Overexpression of both Rubisco and Rubisco activase rescues rice photosynthesis and biomass under heat stress
Funding information: Japan Society for the Promotion of Science, Grant/Award Numbers: 20K21346, 20H05687, 18KK0170, 18H02185, 16H06552
Abstract
Global warming threatens food security by decreasing crop yields through damage to photosynthetic systems, especially Rubisco activation. We examined whether co-overexpression of Rubisco and Rubisco activase improves the photosynthetic and growth performance of rice under high temperatures. We grew three rice lines—the wild-type (WT), a Rubisco activase–overexpressing line (oxRCA) and a Rubisco- and Rubisco activase–co-overexpressing line (oxRCA-RBCS)—and analysed photosynthesis and biomass at 25 and 40°C. Compared with the WT, the Rubisco activase content was 153% higher in oxRCA and 138% higher in oxRCA-RBCS, and the Rubisco content was 27% lower in oxRCA and similar in oxRCA-RBCS. The CO2 assimilation rate (A) of WT was lower at 40°C than at 25°C, attributable to Rubisco deactivation by heat. On the other hand, that of oxRCA and oxRCA-RBCS was maintained at 40°C, resulting in higher A than WT. Notably, the dry weight of oxRCA-RBCS was 26% higher than that of WT at 40°C. These results show that increasing the Rubisco activase content without the reduction of Rubisco content could improve yield and sustainability in rice at high temperature.
1 INTRODUCTION
The global mean temperature has risen by more than 1.2°C owing to human activities since 1880, and this trend is predicted to accelerate in the future (IPCC 2013). The increase in temperature and the duration and frequency of heat waves has reduced crop yields worldwide (Lobell, Schlenker, & Costa-Roberts, 2011; Ruiz-Barradas & Nigam, 2013). Global warming observed at the end of the 20th century has had a negative effect on the growth and yielding of rice (Oryza sativa L.), especially in tropical and subtropical Asia (Welch et al., 2010). This situation requires a practical strategy to breed heat-tolerant crops that maintain high productivity at high temperatures for sustainable food security.
Photosynthesis is fundamental to plant growth and total biomass production, as well as other various factors such as water, nutrients, salinity, pests and diseases (Israel, 1982; Reddy, Reddy, Bidinger, & Blümmel, 2003). Therefore, the photosynthesis condition is considered to be the major factor that influences the yielding in modern agricultural production. Crop productivity can be lowered by heat stress through damage to various aspects of the photosynthetic systems because photosynthesis plays a major role in biomass production in plants. High leaf temperature decreases the CO2 assimilation rate (A) when it is above the optimum value for photosynthesis (Sage, Way, & Kubien, 2008; Yamori, Hikosaka, & Way, 2014). The decrease in A is due partly to the thermal instability of the oxygen-evolving complex of photosystem II (Allakhverdiev et al., 2008; Havaux, Greppin, & Strasser, 1991) and partly to Rubisco deactivation (Yamori, Suzuki, et al., 2006, 2012; Crafts-Brandner & Salvucci, 2000; Kobza & Edwards, 1987; Weis, 1981). Rubisco, one of the enzymes of the Calvin–Benson cycle, catalyses the binding of CO2 to ribulose-1,5-bisphosphate (RuBP) in the presence of Mg2+ (Salvucci & Ogren, 1996; Wang & Portis, 1992). It also binds to RuBP without CO2 and Mg2+, and to sugar phosphates, including carboxyarabinitol 1-phosphate, xylulose-1,5-bisphosphate and 2,3-pentodiulose-1,5-bisphosphate, in a tightly combined malfunctioning conformation, inhibiting the activation of Rubisco (Andralojc et al., 2012; Parry, Keys, Madgwick, Carmo-Silva, & Andralojc, 2008). Rubisco activase mediates Rubisco activation by facilitating the removal of these inhibiting molecules in an ATP-dependent manner (Monson, Stidham, Williams, Edwards, & Uribe, 1982; Salvucci & Ogren, 1996; Wang & Portis, 1992). Rubisco deactivation at high temperature can be explained by the insufficient activity of Rubisco activase (Crafts-Brandner & Salvucci, 2000; Salvucci & Crafts-Brandner, 2004a). In vitro, the ATPase activity of Rubisco activase decreased at a temperature slightly above the optimum for plant growth (Crafts-Brandner & Salvucci, 2000; Salvucci & Crafts-Brandner, 2004a), and its deactivation was accompanied by protein aggregation (Barta, Dunkle, Wachter, & Salvucci, 2010).
In the past two decades, studies have reported the relationship between the thermolability of Rubisco activase and the reduction of A at elevated temperatures (Salvucci & Crafts-Brandner, 2004b). By reducing Rubisco activation, elevated temperatures limit photosynthesis by reducing A, as shown in wheat (Triticum aestivum), rice, cucumber (Cucumis sativus) and cotton (Gossypium hirsutum L.; Carmo-Silva & Salvucci, 2011; Crafts-Brandner & Salvucci, 2000; Kumar et al., 2018; Scafaro et al., 2018; Yamori et al., 2012). Arabidopsis thaliana plants overexpressing thermostable Rubisco activase showed improved biomass production at high temperatures (Kumar, Li, & Portis Jr., 2009; Kurek et al., 2007). These facts imply that overexpression of thermostable Rubisco activase can maintain Rubisco activation in crop plants under heat stress. However, recent studies show that overexpression instead decreased the Rubisco content and A in rice (Fukayama et al., 2018; Suganami et al., 2020; Suganami, Suzuki, Sato, & Makino, 2018). Other attempts at improving the Rubisco activation state, such as overexpression of CA1pase, were also reported to negatively influence Rubisco abundance (Lobo et al., 2019). Therefore, a high level of both Rubisco activation and Rubisco content should be able to improve the photosynthetic and growth performance at high temperatures. In fact, transgenic rice overexpressing both Rubisco activase and Rubisco had increased the Rubisco activation state and A at the optimal temperature (Suganami et al., 2021). However, the growth of such plants under heat stress, which refers to temperature above the optimal range, is yet to be studied. The thermolability of Rubisco activase is also responsible for the inhibition of photosynthesis under moderate heat stress, and C4 plants in general are adapted to higher temperatures than C3 plants; C4 plants have a higher Rubisco activation state at high temperature (Yamori et al., 2014), which may be because the Rubisco activase from C4 plants has a higher optimum temperature than that of C3 plants. Therefore, Rubisco activase from maize, a C4 plant, could be a good choice for high temperature. With this knowledge, we developed a transgenic rice which co-overexpresses rice Rubisco and maize Rubisco activase to improve its thermotolerance. Subsequently, we evaluated the photosynthetic and growth characteristics in three lines differing in their Rubisco and Rubisco activase contents under short-term and long-term extreme high temperatures (40°C). Here, our objective was to examine whether increasing both Rubisco content and Rubisco activation improves the photosynthetic and growth performance of rice at a high temperature beyond the optimal range for growing rice.
2 MATERIALS AND METHODS
2.1 Development of transgenic rice overexpressing Rubisco and Rubisco activase
Transgenic rice (Oryza sativa L. cv. Notohikari) with increased Rubisco content was generated by overexpressing the gene coding the Rubisco small subunit (RBCS) of rice (Suzuki et al., 2007). Respective plasmids containing the sense rbcS cDNA were constructed with the rice rbcS promoter using the Ti plasmid pBI101Hm. We introduced constructs designed to overexpress the Rubisco activase small isoform of maize, in which maize (ZmRca, AB564720) was amplified by RT–PCR and the cDNA was fused to the rice Cab promoter and cloned into a binary vector pIG121Hm (ZmRca, Fukayama et al., 2012), and into calluses derived from the RBCS line via Agrobacterium-mediated gene transfer to generate transgenic rice overexpressing both Rubisco activase (RCA) and Rubisco (oxRCA-RBCS). We introduced the same constructs into calluses derived from wild-type Notohikari (WT) to generate transgenic rice overexpressing Rubisco activase (oxRCA). Hygromycin-resistant transgenic plants were regenerated from the calluses. Primary transgenic plants (T1) showing segregation for overexpressed proteins were selected and grown. Their seeds were sown, and the seeds of T2 plants with high levels of the overexpressed proteins were collected, stocked and used for further analyses. The selection of germplasm for the experiments presented was based solely on the protein abundance of Rubisco and RCA.
2.2 Plant materials and growth conditions
For the analysis of plant growth and gas exchange, we grew WT, oxRCA and oxRCA-RBCS plants in a controlled-environment growth chamber (LPH-0.5PSH; Nippon Medical & Chemical Instrument). Each seedling was planted in a 1.3-L plastic pot containing Akadama soil with 1.0 g of a slow-release fertilizer (Temairazu; Co-Op Chemical Co., Ltd., Tokyo, Japan). Notohikari is a lowland rice which requires a high level of water supply throughout the growing period (Matsumoto, Hatakeyama, Hashimoto, & Nakatani, 1985), so the plants were cultured in pots with a hole at the bottom, and the pots were placed in a container half-filled with water. The water level was checked and maintained at 10 cm every 2 days to ensure the plant was not suffering from drought damage. The pot position was switched randomly to avoid uneven light condition during the water maintenance. The chamber was operated with a day/night temperature of 25/22°C, a relative humidity of 65%, a PPFD of 600 μmol photons m−2 s−1, a 14-hr photoperiod and a CO2 concentration of 400 μmol mol−1. At 30 days after transplanting, half of the plants was transferred to a growth chamber of the same type with a day/night temperature of 40/36°C. All plants were then cultured until the 75th day of transplanting for photosynthetic and biomass characteristics analysis. We placed several fans in the growth chambers to maintain air flow through the plants, avoiding accumulation or uneven distribution of heat, especially caused by the cooling effect of evaporation.
2.3 Gas-exchange and chlorophyll fluorescence measurements
Gas exchange measurements were conducted with the uppermost fully expanded leaves of plants (n = 6–9 per line) grown at 25/22°C at 55–61 days after sowing, using a portable gas-exchange system (LI-6400, Li-COR, Lincoln, NE) in the growth chamber at an air temperature of 25 or 40°C. Plants were first held at 25°C and the first photosynthetic rate measurement was conducted at 25°C; then the plants were treated with a chamber temperature of 40°C for 24 hr and the second photosynthetic rate measurement was conducted at 40°C. The response curve of the CO2 assimilation rate (A) to the intercellular CO2 concentration (Ci) (i.e., A–Ci curve) was measured at 1,500 μmol m−2 s−2 PPFD, which was considered to be sufficient to provide photosynthesis saturation for the glasshouse grown plants (Perdomo, Capó-Bauçà, Carmo-Silva, & Galmés, 2017). The concentration of CO2 was set to be 400 μmol mol−1 at first, and after the readout was stable, switched to 75 μmol mol−1 and gradually increased to 1,500 μmol mol−1. After the measurement, the CO2 concentration was again switched back to 400 μmol mol−1.
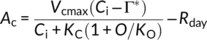 (1)
(1)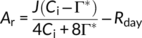 (2)
(2)Temperature dependencies of Rubisco kinetics were obtained in tobacco from Bernacchi, Singsaas, Pimentel, Portis, and Long (2001). A–Ci curves were fitted in KaleidaGraph software (Synergy Software) at low CO2 concentration to estimate Vcmax and at high CO2 concentration to estimate J.
Taking mesophyll conductance (gm) into account, we used the A–Ci curve-fitting procedure described by Ethier and Livingstone (2004). The model was then fitted to the data by an iterative procedure, analyzing the primary limitation to CO2 assimilation with Rubisco kinetic parameters as reported by Bernacchi, Portis, Nakano, Von Caemmerer, and Long (2002) and with temperature dependence of gm in rice as reported by Scafaro, von Caemmerer, Evans, and Atwell (2011). We assumed gm to be similar among oxRCA and oxRCA-RBCS, at 8.2 μmol m−2 s−1 Pa−1 at 25°C, and 21.4 μmol m−2 s−1 Pa−1 at 40°C.
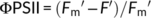 (3)
(3)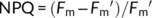 (4)
(4)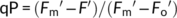 (5)
(5)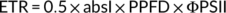 (6)
(6)Gas exchange and chlorophyll fluorescence were also simultaneously measured in rice grown at 25 and 40°C, respectively. A and ETR were measured at a CO2 concentration of 400 μmol mol−1 and a growth light density of 600 μmol photons m−2 s−1.
2.4 Quantification of Rubisco and Rubisco activase contents
Leaf tissues from the same leaf used for gas-exchange measurement were sampled immediately after gas exchange measurements, frozen in liquid nitrogen, and stored at −80°C until analysis. As described by Yamori, Nagai, and Makino (2011), the Rubisco content was determined by formamide extraction of SDS-PAGE-separated, Coomassie Brilliant Blue R-250–stained bands corresponding to the large and small subunits of Rubisco. The Rubisco activase content was also quantified by SDS-PAGE-separated, Coomassie Brilliant Blue R-250–stained bands with Bovine Serum Albumin (BSA) as the standard. The Rubisco activase content was double checked by immunoblotting of SDS-PAGE-separated proteins with Primary anti-activase antibody (Fukayama et al., 2012), and secondary anti-rabbit IgG HRP-linked antibody (GE Healthcare, NA934), on a polyvinylidene difluoride membrane. Rieske FeS protein was quantified by immunoblotting with Primary antibody (Agrisera AS08 330). The chlorophyll concentration in the 80% acetone extract was determined spectrophotometrically (Porra, Thompson, & Kriedemann, 1989).
2.5 Evaluation of Rubisco activation state
Leaf tissues from the same position used for gas-exchange measurement were sampled after high light and ambient CO2 treatment in the Li-COR chamber. The samples were homogenized and extracted within 30 s in 100 mM HEPES/KOH (4-[2-hydroxyethyl]-1-piperazineethanesulfonic acid-sodium hydroxide) (pH 8.0) containing 20 mM MgCl2, 5 mM dithiothreitol and 1 mM ethylenediaminetetraacetic (EDTA), and centrifuged for 16,000g at 4°C for 10 s. The supernatant was used for the Rubisco assay. The initial activity of Rubisco was assayed at 25°C for 1 min in the assay medium containing 100 mM bicine–KOH (pH 8.2), 20 mM MgCl2, 20 mM NaHCO3, 5 mM ATP, 5 mM creatine phosphate, 0.2 mM NADH, 0.5-mM ribulose 1,5-bisphosphate (RuBP), 10 units mL−1 of creatine kinase, 10 units mL−1 of 3-phosphoglyceric phosphokinase (PGK) and 25 units mL−1 of glyceraldehyde-3-phosphate dehydrogenase (GAPDH; all enzymes were purchased from Sigma, St. Louis, MO). Neither of the activity of PGK nor that of GAPDH limited Vcmax at any measurement temperatures (Yamori, Suzuki, et al., 2006). These procedures, from the onset of the extraction to that of the initial activity assay, required about 1 min. Another portion of the extract was used for the determination of the total Rubisco activity. Rubisco was activated for 30 min at 4°C in an activation medium that contained 375 mM HEPES–KOH (pH 7.8), 50 mM MgCl2 and 50 mM NaHCO3, before the measurement of the total activity with the same medium as was used for the determination of the initial activity. After establishing a steady baseline, the carboxylation of Rubisco was started by the addition of 0.6 mM RuBP. The reaction was measured as the decrease in absorbance at 340 nm as a result of the oxidation of NADH. The activation state was estimated as the ratio of the initial to total Rubisco activities (Lilley & Walker, 1974; Usuda, 1985; Yamori et al. 2006, 2012). Recent studies showed that NADH-related Rubisco activation measurement is somehow defective in precise quantification, compared to the 14C-based assay (Sales, da Silva, & Carmo-Silva, 2020), however, we still consider it to be sufficient enough when compared with samples treated identically, especially in qualitative comparisons.
2.6 Dry weight measurement
Plants grown in the 25/22°C chamber and 40/36°C chamber were eventually used for dry weight measurement. Above-ground tissues of the plants were harvested at 75 days after sowing, before the heading stage, dried at 80°C for 72 hr, and then weighed.
2.7 Statistical analysis
The significance of variation in all traits among lines was evaluated by the Tukey–Kramer test. The significance of variation in all traits between 25 and 40°C was evaluated by one-way ANOVA in each line. Statistical analysis was performed in Prism v. 8.0.1 software.
We evaluated the significance of the effects of genotype, measurement temperature and those interactions on A, stomatal conductance, ETR, 1-qP, NPQ, Vcmax, J, Rubisco activation state and plant biomass by using two-way ANOVA. Statistical analysis was conducted using R software version 4. 0. 3 (R Foundation for Statistical Computing, Vienna, Austria).
3 RESULTS
3.1 Rubisco and Rubisco activase contents
We tested two lines each of oxRCA and oxRCA-RBCS (Figure 1a; Figure S1) and present the averages. The Rubisco activase content was 153% higher in oxRCA than in WT but the Rubisco content was 27% lower, consistent with previous results (Fukayama et al., 2012). The Rubisco activase content was 138% higher in oxRCA-RBCS than in WT (p < .05), but the Rubisco content was not significantly lower than in WT. The Rubisco content was 30% higher in oxRCA-RBCS than in oxRCA (p < .05). The ratio of Rubisco to Rubisco activase was 71% lower in oxRCA than in WT, and that of oxRCA-RBCS was 60% lower. It was 38% higher in oxRCA-RBCS than in oxRCA (p < .05; Figure 1c). There was no significant variation in the Rubisco activase content between these overexpressing lines. The contents of chlorophyll and Rieske FeS protein were similar among the three lines (Figure 1b,c).
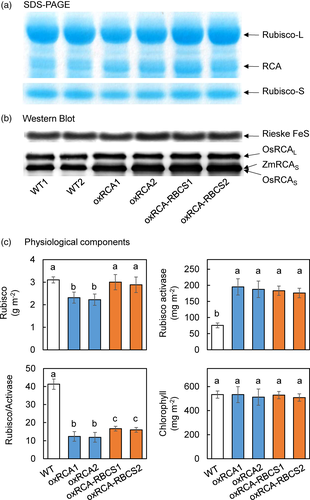
3.2 Photosynthetic characteristics at 25 and 40°C
At 25°C, the CO2 assimilation rate (A) and electron transport rate (ETR) were 18% and 17%, respectively, lower in oxRCA than in WT (p < .05), but there were no significant differences between oxRCA-RBCS and WT (Figure 2). The values of 1 − qP (reduction of plastoquinone pool) and non-photochemical quenching (NPQ) were 20% and 15%, respectively, higher in oxRCA than in WT (p < .05), but there were no significant differences between oxRCA-RBCS and WT. At 40°C, on the other hand, A was 15% higher in oxRCA and 20% higher in oxRCA-RBCS than in WT (p < .05), and ETR was 19% higher in oxRCA and 30% higher in oxRCA-RBCS than in WT (p < .05). The values of 1 − qP were 9% lower in oxRCA and 18% lower in oxRCA-RBCS than in WT, and NPQ was 20% lower in oxRCA and 31% lower in oxRCA-RBCS than in WT (p < .05). There was no significant difference in these four parameters between oxRCA and oxRCA-RBCS. In addition, stomatal conductance (gs) was similar among all three lines at both temperatures (Figure 2). There were no significant effects of the genotype, temperature and those interactions on stomatal conductance (Table S1).
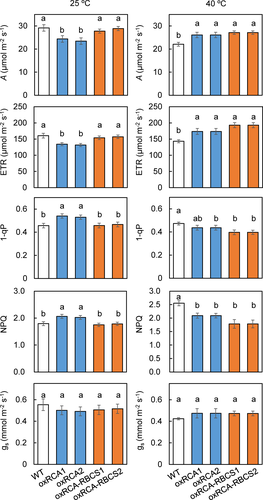
Photosynthetic characteristics differed between 25 and 40°C. In WT, A was 25% lower and ETR was 11% (non-significantly) lower at 40°C than at 25°C; 1 − qP did not differ significantly between temperatures; and NPQ was 42% greater at 40°C than at 25°C (p < .05). In oxRCA, A did not differ significantly between temperatures; ETR was 27% higher at 40°C than at 25°C; 1 − qP was 21% lower at 40°C than at 25°C; and NPQ did not differ significantly between temperatures (p < .05). In oxRCA-RBCS, A did not differ significantly between temperatures; ETR was 20% higher at 40°C than at 25°C; 1 − qP was 16% lower at 40°C than at 25°C; and NPQ did not differ significantly between temperatures (p < .05; Figure 2). The effects of genotype, temperature and those interactions on the CO2 assimilation rate, ETR, 1 − qP and NPQ were significant according to two-way ANOVA (Table S1).
At 25°C, A was lower in oxRCA than in WT and similar in oxRCA-RBCS to that in WT at Ci from 75 to 1,000 μmol mol−1 (Figure 3a); at 40°C, A was higher in both oxRCA and oxRCA-RBCS than in WT (p < .05; Figure 3b). At 25°C and Ci = 400 μmol mol−1, A of WT and oxRCA-RBCS was limited by RuBP regeneration, while that of oxRCA was limited by RuBP carboxylation. At 40°C and Ci = 400 μmol mol−1, on the other hand, A of all three lines was limited by RuBP carboxylation. At 25°C, Vcmax was 27% lower in oxRCA than in WT (p < .05), but not significantly different between oxRCA-RBCS and WT (Figure 3e). At 40°C, Vcmax was 13% (not significantly) higher in oxRCA than in WT, and was 16% higher in oxRCA-RBCS than in WT (p < .05; Figure 3f). J did not differ significantly among the three lines at either temperature (Figure 3g,h). The same trends were shown in the estimation of Vcmax and J assuming finite gm (Figure S2). The effects of the temperature and the interaction between genotype and temperature on Vcmax were significant, while the effects of the temperature on J were significant according to two-way ANOVA (Table S1).
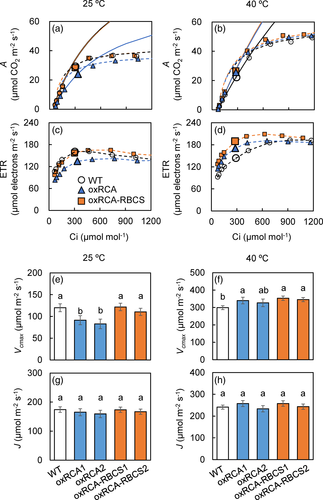
At 25°C, ETR of oxRCA was lower than in WT at Ci < 500 μmol mol−1 (p < .05), and that of oxRCA-RBCS was not significantly different from that in WT (Figure 3c). At 40°C, ETR of oxRCA was higher than in WT at Ci < 500 μmol mol−1, and that of oxRCA-RBCS was higher than in WT at Ci < 700 μmol mol−1 (p < .05; Figure 3d).
3.3 Rubisco activation state at 25 and 40°C
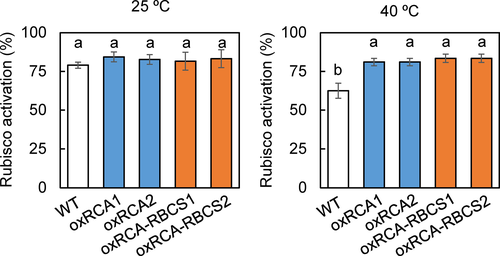
At 25°C, the Rubisco activation state varied from 79 to 84% among the three lines, with no significant difference (Figure 4). At 40°C, it was 60% in WT, 79% in oxRCA and 81% in oxRCA-RBCS, the latter two significantly higher than in WT (p < .05). The effect of genotype on the Rubisco activation state was significant at p < .05 (Table S1).
3.4 Biomass production at 25 and 40°C
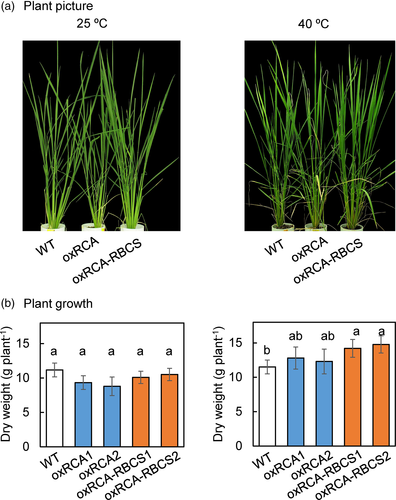
At 25°C, there was no significant difference in the dry weight of the above-ground biomass among the three lines (Figure 5a,b). At 40°C, the dry weight was similar in oxRCA and WT, but was 26% higher in oxRCA-RBCS than in WT (Figure 5b; p < .05). These results were confirmed by the photosynthetic characteristics: A and ETR at the growth light density of 600 μmol m−2 s−1 were higher in oxRCA and oxRCA-RBCS than in WT at 40°C (Figure S3). The effect of temperature but not genotype on plant biomass was significant at p < .05. In addition, the interaction between genotype and temperature was significant at p < .05 (Table S1). However, the p value of the genotype is .0505, indicating a non-statistical but marginable significant difference between genotypes. Plants grown in both the 25/22°C chamber and 40/36°C chamber were eventually used for dry weight measurement. The effect of temperature, not genotype, on plant biomass was significant. In addition, the interaction between genotype and temperature was significant (Table S1).
4 DISCUSSION
Global warming threatens agriculture by reducing crop productivity (Lobell et al., 2011; Ruiz-Barradas & Nigam, 2013), so heat-tolerant crops must be bred for sustainable food security. High temperatures decrease crop productivity through damage to photosynthetic systems. The deactivation of Rubisco, the key enzyme of CO2 carboxylation in the Calvin–Benson cycle, is a major factor in decreased A at high temperatures (Bracher, Whitney, Hartl, & Hayer-Hartl, 2017; Parry et al., 2013). The increased content of Rubisco activase, which mediates Rubisco activation, maintained a high level of Rubisco activation in rice at high temperatures that decreased the Rubisco content, leading to decreased A (Yamori et al., 2012). Here, we show that co-overexpression of Rubisco activase and Rubisco improved the photosynthetic and growth performance of rice at high temperatures, which would be a promising strategy for the improvement of yield capacity and sustainability of crops under global warming.
4.1 Rubisco activase is a promising target for enhancing leaf photosynthesis and biomass productivity in rice at high temperatures
Since photosynthesis is the physiological basis for biomass production, manipulating photosynthetic performance can optimize growth and yield (Yamori et al., 2016). Various studies have attempted to enhance the photosynthetic capacity to improve biomass production and yield (Allen et al., 1987; Sharma-Natu & Ghildiyal, 2005). These studies have identified Rubisco activase and Rubisco as attractive targets for improving photosynthesis (reviewed in Carmo-Silva, Scales, Madgwick, & Parry, 2015). Under moderate heat stress, the thermolability of Rubisco activase is partly responsible for the inhibition of photosynthesis due to Rubisco deactivation (Salvucci & Crafts-Brandner, 2004a). Thus, maintaining Rubisco activation at high temperatures is critical for improving the heat tolerance of crops. This is supported by studies showing that Arabidopsis mutants with more-thermostable Rubisco activase variants had higher photosynthetic and growth rates at high temperatures than the wild-type (Kumar et al., 2009; Kurek et al., 2007). Recent work in rice showed that overexpression of heat-tolerant Rubisco activase from wild rice Oryza australiensis in cultivated rice improved plant growth at 45°C (Scafaro et al., 2018). Thus, increasing Rubisco activase content is critical for improving photosynthesis and plant growth, especially at high temperatures.
On the other hand, the overexpression of Rubisco activase can result in decreased Rubisco content, leading to decreased A (Figure 4; Fukayama et al., 2012, 2018; Suganami et al., 2018). Recent work showed that transgenic rice overexpressing both Rubisco and Rubisco activase could increase the contents of both, resulting in an improvement of the CO2 assimilation rate in the optimal temperature range (Suganami et al., 2021). Here, we confirmed that co-overexpression of Rubisco from rice and Rubisco activase from maize (a C4 plant and therefore better adapted to high temperature than C3 rice) resulted in higher Rubisco activation, maintaining Rubisco content, which contributed to higher A and total dry weight at a higher temperature than in WT (Figures 1 and 3). Thus, our results clearly show that the Rubisco activase content is a promising target for improving the biomass productivity of rice under heat stress.
The thermal stability of Rubisco activase varies depending on species and the climate to which they are adapted. The Rubisco activase of temperate species is more heat-sensitive than that of tropical species (Carmo-Silva & Salvucci, 2011; Henderson, Hazra, Dunkle, Salvucci, & Wachter, 2013). Therefore, we used Rubisco activase from maize, because C4 plants in general are better adapted to higher temperatures than C3 plants, due partly to a higher Rubisco activation state at high temperature (Yamori et al., 2014), but this is not always true since the thermostability of Rubisco activase in Maize or rice could be influenced by cultivars (Perdomo et al., 2017). In addition, the thermostability of Rubisco activase would be divergent among even closely related species adapted to different environments, such as between rice and its close relative O. australiensis, which is endemic to hot environments in northern Australia (Scafaro et al., 2016). The interspecific diversity in the thermostability of Rubisco activase is being explored for genetic improvement: Rubisco activase from Agave tequilana was found to have much higher thermostability as well as faster enzymatic velocity than rice (Shivhare & Mueller-Cajar, 2017); and the introduction of a single mutation into Rubisco activase of bread wheat (Triticum aestivum L.) altered its optimal temperature range and catalysis activity in vitro (Degen, Worrall, & Carmo-Silva, 2020). CAM plant species would also be a useful source of highly functional and thermostable Rubisco activase for improved heat tolerance of crop photosynthesis (Shivhare & Mueller-Cajar, 2017). Thus, the overexpression of highly functional and thermostable Rubisco activase without reduction of the Rubisco content has great potential to improve the biomass production of rice under heat stress. High temperature would influence rice growth in a more complicated manner such as accelerating leaf emergence, senescence and other physical processes (Krishnan, Ramakrishnan, Reddy, & Reddy, 2011). How this effect influences biomass accumulation or plant metabolism is yet to be discussed.
4.2 Rubisco activase limits leaf photosynthesis in rice at high temperature
The primary limitation to CO2 assimilation at high temperatures has been controversial because a decline in electron transport capacity can limit CO2 assimilation indirectly by downregulating Rubisco activase activity (Sharkey, 2005). The C3 photosynthesis model is a powerful tool to determine whether it is limited by RuBP carboxylation or regeneration (Farquhar et al., 1980). CO2 response analysis of A and ETR showed that at Ci = 400 μmol mol−1, A in WT was limited by RuBP regeneration at 25°C, but by RuBP carboxylation at 40°C (Figure 3). The C3 photosynthesis model taking account of gm supported this conclusion (Figure S3). This is consistent with the results that the photosynthetic rate above 33°C was Rubisco-limited in both O. sativa and wild rice O. meridionalis (Scafaro et al., 2012). In addition, the variation in ETR at 40°C corresponded to that in the Rubisco activation state among the three lines (Figures 3 and 4). These results suggest that the thermolability of Rubisco activase is responsible for the inhibition of photosynthesis under moderate heat stress, which would downregulate the electron transport capacity. Therefore, improved Rubisco activity would enhance the photosynthesis and biomass production of crops at high temperatures.
There is considerable interspecific variation in the conditions that limit A across a broad temperature range (Yamori, Noguchi, Hikosaka, & Terashima, 2009, 2010). In some species, RuBP carboxylation limits A at high temperatures (Busch & Sage, 2017; Crafts-Brandner & Salvucci, 2000; Salvucci & Crafts-Brandner, 2004; Yamori, Noguchi, Hanba, & Terashima, 2006, Yamori, Suzuki, et al., 2006, Yamori, Noguchi, Kashino, & Terashima, 2008), but in other species, RuBP regeneration limits A at high temperature (Makino & Sage, 2007; Wise, Olson, Schrader, & Sharkey, 2004). In the latter case, Rubisco deactivation at high temperature is attributable to a regulatory response to other limitations than Rubisco activase thermolability (Cen & Sage, 2005; Sage & Kubien, 2007; Sharkey, 2005). Like Rubisco activation, reactions associated with the thylakoid membranes are also susceptible to heat stress; specifically, thylakoid membrane integrity and PSII are less stable at higher temperature (Carmo-Silva et al., 2012; Yamane, Kashino, Koike, & Satoh, 1998; Yamasaki et al., 2002). Thus, it is possible that the susceptibility of Rubisco activase and electron transport systems depend on plant species, which would underlie the interspecific variation in the conditions limiting photosynthesis at high temperatures.
4.3 Relationship between contents of Rubisco and Rubisco activase in rice
The overproduction of Rubisco activase decreased the Rubisco content (Figure 1; Fukayama et al., 2012, 2018). Interestingly, the overproduction of Rubisco in rice plants caused by overexpressing the gene encoding the Rubisco small subunit (RBCS) tended to decrease the Rubisco activase content, decreasing the Rubisco activation state, whereas an RBCS antisense line had an increased Rubisco activase content, increasing the activation state (Suganami et al., 2018, 2020). In addition, overexpression of 2-carboxy-d-arabinitol-1-phosphate phosphatase (CA1Pase), which dephosphorylates tight-binding inhibitors of Rubisco, improved the activation state but decreased Rubisco abundance (Lobo et al., 2019). These results indicate a trade-off between the amounts of Rubisco and Rubisco activase in transgenic rice.
What is the physiological basis underlying this trade-off? Recent studies showed that the mRNA levels of genes for the Rubisco small subunit (RBCS) and the Rubisco large subunit (RBCL) remain unchanged although the Rubisco content decreased in Rubisco activase overexpressing individuals planted in moderate temperature (Fukayama et al., 2012, 2018; Suganami et al., 2018, 2020), suggesting that the Rubisco content was regulated post-transcriptionally. It has been suggested that unassembled RBCL protein could interact with RBCL mRNA, reducing its polysome loading and suppressing its translation in tobacco plants (Rodermel, Haley, Jiang, Tsai, & Bogorad, 1996). This assembly-dependent translational regulation is termed ‘control by epistasy of synthesis’ (CES) (Choquet & Vallon, 2000). In previous studies using rice (Suzuki & Makino, 2012, 2013), the loading of RBCL on polysomes decreased in senescent leaves, although regulation of the transcriptional level remained unchanged. This suggests that the RBCL protein synthesis is further regulated by CES post-transcriptionally, which could possibly become the bottleneck of final RBCL synthesis, especially in a senescent rice leaf. On the other hand, Fukayama et al. (2018) indicated that the translation activities of RBCS and RBCL did not change greatly in Rubisco activase–overexpressing rice plants. Further study is required to elucidate the mechanisms underlying the trade-off between the amounts of Rubisco and Rubisco activase.
ACKNOWLEDGMENTS
This work was supported by KAKENHI to W.Y. (Grant Number: 16H06552, 18H02185, 18KK0170, 20H05687, and 20K21346) from the Japan Society for the Promotion of Science (JSPS).
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data available on request from the authors.




