Hawk and Sacramento genotypes, seeds were planted in Sure-Mix potting soil (Michigan Grower Products, Inc., Galesburg, MI) in three-gallon plastic nursery pots (Nursery Suppliers, Inc., Chambersburg, PA) in a completely randomized design. The plants were grown in a greenhouse with average day/night temperature of 27/20°C and fertilized twice per week with ½-strength Hoagland's solution starting at V1 growth stage. Plants subjected to heat stress were transferred to a greenhouse with an average day/night temperature of 34/27°C about 25 days after sowing (DAS), upon appearance of the first flower buds. The control plants remained in the original greenhouse with a day/night temperature of 27/20°C. During the treatment, heat-treated and control plants were watered daily to eliminate the effect of water limitation on reproductive development. Five days after the plants were subjected to heat treatment, a source leaf from the fourth node from the base of the plant was collected from control and heat-treated plants and immediately frozen in liquid nitrogen. The leaf harvest was performed within a window of 5 hr during the middle of the light period. Flowers at 5 days before anthesis were collected daily from both control and heat-treated plants and flash frozen in liquid nitrogen. The number of biological replicates for each experiment is indicated in the figure legends.
2.2 Collection of leaf exudates
After 5 days of exposure to elevated temperature, source leaf exudates were collected from heat stressed and control plants according to Zhang, Garneau, Majumdar, Grant, and Tegeder (2015) with some modifications. Briefly, one trifoliate source leaf with at least 4 cm of petiole was cut from plants in water containing 20 mM Na-EDTA to chelate calcium and prevent occlusion of phloem sieve tubes (Knoblauch, Peters, Ehlers, & van Bel, 2001). The leaves were immediately covered with a wet paper towel and plastic wrap to prevent transpiration and the petiole was re-cut while submerged in 20 mM Na-EDTA. The leaves were then allowed to sit in the EDTA solution for 5 min before the petioles were inserted into a 1.5 ml microcentrifuge tube with 600 μl of 20 mM Na-EDTA. The leaves were allowed to bleed in a box with high humidity for 1 hr. After bleeding, the leaves were carefully removed and 50 μl of 1 N HCl was added to the exudates, which were then vortexed, and centrifuged at 4°C and 15,000 x g for 30 min. A 200 μl aliquot was transferred to a new tube and vacuum dried (Savant Instruments, Hyderabad, India). The residue was then re-dissolved in 20 μl milliQ water and used for sucrose analysis.
2.3 Transport studies with 14CO2
To test if sucrose phloem loading and allocation to anthers are affected by elevated temperature and if differences exist between bean cultivars, a radioactive tracer experiment was performed. Gaseous 14CO2 was prepared from a reaction between 1 M acetic acid and a sodium bicarbonate (NaH[14C]O3) solution. The radioactive 14CO2 was first pulled into a previously evacuated stainless-steel tank. The tank was then pressurized with 5% 12CO2 in air. The final specific activity was ~1 Ci/mol. On the day of the experiment, a fully expanded source leaf was clamped into the fluorescence head attachment of an LI-COR 6800 portable photosynthesis system (LI-COR, Lincoln, NE) and fed with 40 Pa unlabelled CO2 until stomatal conductance and net photosynthetic rate stabilized under the following conditions: 1000 μmol m−2 s−1 light, 500 μmol s−1 air flow rate, 25°C Tleaf, 1 kPa leaf to ambient air water vapour pressure difference (VPD). Once stabilized, 14CO2 was fed to leaves by simultaneously turning on the tank with 14CO2 connected to the LI-COR 6800 and turning off the machine's 12CO2 mixer. The leaf was then fed with radioactive CO2 for 10 min for tracer analysis. Flowers on the same branch as the leaf that was fed with 14CO2 were harvested after 24 hr. The anthers were subsequently collected from these harvested flowers. Thirty anthers per plant were treated with 10 ml of Safety-Solve Complete Counting Cocktail (Research Products International, Mount Prospect, IL) and then the radioactivity was measured by Perkin Elmer Tri-Carb scintillation counter (Waltham, MA). These data are presented as disintegrations per minute (DPM).
2.4 Anther respiration measurement
The LI-COR 6800 was fitted with an adapter (LI-COR part # 6800-19) with the insect respiration chamber attached (LI-COR part # 6800-89) prior to respiration measurements. Flowers that developed under control and heat stress conditions were collected from plants at 5 days before anthesis and the anthers were carefully excised and weighed. After weighing, the anthers were transferred to a strip of moist paper towel and then inserted into the respiration chamber. The respiration chamber was exposed to 1,000 μmol m−2 s−1 white LED light during the measurement. The flow rate, chamber temperature, VPD and relative humidity were maintained as described above (see Section 2.3).
2.5 Pollen count and viability
Flowers that developed at control or elevated temperature were collected and anthers were excised. Nine anthers, each from an individual flower, were placed on a microscope slide with a drop of Lugol's solution and squished with a cover slip. Once the pollen grains were released from the anthers, the glass slide was placed under a stereomicroscope (Zeiss SteREO Discovery.V8, Karl Zeiss Microscopy, GmBH, Jena, Germany) and pollen count was performed.
Pollen viability was assayed by collecting flowers and anthers as described above. However, instead of using Lugol's solution, the anthers and pollen grains were stained with 1% triphenyl tetrazolium and then incubated for 1 hr in 30°C (Prasad, Boote, & Thomas, 2002). Viable pollen grains appeared as reddish-purple colour due to the formation of insoluble red formazan.
2.6 Carbohydrate extraction and analysis
Sucrose, glucose and starch were extracted following the method of Lu, Steichen, Weise, and Sharkey (2006). Briefly, frozen flower and anther tissues were ground to a fine powder and lyophilized. This lyophilized tissue was then weighed and placed in a microcentrifuge tube. Soluble carbohydrates were then extracted by adding 3.5% perchloric acid at 2 μl per mg tissue. The solution was homogenized by vortexing and allowed to sit on ice for 5 min. After incubation, samples were centrifuged at 18,000× g for 10 min at 4°C. The supernatant was transferred to a new microcentrifuge tube, while the remaining pellet was further extracted for starch. Neutralizing buffer (2 M KOH, 150 mM Hepes, 10 mM KCl) was added at ¼ the volume of the supernatant to bring up the pH to approximately pH 7 and mixed by vortexing. The mixture was then frozen in liquid nitrogen and thawed twice to precipitate the K-perchlorate, centrifuged at 18,000× g for 10 min at 4°C, and then the supernatant was transferred to a fresh 1.5 ml tube for soluble sugar analysis. Carbohydrate concentrations were determined using NADP(H)-linked assays, as described by Lu and Sharkey (2004).
2.7 Hydrogen peroxide measurement
Anther tissues were collected, as described above and immediately frozen in liquid N2. A pool of anthers from 25 flowers represents each biological replicate. The anthers showed no significant difference in weight (Figure S1). The frozen tissues were transferred to 2 ml microfuge tubes with stainless steel beads and then ground using a tissue homogenizer. Hydrogen peroxide was extracted with 500 μl of 5% trichloroacetic acid in 50 mM sodium phosphate buffer (pH 7.4) and then vortexed. For hydrogen peroxide extraction and measurement with Amplex-Red reagent, a buffer of pH 7.4 was used as recommended (Chakraborty et al., 2016). The samples were then centrifuged at 14,000 x g and the supernatant was transferred to a new 1.5 ml tube. Hydrogen peroxide was quantified in 40 μl of tissue extract using the Amplex Red Hydrogen Peroxide/Peroxidase Assay Kit (ThermoFisher, Waltham, MA). The Amplex Red reagent reacts with H2O2 in a 1:1 stoichiometry producing a red-fluorescent oxidation product called resorufin in the presence of peroxidase (Zhou, Diwu, Panchuk-Voloshina, & Haugland, 1997). This kit had been shown to be sensitive enough as it was able to measure as little as 10 picomoles of H2O2 in a 100 μl sample volume. Absorbance was measured at 560 nm and H2O2 concentration was calculated using the molar absorptivity of resorufin (58,000 M−1 cm−1).
2.8 RNA extraction and expression analyses of PvSUT1.1
RNA-seq on leaf tissue was previously performed by Soltani et al. (Soltani et al., 2019; Figure S1). To verify the RNA-seq result on PvSUT1.1 expression, quantitative real time PCR (qRT-PCR) was carried out using an Eppendorf Realplex2 Mastercycler (Eppendorf North America, Hauppauge, NY). Total RNA was extracted from leaves that were collected and immediately frozen in liquid nitrogen and ground into fine powder. Approximately 200 μl equivalent of frozen powder was aliquoted in a nuclease-free 1.5 ml microcentrifuge tube. Total RNA was extracted using E.Z.N.A. Plant RNA Kit (Omega Bio-Tek, Norcross, GA) and cDNA was synthesized using oligo(dT) primers and Moloney murine leukemia virus reverse transcriptase (Invitrogen, Waltham, MA; Santiago & Tegeder, 2016). A subsequent polymerase chain reaction was performed for 24 cycles. At least three biological replicates, consisting of 1:10 diluted cDNA, were used per run. Each reaction mix consisted of 1X Platinum Taq DNA polymerase buffer (Invitrogen, Waltham, MA), 200 μM deoxynucleotide diphosphate (Fisher Bio-Reagents, Waltham, MA), 2.5 mM MgCl2 (Invitrogen), 0.25X SYBR Green I (Thermo Fisher Scientific, Waltham, MA), 50 nM ROX reference dye (Thermo Fisher Scientific, Waltham, MA), 0.5 U of Platinum Taq DNA Polymerase (Invitrogen, Waltham, MA), and 0.25 μM primers. The Phaseolus vulgaris ACTIN11 (PvACT11; Pélissier & Tegeder, 2007) gene was used as a control to determine the relative expression of PvSUT1.1.
2.9 Enzyme extraction and activity assays
Crude enzyme extracts were obtained from isolated anthers derived from flowers that had developed in control or elevated temperature conditions. Up to five biological replicates were made by collecting pools of anthers from 10 flowers at 5 days before anthesis stage. At this stage, the flower bud petals were still unopened and light green in colour. The anthers were extracted on ice with 400 μl of phosphate buffer (50 mM sodium phosphate buffer [Na2HPO4 + NaH2PO4] at pH 7.0, 1% polyvinylpolypyrrolidone) and macerated with a micropestle. The homogenized mixture was then centrifuged at 14,000× g for 5 min at 4°C and the supernatant transferred to a fresh 1.5 ml tube. The anther extract was then used immediately for the enzyme assay. Catalase activity was measured colorimetrically using 50 μl of enzyme extract and a catalase assay kit (Megazyme, County Wicklow, Ireland). For this kit, catalase activity was determined by measuring the decrease in H2O2 concentration in a two-step reaction. The first reaction involves incubation of the sample with a known concentration of H2O2 for 5 min at 25°C. The reaction is then stopped by addition of 15 mM sodium azide. The samples are then assayed for the formation of quinoneimine at 520 nm in a second reaction involving an enzyme-linked decomposition of H2O2 with 3,5-dichloro-2-hydroxy-benzenesulfonic acid, 4-aminoantipyrine and peroxidase. Glutathione reductase (GR) activity was measured following Cakmak and Marshner (1992). Briefly, 50 μl of enzyme extract was mixed with 25 mM EPPS buffer (pH 7.8), 0.5 mM oxidized glutathione, and 0.12 mM NADPH (dissolved in 100 mM carbonate buffer, pH 10.6) in a total volume of 200 μl. NADPH oxidation was measured by the decrease in absorbance at 340 nm over a period of 20 min using a 96-well plate reader (Molecular Devices Filter Max 5, San Jose, CA). The activity of GR was normalized by the total protein content of the anther extract. G6PDH activity was measured according to Preiser, Fisher, Banerjee, and Sharkey (2019) with some modifications. The reaction mixture included 50 mM Tris HCl (pH 7.4), 5 mM MgCl2, 0.5 mM glucose 6-phosphate, 0.5 mM NADP, 10 mM DTT and 50 μl anther enzyme extract to a total volume of 1 ml. For this assay, an increase in absorbance due to NADPH production was measured over time using a spectrophotometer (Thermo Scientific GENESYS™ 10S UV–Vis, Waltham, MA). G6PDH activity was normalized by the total protein content of the anther extract. For measurement of glutathione peroxidase (GPX), ascorbate peroxidase (APX) and dehydroascorbate reductase (DHAR) activities, spectrophotometric enzyme assays were performed according to Noctor, Mhamdi, and Foyer (2016).
2.10 Statistical analysis
Graphs in each figure represent results from one plant growth set. Each experiment was repeated using tissues from an independently grown set of plants to ensure repeatability of the results. Each set of plants had biological replicates per genotype in each temperature condition. Data are presented as mean ± SEM. To determine the differences between population means, two-way ANOVA and Tukey HSD post hoc tests were performed using SigmaPlot version 14.0 (Systat Software, Inc., San Jose, CA).
3 RESULTS
3.1 Red Hawk produced fewer pollen grains than Sacramento at elevated temperature
Under control conditions, heat-tolerant (Sacramento) and heat-sensitive (Red Hawk) common beans appear to have the same amount of pollen produced and the anthers were able to release the pollen grains (Figure 1a). When these plants were subjected to heat treatment, Sacramento anthers appeared to produce similar amounts of pollen as anthers in control conditions (top, right panel; Figure 1a), whereas Red Hawk produced very few pollen grains (bottom, right panel; Figure 1a). Quantification of pollen grains show both Sacramento and Red Hawk produced similar amounts of pollen grains under at control temperatures. When exposed to heat stress, both genotypes exhibited a reduction in pollen production (Figure 1b). However, under heat stress Sacramento produced over twice as many pollen grains as did Red Hawk. Viability of the pollen grains that were produced under heat stress conditions was reduced to a similar degree in both Sacramento and Red Hawk (Figure 1c).
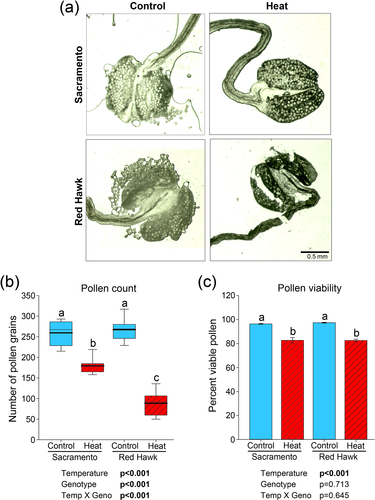
3.2 H2O2 accumulates in heat-sensitive bean anthers but not in the tolerant variety
To test if differences in anther ROS exists between bean genotypes, hydrogen peroxide content of anthers collected from heat-tolerant Sacramento and heat-sensitive Red Hawk was measured. Under the control temperature treatment, Red Hawk had a lower H2O2 content than Sacramento (Figure 2a). However, when subjected to heat stress, H2O2 content increased in Red Hawk to the levels observed in Sacramento under control conditions. For Sacramento, no change in ROS content was observed within the genotype when exposed to elevated temperature. As H2O2 can be degraded enzymatically, we next investigated anther catalase activity. In the control treatment, Sacramento had higher catalase activity compared with Red Hawk, even though anther hydrogen peroxide levels were slightly elevated (Figure 2b). Catalase activity responded differentially across the treatments for each cultivar, with it increasing in Sacramento but decreasing in Red Hawk in response to elevated temperature. As a result, Sacramento had a much higher catalase activity than Red Hawk in the heat treatment. Together, these data support the idea that Sacramento anthers are more heat-tolerant than Red Hawk. Further, these data suggest that Sacramento anthers are more responsive to increases in temperature than Red Hawk.
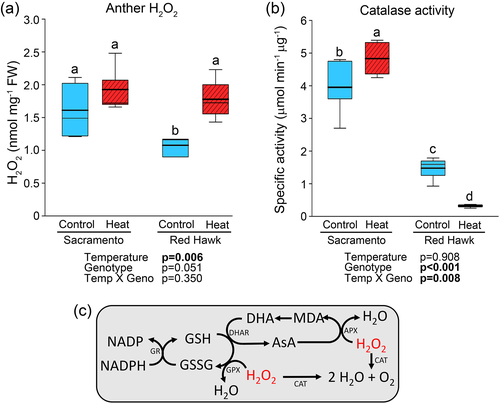
3.3 Effects of heat on enzymes related to ascorbate regulation of H2O2
In addition to catalase, H2O2 can also be removed through the glutathione and ascorbate systems (Figure 2c). The glutathione system depends on GPX, GR and NADPH (from G6PDH activity) while the ascorbate system depends on APX and DHAR activities. To determine if APX was involved in H2O2 quenching at elevated temperature in bean anthers, enzyme activity assays were performed. At control conditions, APX activity was significantly higher in Red Hawk than in Sacramento (Figure 3a). At high temperature, APX activity was increased in Sacramento but there was no change in Red Hawk. Further analysis of dehydroascorbate reductase (DHAR) activity showed similar activities between genotypes at control conditions (Figure 3b). At elevated temperature, enzyme activity was increased in both bean cultivars compared with controls but no significant difference was observed between genotypes.
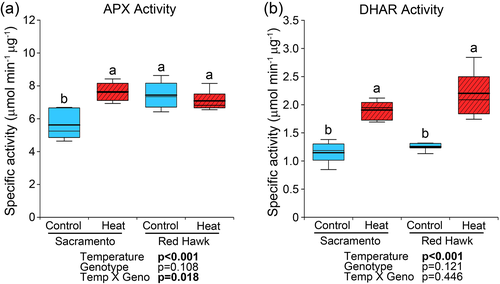
3.4 Effects of heat on enzymes related to glutathione regulation of H2O2
Antioxidant enzymes require reductants from the OPPP, which is limited by the enzyme G6PDH. The activity of anther G6PDH was measured at control and high temperature conditions. Under control conditions, between the genotypes, the heat-sensitive Red Hawk had higher G6PDH activity than Sacramento (Figure 4a). In the elevated temperature treatment, G6PDH activity increased in Sacramento (about 78%) while it decreased in Red Hawk by about 37%. This resulted in Sacramento having higher G6PDH activity than Red Hawk at elevated temperature.
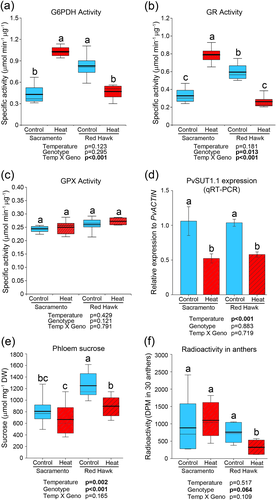
The NADPH produced by G6PDH is used by the glutathione reductase (GR) to cycle glutathione from the oxidized to the reduced form. Patterns of GR activity mirrored those of G6PDH activity (Figure 4b). Red Hawk had higher GR activity than Sacramento in the control treatment. However, Sacramento GR activity greatly increased and Red Hawk GR activity greatly decreased in the heat treatment (Figure 4b). As a result, Sacramento had higher GR activity than Red Hawk at elevated temperature. As GPX is connected to the glutathione cycle, we next measured GPX activity (Figure 4c), which showed no significant differences within and between genotypes and temperature conditions.
3.5 Leaf carbohydrate export correlates with heat sensitivity
The expression of a sucrose transporter gene hypothesized to be involved in phloem loading of sucrose (Santiago, Ward, & Sharkey, 2020; Soltani et al., 2019) was measured by qRT-PCR in both heat-sensitive and heat-tolerant genotypes. The expression of PvSUT1.1 was significantly reduced at high temperature in both bean varieties (Figure 4d; Figure S1). Interestingly, the expression of other characterized (PvSUF1/PvSUT1.4 and PvSUT1/PvSUT1.5) and putative sucrose transporters were not changed in both heat-tolerant Sacramento and heat-sensitive Red Hawk (Table S1). Leaf-exudate sucrose level was significantly different between genotypes at control conditions where the heat-sensitive Red Hawk's leaf exudate had higher sucrose content than Sacramento (Figure 4e). At high temperature, Sacramento did not show significant change versus control but a significant reduction of sucrose was detected in Red Hawk. Leaf-to-anther transport of carbon was assessed by 14CO2 feeding. The two bean genotypes showed no significant difference in radioactivity at control conditions (Figure 4f). When plants were exposed to elevated temperature, no change in radioactivity was observed in Sacramento while a significant reduction was seen in Red Hawk.
3.6 Sucrose accumulation is altered in flowers and anthers of heat-treated plants
To determine the effect of reduced carbon export on sink soluble sugar levels under heat stress, sucrose and glucose were measured in floral tissues of both bean genotypes (Figure 5). In whole flowers, the temperature treatment did not affect sucrose levels in Sacramento, but a significant reduction was observed in Red Hawk (Figure 5a). As pollen grains develop inside the anther tissue, the amount of sucrose was also measured in anthers. In control conditions, Red Hawk had a higher sucrose content (Figure 5b). After exposure to elevated temperatures, no significant change was observed in Sacramento, while a significant reduction in sucrose level was found in heat-treated Red Hawk plants. This resulted in Sacramento having higher anther sucrose levels than Red Hawk in the elevated temperature treatment. Since sucrose is cleaved into glucose and fructose in sink tissues, we also quantified anther glucose content. Similar to sucrose levels, Red Hawk had higher glucose content than Sacramento at control conditions. When heat-treated, anther glucose was increased in Sacramento, while in Red Hawk anther glucose declined (Figure 5c). Sacramento had higher glucose content than Red Hawk in the elevated temperature treatment.
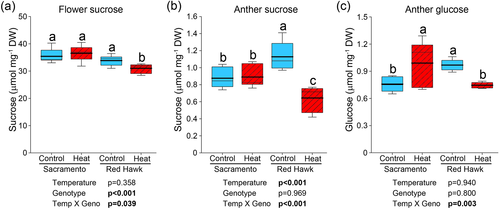
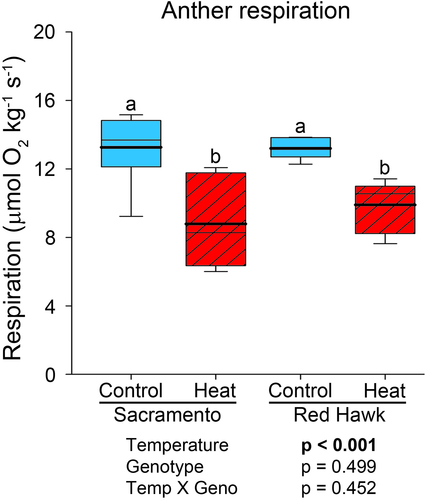
3.7 Anther cellular respiration rate is reduced in both common bean genotypes when subjected to high temperature stress
Genotype- and temperature-specific changes in free glucose accumulation in anthers suggest differences in its use during heat stress. Two parallel pathways that glucose is channelled to are the OPPP and glycolysis (and subsequent cellular respiration). We hypothesize competing allocation of glucose between these pathways. To test for changes in cellular respiration in both bean varieties, anther respiration rate was measured (Figure 6). At control conditions, respiration rate was similar between bean genotypes. After exposure to high temperature, the respiration rates in both genotypes were reduced. However, there was no significant difference between genotypes within high temperature treatment.
4 DISCUSSION
Heat stress is one of the biggest problems in crop production today since it can drastically reduce plant yield. While many studies reported the effect of heat stress in crop plants including tomato (Solanum lycopersicum; Pressman et al., 2002), rice (Oryza sativa; Jagadish, Craufurd, & Wheeler, 2007) and pepper (Capsicum annuum; Aloni et al., 1991), there is limited information on the effects of high temperature on anther metabolism, pollen formation and heat resilience of male reproductive tissues in common beans. We hypothesized that carbon metabolism in anthers via the OPPP enzyme G6PDH helps maintain the ROS balance in common bean anthers at elevated temperature, and thus contributes to heat resilience. This study provided a new understanding of the role of anther-localized G6PDH in contributing to maintaining H2O2 balance, male reproductive development and heat tolerance in common beans. The common bean varieties used in this study, Sacramento and Red Hawk, were previously described as heat-tolerant and sensitive, respectively, by Soltani et al. (2019). Leaf physiological and biochemical analyses revealed that the two genotypes are physiologically very similar at the flowering stage under control conditions. Specifically, analysis of stomatal conductance, cellular respiration, internal CO2 concentration, total chlorophyll content, leaf area, leaf mass, leaf sucrose, hexose and starch content show no significant differences between the two genotypes at the flowering stage under control temperature (Soltani et al., 2019). Some of the striking changes that occur in anthers exposed to heat stress is a reduction in anther carbohydrates (Begcy et al., 2019; Pressman et al., 2002), premature degradation of the tapetum cell layer (Suzuki et al., 2001), and deformed pollen grains (Djanaguiraman et al., 2018; Porch & Jahn, 2001). Many studies have investigated each phenomenon individually, but how these events are connected is unclear.
In male reproductive tissues at normal temperature, a well-timed ROS accumulation is beneficial for tapetum degradation and pollen viability (Yi et al., 2016). During heat stress, however, ROS accumulation in anthers during early development most likely contributes to the accelerated onset of tapetum degeneration and loss of pollen formation and viability (Liu, Shi, Li, Zhang, & Song, 2018; Porch & Jahn, 2001; Saini et al., 1984; Sakata et al., 2000; Suzuki et al., 2001).
4.1 Hydrogen peroxide is scavenged via multiple pathways in common bean anthers
When H2O2 accumulation exceeds a plant cell's capacity to scavenge the ROS, the cells experience oxidative damage and initiate programmed cell death (Gill & Tuteja, 2010; Vacca et al., 2004). This has been demonstrated in cultured tobacco BY2 cells, where high temperatures increased levels of H2O2 and superoxide anions, which in turn induced programmed cell death (Vacca et al., 2004). There are multiple mechanisms by which H2O2 is scavenged to prevent cellular damage and programmed cell death. One of these pathways is through the enzyme catalase, where its overexpression was found to confer heat tolerance in plants (Chiang et al., 2014). This finding suggests that the ability to scavenge H2O2 via catalase during high temperature stress is a marker of a plant's heat tolerance capacity. This idea is further supported by our results, which found increased anther catalase activity in heat-tolerant Sacramento and reduced activity in heat-sensitive Red Hawk (Figure 2b).
Another way to scavenge H2O2 is through ascorbate and glutathione. These two antioxidants were traditionally considered to function in an integrated fashion, but recent work on Arabidopsis revealed that they have independent and specific functions in plants (see Foyer & Noctor, 2011 and references therein). As part of the ascorbate pathway, APX and DHAR enzyme activities were usually measured in response to various abiotic stresses (Badawi et al., 2004; Kaushal et al., 2011; Sairam, Srivastava, & Saxena, 2000). For heat stress, leaf APX activity was found to be lower in a heat-sensitive wheat (Triticum aestivum) genotype than the tolerant genotype (Sairam et al., 2000). As APX is considered a primary enzyme involved in H2O2 removal (Noctor & Foyer, 1998; Smirnoff, 2000), lower APX activity suggests heat-sensitive plants have lower ROS scavenging capacity. This, however, is not supported by our anther APX activity data in heat-sensitive Red-Hawk, and it is unclear why this is the case in Red-Hawk anthers. Conversely, plants with high APX activity were regarded as having higher ROS scavenging capability and stress tolerance, which agrees with our data for the heat-tolerant Sacramento bean variety. Between Sacramento and Red Hawk, no significant difference in APX activity was observed indicating that APX activity is not an important determinant of thermotolerance in anthers via H2O2 scavenging, as it is in leaves. This is further supported by the total ascorbate measured in anthers, where no significant difference was observed between temperature treatments within each common bean genotype (Figure S2).
The reduced (GSH) and oxidized (GSSG) forms of glutathione are interconverted by the GR enzyme. Like ascorbate, glutathione is an important antioxidant when plants experience stress, and the activity of the GR enzyme is a signature of oxidative stress resilience (Aono, Kubo, Saji, Tanaka, & Kondo, 1993; Foyer et al., 1995; Shu, Wang, Duan, Deng, & Meng, 2011). Consistent with previous reports that higher or lower GR activity is related to oxidative stress tolerance, we found higher GR activity in the more heat tolerant Sacramento cultivar in the elevated temperature treatment (Figure 4b). Interestingly, heat-tolerant Sacramento had lower GR activity at control temperature (Figure 4b). Based on this, it can be assumed that the heat-sensitive Red Hawk may always metabolically be on guard to stress by allocating higher resources (Figures 4e and 5b,c) to anthers at unstressed conditions. This is further supported by the fact that GR is involved in redox balance in plant cells (Foyer et al., 1995; Rao & Reddy, 2008).
In plants, GSH, rather than GSSG, is what is responsible for ROS balance and other biological processes including protection from effects of various environmental stress, and regulation of stress defense gene expression (Hasanuzzaman, Nahar, Anee, & Fujita, 2017; Wingate, Lawton, & Lamb, 1988). Ultimately, a high GSH:GSSG ratio prevents the formation of mixed disulphides, and is essential for maintaining glutathione as an antioxidant (Foyer, Lopez-Delgado, Dat, & Scott, 1997; Szalai, Kellős, Galiba, & Kocsy, 2009). GR activity is important for maintaining the cellular GSH pool and is an important determinant of GSH pool size. Therefore, it is likely that heat-tolerant bean anthers with high GR activity, like those of the Sacramento cultivar, also have a high GSH content that helps protect the developing tissue during heat stress. In contrast, the reduced GR activity at high temperature in Red Hawk implies that its anthers do not have enough GSH to protect them from heat stress. Regardless of environmental condition, antioxidant capacity is linked with carbon metabolism because GR activity is dependent on NADPH produced by the OPPP via the G6PDH enzyme. This relationship is especially crucial in sink tissues such as heat-sensitive anthers that receive carbon from source tissues like leaves. This idea is supported by studies that have found that heat-tolerant plants are able to export sucrose out of leaves for delivery to sinks, while heat-sensitive plants have reduced sucrose export at high temperatures (Basu & Minhas, 1991; Dinar & Rudich, 1985). Our results are consistent with these patterns, as the thermotolerance status of bean genotypes was correlated with their sucrose export capacity (Figure 4e,f). Taken together, our results indicate that thermotolerance of common anthers can be traced back to catalase and glutathione-dependent scavenging of ROS, and that the ascorbate pathway seems to play a minor role. The importance of glutathione in anther ROS levels, and by extension thermotolerance, appears to be connected with the supply of sugars from leaves and sugar metabolism in anthers, specifically through the OPPP.
4.2 Anther G6PDH activity at high temperature contributes to heat stress tolerance in heat-tolerant bean genotype
To date, a few carbon metabolism enzymes have already been shown to be involved in heat stress tolerance including invertase (Jiang et al., 2020; Rezaul et al., 2019; Sato et al., 2006), fructokinase and hexokinase (Karni & Aloni, 2002), sucrose-phosphate synthase and sucrose synthase (Kaushal et al., 2013), and glyceraldehyde-3-phosphate dehydrogenase (Kim, Guo, & Wang, 2020). In addition to these enzymes, we found that G6PDH contributes to heat tolerance in bean anthers. G6PDH catalyses the rate-limiting reaction in the OPPP and was previously shown to be involved in abiotic stress tolerance attributed to maintenance of H2O2 homeostasis as a result of increased reductase activity and ascorbate content (Cardi et al., 2015; Gong et al., 2012; Landi et al., 2016; Liu, Wang, Hu, Hu, & Bi, 2013). However, previous studies on G6PDH and stress tolerance were focused on the activity of the enzyme in leaves, roots, or callus tissue under drought and salt stresses. To the best of our knowledge, the role of G6PDH in anther resilience at high temperature is yet to be examined.
In general, G6PDH activity is regulated by substrate (G6P) availability (Hauschild & von Schaewen, 2003) and redox (Preiser et al., 2019). In our study, we found genotypic differences in G6PDH activity, even in the control treatment (Figure 4a). We also found that this is not due to substrate limitation since G6PDH activity was assayed in vitro with the same substrate (G6P) concentration in the reaction mixture for all samples (Figure 4a; see Materials and Methods). For potential differences in redox state of G6PDH between genotypes at high temperature, DTT was added to the assay reaction to fully reduce all enzyme extracts (see Materials and Methods). These conditions indicate that the response was seen in this study (Figure 4) is not due to differences in the substrate availability or redox state of the enzyme. Together, this means that the differences in G6PDH activity are due to differences in enzyme abundance between bean genotypes at elevated temperature. However, further studies will be needed determine whether the difference between the genotypes is due to differences in Km or Vmax. Our conclusion that G6PDH enzyme abundance might differ between genotypes is supported by a recent pollen proteomics study that revealed that high temperature negatively affects the protein synthesis and degradation machinery in plants (Jegadeesan et al., 2018). Based on that proteomics study by Jegadeesan et al. (2018), we hypothesized that the difference in G6PDH enzyme abundance is due to inherent differences in anther protein turnover between Sacramento and Red Hawk.
In addition to G6PDH, studies on other carbohydrate metabolism enzymes were reported to play a role in heat tolerance in plants and in pollen fertility. For example, repression of anther-localized cell wall invertase (CWIN; Goetz, Godt, Guivarc'h, Chriqui, & Roitsch, 2001; Sato et al., 2006) and sucrose-phosphate synthase (Kaushal et al., 2013) can cause male-sterility. Further, higher expression levels of invertase genes were observed in the tolerant versus sensitive varieties of rice (Jiang et al., 2020). The heat-tolerant rice cultivar in that study had higher NAD(H) and ATP content in the spikelets than the sensitive genotype, suggesting the heat-tolerant rice cultivar was able to better maintain energy homeostasis at elevated temperature. These findings are consistent with our results, especially for the heat-tolerant Sacramento. While we did not measure NADPH levels in anthers, the increased G6PDH activity and higher sugar supply within Sacramento points to better redox homeostasis of this genotype in the high temperature treatment than Red Hawk. Elevated NADPH levels theoretically can promote GR activity, which uses NADPH to cycle glutathione, and in turn helps regulate ROS levels. The connection between G6PDH and GR activities, and ROS regulation through redox balance is supported by a study in rice, where G6PDH was shown to be involved in the maintenance of cellular redox in cells subjected to salt stress (Zhang, Liu, Wang, & Bi, 2013).
Our study, together with studies on other carbon metabolism enzymes, emphasizes the importance of maintaining carbon metabolism in male reproductive tissues during periods of high temperature stress to prevent infertility. While our current data suggest a positive role of G6PDH in heat stress tolerance, further physiological and biochemical studies using g6pdh mutants are needed to confirm the role of the enzyme in heat stress tolerance of anthers.
4.3 Is there a shift in carbon flux between respiration and OPPP?
After the arrival of sucrose in the anthers, it is cleaved into fructose and glucose. Under normal conditions, glucose is channelled to both cellular respiration through glycolysis and the OPPP, which specifically produces NADPH, that can be used for ROS quenching and synthesis of amino acids, fatty acids etc. Between bean cultivars used in this study, differences in leaf sucrose export (Figure 1a), anther sucrose and glucose accumulation (Figure 2b–c), ROS accumulation (Figure 4), G6PDH and GR activities (Figure 5b,c), and anther respiration rate (Figure 6) suggest a shift in carbon flux within the anthers, at least for the heat-tolerant Sacramento genotype. Since leaf sucrose export and anther carbohydrates were unchanged but anther respiration was reduced in Sacramento, these results are consistent with our hypothesis that the partitioning of carbon between glycolysis and the OPPP is shifted in favour of the OPPP at high temperature (Figure 7). This is further supported by the increased G6PDH activity in Sacramento at elevated temperature, presumably to maintain ROS balance in anthers and prevent oxidative damage. In contrast, in heat-sensitive Red Hawk the reduced sucrose arrival in anthers means that all downstream pathways will also be negatively affected. In our study, these negative downstream effects included the accumulation of anther ROS, reduced anther respiration rate and decreased activities of G6PDH and GR. Reduced protein synthesis in anthers also might have contributed to reduced G6PDH activity in Red Hawk.
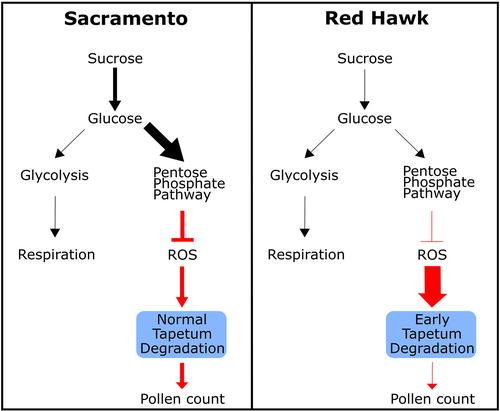
5 CONCLUSION
Studying the effect of elevated temperature on plant reproduction is extremely complicated, since high temperature can negatively affect diverse metabolic pathways (Soltani et al., 2019). Our study focused on the role of carbon metabolism and found that catalase and glutathione pathways, but not ascorbate, are involved in H2O2 balance at high temperature in bean anthers, at least in the two bean genotypes studied. Sacramento was able to tolerate heat stress better than Red Hawk due to higher antioxidant capacity in anthers, which translates, presumably, to better timing of tapetum degradation. We also show that the flux of sucrose from leaves to anthers is important for maintaining the glutathione pathway via G6PDH in anthers, promoting H2O2 scavenging. Finally, in heat-tolerant bean anthers at high temperature, glucose partitioning between respiration and the OPPP is shifted in favour of OPPP, which supports glutathione scavenging of H2O2 (Figure 7). Although g6pdh mutant analysis is needed to strengthen this observation, this study provides an insight into the role of G6PDH on anther heat tolerance and pollen grain production at elevated temperature. Further, since anther wall and connective cells were shown to accumulate starch, sucrose and glucose (Clément et al., 1994; Clément & Audran, 1995), cell-type-specific analysis of carbon metabolism and antioxidant activity during anther development would resolve the contribution of specific anther cell types on ROS scavenging during high temperature stress.
ACKNOWLEDGMENTS
The authors thank MSU Plant Resilience Institute for funding this research. We also thank Mr. Jim Klug and Mr. Cody Keilen for overall greenhouse maintenance and pest management during plant cultivation. Partial salary support for TDS came from Michigan AgBioResearch.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
James P. Santiago and Thomas D. Sharkey conceived and designed the experiments; James P. Santiago performed most of the experiments; Ali Soltani and David B. Lowry performed and analysed the RNA-Seq data and edited the manuscript; Madeline M. Bresson helped with tissue collection and performed the hydrogen peroxide and flower sucrose measurements; James P. Santiago and Thomas D. Sharkey wrote the article. Alyssa L. Preisera advised on G6PDH activity assay and helped with G6PDH data interpretation and editing the article.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




