Responses of leaf respiration to heatwaves
Funding information: Centre of Excellence in Plant Energy Biology, Australian Research Council, Grant/Award Number: CE140100008; Grains Research and Development Corporation, Grant/Award Number: National Wheat Heat Tolerance Project (US00080)
Abstract
Mitochondrial respiration (R) is central to plant physiology and responds dynamically to daily short-term temperature changes. In the longer-term, changes in energy demand and membrane fluidity can decrease leaf R at a common temperature and increase the temperature at which leaf R peaks (Tmax). However, leaf R functionality is more susceptible to short-term heatwaves. Catalysis increases with rising leaf temperature, driving faster metabolism and leaf R demand, despite declines in photosynthesis restricting assimilate supply and growth. Proteins denature as temperatures increase further, adding to maintenance costs. Excessive heat also inactivates respiratory enzymes, with a concomitant limitation on the capacity of the R system. These competing push-and-pull factors are responsible for the diminishing acceleration in leaf R rate as temperature rises. Under extreme heat, membranes become overly fluid, and enzymes such as the cytochrome c oxidase are impaired. Such changes can lead to over-reduction of the energy system culminating in reactive oxygen species production. This ultimately leads to the total breakdown of leaf R, setting the limit of leaf survival. Understanding the heat stress responses of leaf R is imperative, given the continued rise in frequency and intensity of heatwaves and the importance of R for plant fitness and survival.
1 INTRODUCTION
Mitochondrial respiration (R) is a metabolic process of interlinked enzyme- and membrane-dependent reactions necessary for cellular function (Amthor, 2000; Atkin, Millar, Gardeström, & Day, 2000). R strongly influences the extent of net carbon gain by individual plants, with 20–80% of daily photoassimilates being respired each day (Atkin, Scheurwater, & Pons, 2006; Bunce, 2005; Poorter, Remkes, & Lambers, 1990). Leaf R contributes approximately half of all plant R CO2 release (Poorter et al., 1990). R catabolizes photoassimilates to generate and transport chemical energy held in ATP and NAD(P)H covalent bonds. ATP and NAD(P)H provide the energy required to power cellular processes, such as active transport of ions and molecules, DNA synthesis and transcription, and protein synthesis and degradation. R is also central to the synthesis and recycling of primary metabolites such as amino acids, nitrogenous compounds and growth regulatory factors. Thus, while CO2 assimilation only occurs in the day when irradiance is captured, R continues through day and night and must function under extreme biotic or abiotic stress; in its absence, death will occur. How R responds to changes in the surrounding environment – such as heatwaves – can, therefore, be crucial to plant growth and survival.
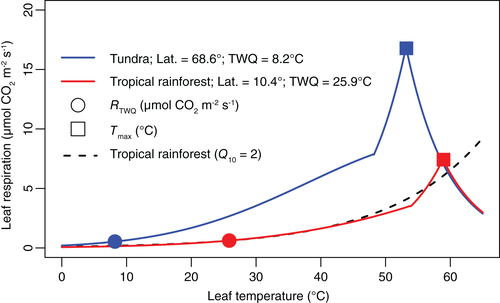
Over the past 50 years, heatwaves have occurred more frequently, and increased in intensity and duration (Perkins-Kirkpatrick & Lewis, 2020). The implications of rising air temperature on R are, therefore, a mounting concern. Although plants can survive and indeed thrive in environments in which air temperatures fluctuate by upwards of 20°C over the course of a day (R. L. Davidson, 1969), the likelihood is that more plants, for longer periods of time, will experience heat stress. For the purposes of this review, we define heat stress as a leaf temperature that exceeds the optimum for net photosynthetic CO2 uptake. The leaf temperature at which photosynthesis is optimal typically aligns with the mean daily temperatures that the leaf has experienced in the preceding days/weeks. Above this temperature optimum of net assimilation, rates of photoassimilate generation decline (Berry & Björkman, 1980; Yamori, Hikosaka, & Way, 2014), as does growth (Poorter et al., 1990). More severe heat stress results in a rapid ‘burst’ in respiratory CO2 release (Hüve, Bichele, Rasulov, & Niinemets, 2011; O'Sullivan, Weerasinghe, et al., 2013), followed by leaf R reaching a peak at a maximum temperature (Tmax; Figure 1). Beyond Tmax, typically in the 50–60°C range (O'Sullivan, Heskel, et al., 2017), rates of leaf R irreversibly decline as mitochondrial function is lost, and a rapid onset of tissue death ensues (O'Sullivan, Weerasinghe, et al., 2013). Leaf R can thermally acclimate, a phenomenon characterized by reduced leaf R rate at a given measuring temperature and an increase in the Tmax of leaf R. (Atkin & Tjoelker, 2003; O'Sullivan, Heskel, et al., 2017; Yamori et al., 2014; Zhu et al., 2020). The extent of the difference in rates of leaf R in plants that have thermally acclimated is evident in Figure 1, with cold-acclimated tundra species exhibiting higher rates of leaf R across a broad range of leaf temperatures and lower Tmax values than warm-acclimated tropical rainforest species. It is striking that rates are similar when compared at the respective growth temperatures (i.e. homeostasis is observed) of the tundra and rainforest environments, despite an 18°C difference in growth temperature.
In this review, we explore mechanisms underpinning changes in leaf R rate during the onset of a heatwave; we do this through following its theoretical progression over the course of an extremely hot day for leaves that are acclimated either to initially cooler or warmer temperatures. The physical implications of rising temperature on membranes and proteins are characterized in detail, as both are central to leaf R function and fundamentally altered by heat. We also examine the implications of rising temperature for the factors that regulate leaf R rate, such as how rising temperatures affect respiratory substrate supply and demand for respiratory products. We aim to build a comprehensive picture of the factors that influence variability in leaf R rate, and how these will affect leaf and, ultimately, whole plant function in a warmer world of more frequent and severe heatwaves.
2 LEAF RESPIRATION OVER THE COURSE OF A HOT DAY
The temperature range that a leaf experiences within a single day is dependent on many environmental variables. Factors that lead to a more dynamic range in daily temperature include whether a plant is: growing in a high or low latitude region; adjacent to a large body of water that buffers air temperature and growing in a more exposed microenvironment (e.g. open field) rather than a sheltered site (e.g. forest understory). Irrespective of these variables, the increasing frequency, intensity and duration of heatwaves will require leaves to function over a greater temperature range and at higher maximum temperatures. With this in mind, we explore the consequences of an extremely hot day for leaf mitochondria. For simplicity, we initially consider only temperature-related external factors impacting on a leaf, putting aside changes in irradiation and moisture availability. In later sections, we briefly explore these other interacting stimuli and the profound implications that they have on leaf R performance.
2.1 The starting point
Respiratory machinery is geared towards the temperatures that are most commonly experienced in a given season and environment (N. G. Smith & Dukes, 2018). As indicated in Figure 1, acclimation of leaf R to warmer temperatures will involve a higher Tmax and reduction in rates of leaf R at a given temperature, to the extent that R rates of warm- and cold-grown leaves can match one another (homeostasis) when measured at their respective growth temperatures (Heskel et al., 2016; Zhu et al., 2020). Heatwaves usually occur when leaf R has acclimated to these warmer summer months.
A higher Tmax of leaf R can be attributed to membrane characteristics. Across all organisms, structurally sound membranes are critical in determining maximum thermal tolerance. For example, an increase in thermal limits of growth from 20 to 45°C in different yeast strains closely aligned with a reduction in cellular membrane unsaturated fatty acids from 90% to below 40% (Arthur & Watson, 1976). Similarly, plants increase the relative amount of saturated fatty acids in their cellular membranes during warmer seasons or following direct application of heat in a controlled setting; this increase in fatty acid saturation results in membranes that are more stable at hotter temperatures (Zhu et al., 2018).
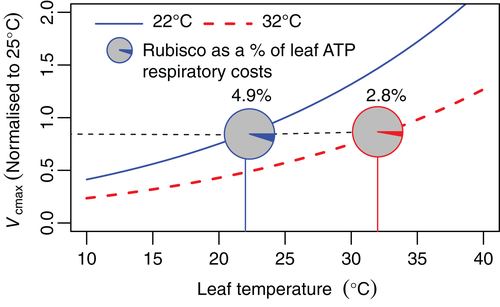
The reduction in leaf R rate at a given temperature that is exhibited by warm acclimated leaves (compared to their cool-acclimated counterparts; Figure 1) is likely explained by: (1) the initial effect of heat on enzyme reaction rates and (2) the effect of sustained high temperature on protein abundance and energy demand. Catalytic rates increase at higher temperatures that are not severe enough to denature proteins, and less protein may therefore be required to perform the same catalytic function. Rubisco, the most abundant leaf protein, accounting for 4.9% of total leaf respiratory ATP maintenance costs in 22°C-grown Arabidopsis (Arabidopsis thaliana (L. Heynh.)) (Li et al., 2017), provides a good example of this. Based on its maximal carboxylation velocity (Vcmax) (Walker, Ariza, Kaines, Badger, & Cousins, 2013), Arabidopsis grown at 32°C can reduce the Rubisco ATP respiratory cost to 2.8% whilst maintaining the same level of CO2 fixation capacity (Figure 2). Observations support this theoretical example; when analysed at a standardized temperature, there is a tight and predictable relationship between thermal acclimation-dependent declines in Vcmax and acclimation-dependent declines in rates of leaf R across 899 species across the globe (H. Wang et al., 2020). Thus, leaf R and Rubisco are closely related, and by simply reducing Rubisco abundance, substantial savings in total leaf respiratory ATP requirements could potentially be achieved without sacrificing photosynthetic performance. If this example is indicative of a general heat response of plant proteins, it is possible that the observed decline in leaf R rate under elevated growth temperature could be largely accounted for.
2.2 Rising temperature and the physical and regulatory response of leaf respiration
While leaf R increases with rising temperature up until severe heat approaching 60°C (Figure 1), photosynthetic CO2 assimilation peaks and declines in response to moderate heat stress at temperatures as much as 30°C below the Tmax (Berry & Björkman, 1980; Way & Yamori, 2014). Heat-dependent declines in CO2 assimilation during moderate heat stress can be attributed, in part, to loss of Rubisco carboxylation activity due to the heat-labile nature of its regulatory protein Rubisco activase (Rca) (Busch & Sage, 2017; Law & Crafts-Brandner, 1999; Salvucci & Crafts-Brandner, 2004). Heat damage to the thylakoid membranes of chloroplasts also contributes to a sharp decline in assimilation at temperatures more than 10°C below Tmax (Allakhverdiev et al., 2008; O'Sullivan, Heskel, et al., 2017; Takahashi & Badger, 2011; Yoshioka et al., 2006). The impacts of heat on vapour pressure deficit (VPD) between leaves and the surrounding air can lead to stomatal closure, further contributing to reductions in CO2 assimilation (Grossiord et al., 2020) – see Section 3.2 for further details.
Not only does heat reduce the acquisition of atmospheric CO2 through supressed photosynthesis, but heat also impedes the activity of sucrose-synthesizing enzymes; examples include sucrose-phosphate synthase, ADP-glucose pyrophosphorylase, invertase and sucrose transporters (Kaushal et al., 2013; Wardlaw, Moncur, & Patrick, 1995). For these reasons, as the day begins to heat up and surpass the photosynthetic optimum, reduced CO2 assimilation and sucrose metabolism can lead to a decline in the production of soluble sugars in the leaf (Julius, Leach, Tran, Mertz, & Braun, 2017), while leaf R continues to increase. Thus, sugar supply may decline at the same time that sugar demand increases to support stimulated leaf R. In all likelihood, breakdown of starch (i.e. stored carbon) fills this gap between sugar supply and demand in the short term (Hüve et al., 2012; Rashid, Crisp, et al., 2020). However, growth is highly sensitive to sugar and starch depletion (A. M. Smith & Stitt, 2007). It is therefore likely that, as temperatures rise above the optimum, the suppression of growth will reduce assimilate demand and the export of sucrose from leaves to growing tissues.
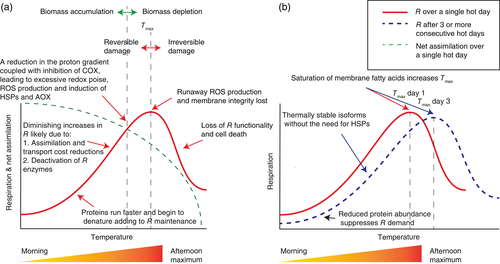
With decreased export of carbohydrates, leaf starch and sugar concentrations may remain constant, despite a decline in photosynthesis and rise in R. Such a situation was postulated to occur in rice (Oryza sativa L.) leaves transferred from 30°C to 40°C (Rashid, Crisp, et al., 2020). Reduced sugar export can lead to substantial leaf R reduction, with as much as 29% of leaf R rate attributed to leaf starch breakdown and export costs (Bouma, De Visser, Van Leeuwen, De Kock, & Lambers, 1995). This decline in assimilate-related metabolism (i.e. reduced rates of photosynthesis, sucrose synthesis and sucrose export) may ultimately contribute to a reduction in demand for respiratory ATP, with a result that leaf R becomes adenylate restricted (i.e. limited by high ATP/ADP ratios and low ADP concentrations). Thus, the fact that the short-term temperature-sensitivity of leaf R is less than exponential (Figures 1 and 3) may be partially accounted for by the way in which heat reduces assimilate acquisition and transport. Depletion of starch reserves and reduced sugar transport to storage tissue may have longer-term implications on plant fitness. Stored starch is commonly utilized by many plants in the reproductive life-stage to develop seeds and other sexual organs (Impa et al., 2019; Morita & Nakano, 2011). Ultimately, when a certain level of heat is reached, net CO2 assimilation rate declines to a negative value as CO2 release outpaces CO2 assimilation, primarily through increased rates of leaf R (Figure 3). The biomass of source leaves may decline as stored carbon, such as lipids and organic acids, is catabolized for R maintenance.
As leaf temperatures rise even higher above the optimum for net CO2 assimilation, proteins begin to denature and lose efficiency. The costs of stabilizing and replacing denatured proteins increase the demand for respiratory energy associated with cellular maintenance. In leaves of higher plants, ATP energy costs associated with protein synthesis and maintenance can account for up to 42% of total ATP demand, and approximately 11% of which are Rubisco-associated ATP costs, as previously discussed (Li et al., 2017). Similarly, an ATP budget was calculated in a study of rice coleoptiles under standard or anoxic abiotic stress conditions – the latter was characterized by stalled growth (Edwards, Roberts, & Atwell, 2012). Half of all respiratory ATP was needed to support protein synthesis; by comparison, cell wall synthesis accounted for ~8%, while carbohydrate and nitrate imports from seed accounted for ~14%. The above studies demonstrate the extent to which proteins are a huge sink for the products of respiration in various plant tissue types, including both growing and non-growing tissue. Clearly, the respiratory requirements to build, maintain and degrade proteins are substantive, which reflects the fact that proteins are largely composed of nitrogen (N), and that greater molecular N content increases ATP synthesis requirements (De Vries, Brunsting, & Van Laar, 1974). To emphasize this point, a greater need for protein synthesis and turnover comes at a cost to biomass accumulation in Arabidopsis (Ishihara et al., 2017).
The less-than-exponential increase in R with short-term temperature rise could simply reflect enzymes of the respiratory pathway becoming deactivated by heat. In support of this, the deactivation profile of malate dehydrogenase from barley (Hordeum vulgare L.) and citrate synthase from pig (Sus scrofa L.) heart are consistent with the overall temperature profile of leaf R (Jaindl & Popp, 2006; Senisterra, Soo Hong, Park, & Vedadi, 2008). Alternatively, stabilizing of proteins, rather than de novo synthesis, may be a means of reducing energy costs as a leaf acclimates to rising temperature over the course of a day. Along with reduced demands for respiratory energy associated with lower rates of CO2 assimilation and sugar export, this stabilization of proteins (and thus reduced demand for respiratory energy) may contribute to the less-than-exponential rise in leaf R with heat stress approaching the Tmax (Figure 3). Heat shock proteins (HSPs) such as HSP70, HSP90, Cpn60 and a myriad of small heat shock proteins (sHSPs) are expressed and increase in abundance within hours of heat stress imposition and can stabilize and refold denatured proteins (Santhanagopalan, Basha, Ballard, Bopp, & Vierling, 2015; W. Wang, Vinocur, Shoseyov, & Altman, 2004). However, HSPs are characterized by having an ATP-binding domain and hydrolyze ATP to perform their function (Hartl, 1996). It is therefore likely that as long as HSPs are required, there will be significant heat-related energy costs to stabilizing proteins. This may increase the demand for respiratory ATP, with consequences for the availability of resources to drive growth.
Another factor that is likely to affect the demand for respiratory energy as leaves are heated is the effect that rising temperature has on membrane fluidity. Membranes are liable to become ‘leaky’ during hot weather, particularly in cells whose fatty acid profile is not highly saturated. While membrane fatty acid saturation can increase in response to sustained exposure to higher temperatures, this increase typically takes several days (Larkindale & Huang, 2004). Therefore, on a day when air and leaf temperatures rise quickly, membranes may become overly fluid as day temperature steadily increases, potentially resulting in leakage of ions and protons across membranes (Allakhverdiev et al., 2008; Niu & Xiang, 2018). Leakage of protons from the mitochondrial inter-membrane space to the mitochondrial matrix can significantly impede the efficiency of ATP synthesis as more respiratory substrates and reducing equivalents are consumed in maintaining the level of proton motive force needed to generate ATP (Brookes, 2005). A loss of the proton motive force reduces ATP synthase activity, resulting in an increase in the concentration of ADP and lower ATP/ADP ratios. Together, these factors can result in reduced adenylate restriction of the mitochondrial electron transport chain (ETC), which in turn can increase electron flux through the ETC (O'Leary, Asao, Millar, & Atkin, 2019). Thus, heat has the potential to stimulate mitochondrial O2 uptake while also reducing the efficiency of mitochondrial ATP synthesis.
In addition to reducing the efficiency of ATP synthesis, heat stress can lead to the functionality of the membrane-bound cytochrome c oxidase (COX) being reduced due to membrane disruption. Furthermore, COX and the ATP synthase are both particularly susceptible to oxidative stress via heat-induced peroxidation that inhibits activity (Buchert, Schober, Römpp, Richter, & Forreiter, 2012; Pan, Jones, & Hu, 2014; Paradies, Ruggiero, Petrosillo, & Quagliariello, 1998; Sweetlove et al., 2002). Reduced COX activity can lead to over-reduction of the mitochondrial ETC ubiquinone pool (Møller, 2001; Rhoads, Umbach, Subbaiah, & Siedow, 2006). The excessive reducing potential in the ETC is transferred as single electrons to oxygen and hydrogen peroxide, causing the production of reactive oxygen species (ROS) such as peroxides, superoxide, hydroxyl radical and singlet oxygen (Suzuki & Mittler, 2006; Turrens, 2003). The build-up of ROS damages nuclear DNA, proteins and membranes (J. F. Davidson & Schiestl, 2001; Suzuki, Koussevitzky, Mittler, & Miller, 2012). Thus, the production of ROS with reduced membrane integrity will further exacerbate the degradation of membranes and COX, further accelerating the production of ROS in a detrimental feedback loop (Figure 4).
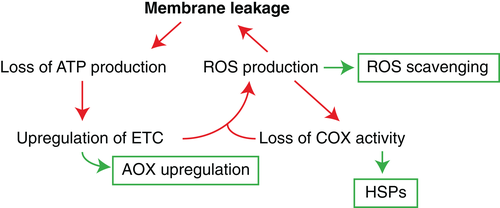
Mitochondria – being a site of high energy and electron flow – have advanced mechanisms to convert ROS to harmless molecules, protect cellular machinery from ROS damage and limit the production of ROS (Figure 4). Removal of ROS is achieved through the use of superoxidase dismutase that can convert superoxide to hydrogen peroxide, followed by conversion of hydrogen peroxide into water and oxygen by ROS scavengers including ascorbate peroxidase and catalase (Giannopolitis & Ries, 1977). An increase in these ROS scavenging enzymes is associated with reduced chlorophyll and membrane damage under hot growing conditions (Almeselmani, Deshmukh, Sairam, Kushwaha, & Singh, 2006). HSPs also appear to provide protection from ROS damage. Arabidopsis mitochondrial HSP70 knockout lines have severely reduced growth, increased ROS and impaired COX assembly and activity (Wei et al., 2019), emphasizing the importance of HSP70 in maintaining COX and limiting ROS production. Supplementation of apple (Pyrus pumila [Mill.] K. Koch) mitochondria with sHSPs enabled a significant increase in ETC activity at 40°C but not 28°C, highlighting sHSPs role in protecting the ETC complexes during heat stress (Downs & Heckathorn, 1998). HSP22, a mitochondria-located sHSP, protects mitochondria from ROS damage in tomato and maize (Banzet et al., 1998; Lund, Rhoads, Lund, Cerny, & Elthon, 2001). However, ROS scavenging pathways and HSP protection cannot alone cope with excessive ROS production under extreme heat, so engagement of metabolic pathways that prevent the production of ROS is necessary. The alternative oxidase pathway that uncouples respiratory oxidation from ATP production – in particular the alternative oxidase (AOX) of the mitochondrial ETC – is one such mechanism by which a leaf can dissipate excessive reducing equivalent and limit ROS production (Millar, Whelan, Soole, & Day, 2011). AOX activity appears to increase under conditions that cause oxidative stress and reduced growth (Saha, Borovskii, & Panda, 2016), helping to dissipate an overly reduced UQ pool without forming ROS (Millenaar & Lambers, 2003). Indeed, a growing number of in vivo studies show AOX activity increasing under stress, including under heat stress (Del-Saz et al., 2018). An example is the involvement of AOX in enabling heat stress tolerance of wheat leaves within a 24-h period of heat-shock (Triticum aestivum L.) (Borovik & Grabelnych, 2018). In rice seedlings exposed to heat stress, the over-expression of AOX improved seedling growth (Murakami & Toriyama, 2008). This may seem counterintuitive, as AOX upregulation would come with a reduction in ATP synthesis presumably needed for growth, but it implies that the improvement to growth came through a reduction in the detrimental effects of ROS on growth.
2.3 Rates of leaf respiration at critically high temperatures
As the daily maximum air temperature is reached, leaf temperatures may approach and even exceed the Tmax. As noted earlier (Figure 1), there is often a spike in the rate of respiratory CO2 efflux at temperatures just below the Tmax (Hüve et al., 2012; O'Sullivan, Weerasinghe, et al., 2013; O'Sullivan, Heskel, et al., 2017). While this could be seen as reflecting greater leaf respiratory demand driven by stress-related energy costs – such as synthesizing degraded proteins – it is unlikely that the spike in respiratory CO2 release so close to loss of leaf R function is coupled with concomitantly higher rates of ATP production. This is because, at such high temperatures, severe heat makes membranes overly fluid and prone to membrane fusion (Hazel, 1995). Approaching the Tmax, the spike in respiratory CO2 release may be attributed to a loss of mitochondrial membrane integrity and subsequent removal of feedback controls by adenylate limitations on ETC activity (Dry, Bryce, Wiskich, & Davies, 1987; Hüve et al., 2012). For example, exposure of mitochondria – extracted from bean (Phaseolus vulgaris L.) hypocotyl tissue – to 40°C for 5 min resulted in near total loss of the ADP:O ratio (the amount of ADP required per O2 consumed during oxidative phosphorylation) despite continued O2 consumption, implying loss of a proton gradient (Lin & Markhart, 1990). Thus, the CO2 spike likely demonstrates a critical breakdown in cellular structures, particularly membranes, during severe heat stress.
As the thermal limits of leaf R are approached and ROS production increases, proteins denature, aggregate and become increasingly non-functional. The associated costs of replacing denatured proteins would contribute to greater respiratory maintenance costs during heat stress. However, as previously mentioned, adenylate supply under heat stress will be limited by loss of membrane integrity and will unlikely meet the ever-increasing demand associated with the costs of heat-induced protein synthesis and degradation and membrane repair.
The range of 50–60°C in which Tmax falls experimentally (Figure 1) may be an overestimation of the in situ Tmax. Laboratory-based experimental studies of Tmax in plants have typically ramped temperatures by 1°C/min, meaning that each temperature is only briefly experienced by the plant (Hüve et al., 2012; O'Sullivan, Weerasinghe, et al., 2013; O'Sullivan, Heskel, et al., 2017). By contrast, in nature, daily temperatures generally rise and are held over a longer duration of hours. As damage to the leaf is a combination of duration and severity (e.g. a greater ROS build-up with sustained heat), the short-term experimental approach will likely overestimate Tmax. Indeed, O'Sullivan, Weerasinghe, et al. (2013) demonstrated that increasing the duration of heat exposure from 5 to 60 min significantly reduced Tmax.
If the Tmax is surpassed, damage is likely to be permanent, given that leaf R function declines rapidly. Interestingly, there are no experimental observations of leaf functionality during recovery after the Tmax has been surpassed. This has important ramifications on whether the leaf will remain viable after temperature begins to fall as night approaches. However, although Tmax represents the temperature at which leaf R and cellular functionality are impaired, it should not necessarily be considered as the definitive upper limit of survival. It is now becoming apparent in animal studies that the experimental Tmax may be well above the temperature at which survival declines for organisms in their natural environment (Rezende, Bozinovic, Szilágyi, & Santos, 2020). Damage experienced prior to the Tmax may increase plant susceptibility to other biotic and abiotic stresses, making the plant non-viable in the context of longer term growth and reproductive success in a natural setting.
2.4 Acclimation of respiration to consecutive heatwave days
On the subsequent days after a heatwave begins, the extended duration of heat stress allows thermal acclimation to occur (Figure 3). Specifically, genes that were induced on the first day of the heatwave may lead to synthesis of thermally stable isoforms of proteins by the second and following days (Somero, 1995). An example of this is evident in the previously mentioned thermolabile photosynthetic enzyme, Rca. Thermally stable isoforms of Rca exist and are induced by heat stress. However, it takes time for gene expression to culminate in a change in protein abundance. In wheat, a 40-fold increase in gene expression of a heat stable isoform of Rca occurred within 4 h of heat application, yet it took 7 days to register a significant increase in protein abundance of the thermally stable isoform (Degen, Orr, & Carmo-Silva, 2020). More applicable to leaf R is mitochondrial malate dehydrogenase isoforms from beach pea (Lathyrus japonicus Willd.) and Arabidopsis. In both species, malate dehydrogenase from genotypes adapted or acclimated to warmer temperatures had faster activity at warmer temperature and greater thermal stability (Simon, 1979; Simon, Potvin, & Blanchard, 1983). Changes in the proteome are likely to have a greater impact on the efficiency of leaf R, as heat-inducible isoforms of proteins that are more thermally stable do not require the added energy costs associated with HSPs. Furthermore, the catalytic efficiency of heat-induced enzymes is optimized to warmer growth temperatures, and a more rigid protein structure may prolong protein half-life (Somero, 1995). This will likely reduce the respiratory maintenance costs of leaves and enable a greater contribution of leaf R to growth, thus increasing the plants optimal growth temperature. Further study is required in order to quantify the level of genetic diversity in thermally stable and efficient isoforms of mitochondrial proteins, and proteins more generally, particularly in terms of what proteins and in which species. As with proteins, membrane structures can be synthesized de novo over the days that a heatwave progresses (Los & Murata, 2004). As previously discussed, increases in fatty acid saturation may raise the Tmax so that the thermal safety margin (the difference between the leaf temperature and Tmax) will increase (O'Sullivan, Heskel, et al., 2017; Zhu et al., 2020). Consequently, there will be less chance that the maximum daily temperatures approach or exceed the Tmax after longer-term acclimation (Figure 3).
In the dark, leaf R is reliant on substrate supply from stored reserves (e.g. starch, sugars, organic acids; though is starch the primary storage form), which are broken down and depleted during the night to fuel respiration (A. M. Smith & Stitt, 2007). If a heatwave is severe and protracted, limits on photosynthetic CO2 assimilation coupled with stimulated leaf R (for maintenance of cells stressed by heat) may deplete starch reserves, as less is produced and more is consumed. The extent to which heat-depletion of starch reserves results in substrate-limitation of leaf R, as opposed to leaf R becoming reliant on increases in ATP demand, is still an unresolved question. Although both could contribute to heat-induced changes in leaf R rate, it seems more likely that leaf R is driven to a greater extent by changes in energy demand and the efficiency of ATP synthesis. The TCA cycle and ETC are regulated by negative feedback from the inhibition of PEP, malate and ATP, rather than by the glycolysis substrates, glucose and fructose (O'Leary et al., 2019; Plaxton & Podestá, 2006). It has been postulated that, with protein denaturation under stress, proteins are broken down and used as an alternative carbon source for respiration and helping to maintain ATP production (Araújo, Tohge, Ishizaki, Leaver, & Fernie, 2011). Supplying metabolites, such as amino acids and organic acids, directly to leaf tissue does stimulate R as much as supplying sugars (O'Leary et al., 2017). These factors all suggest that sugar supply plays a limited role in regulation of respiratory flux in leaves. Determining respiratory quotients (the ratio of CO2 to O2 R flux) may provide insight by determining the extent to which respiratory substrates switch from sugars to other primary metabolites under varying levels of heat stress and acclimation.
3 ENVIRONMENTAL STIMULI INTERACT WITH THE HEAT RESPONSE OF RESPIRATION
The impacts of air temperature on leaf R do not occur in isolation from other abiotic stimuli. In the context of a hot day, irradiance and water availability are two other abiotic factors that will have a significant influence on leaf R and its interaction with temperature. Irradiance is important because the leaf will transition from dark to light within a day, and it is well known that leaf R flux differs depending on whether it is light or dark (Krömer, 1995; Lambers & Ribas-Carbo, 2005; Tcherkez et al., 2017). Leaf R is significantly altered by the presence or absence of photosynthesis, particularly in cells that are photosynthetically active (N. G. Smith, Li, & Dukes, 2019). Water availability is especially important when considering extremely hot days and heatwaves, as drought and heat often occur concurrently due to the evaporative effects of heat on soils and the larger VPD between leaves and the surrounding air on hot days (Sadok, Lopez, & Smith, 2020; Teskey et al., 2014). With this in mind, we consider how dark to light transitions, and water limitations, would each impact the high temperature response of leaf R.
3.1 Light versus dark respiration
For reasons that are not fully understood but are related to the absence of photosynthesis in the dark, the presence of photorespiration in the light and how light places demands on TCA intermediates, leaf R in the light is lower than it is in the dark, even when accounting for refixation of respiratory CO2 by chloroplasts (Tcherkez et al., 2017). The production of NADH in the photorespiratory conversion of glycine to serine, as well as excess ATP and NADH generated from the chloroplast ETC, likely supplement mitochondrial and cytosolic energy requirements (Shameer, Ratcliffe, & Sweetlove, 2019). Although it is generally accepted that leaf R is supressed in the light, whether the temperature-response of leaf R in the light is the same as that of the dark is unclear. Previous work on Eucalyptus pauciflora Sieb. Ex Spreng suggests that leaf R is less responsive to temperature in the light (Atkin, Evans, Ball, Lambers, & Pons, 2000). However, a recent study in Eucalyptus tereticornis Sm. that used two alternative methods showed no significant difference in the temperature response of leaf R when plants were in the light or dark (Way et al., 2019). Similarly, the artic species Eriophorum vaginatum L. and Betula nana L. have a less sensitive or similar temperature response of leaf R in the light relative to darkness, respectively (McLaughlin, Xu, Rastetter, & Griffin, 2014). Thus, it is unclear whether leaf R in the light is less responsive to short-term changes in air temperature or whether it has a similar short-term response to leaf R in the dark, with the distinction seemingly highly species specific. With regards to how sustained changes in growth temperature interact with the day/night cycle, a recent study on rice showed no significant interaction between time of day/night and the extent to which leaf R acclimated to air temperatures ranging from 25/20 to 40/35°C (day/night) (Rashid, Scafaro, et al., 2020). Growth temperature had a minimal effect on leaf R metabolite pools over a day/night cycle (no significant interaction), despite significant shifts in metabolite pools with growth temperature and over a day/night cycle, when analysed individually. With this in mind, it seems valid to assume that the response of leaf R in the light over the course of a day, or its acclimation over several successive hot days, will follow a similar pattern to that of leaf R measured in the dark, albeit with reduced absolute rates of leaf R at a given temperature and potentially with less sensitivity to air temperature.
Of further interest, it is becoming apparent that nighttime air temperature is potentially more important than daytime air temperature in determining the susceptibility of plant growth to heat (Anderegg et al., 2015; Turnbull, Murthy, & Griffin, 2002). The impacts of night warming on growth are thought to be heavily related to stimulation of leaf R and a loss of stored assimilates (Sadok & Jagadish, 2020). Further research is needed to ascertain why night more than day heat is critical in regards to leaf R and biomass accumulation. With this in mind, the extent to which the night warms during a heatwave may be equally as important a consideration as the daytime maximum reached.
3.2 Water stress
An increase in global surface temperatures and heatwaves does not necessarily coincide with reduced precipitation events and drier soils (Alexander et al., 2006). However, rising air temperatures will certainly increase the VPD of the air, driving greater leaf transpiration (Grossiord et al., 2020). Changes in transpiration directly influence leaf and mitochondrial temperature through latent heat transfer and thus the difference between air temperature and Tmax. Increased transpiration leads to greater water loss and can promote stomatal closure (Eamus, Taylor, Macinnis-Ng, Shanahan, & De Silva, 2008). However, without sufficient transpiration, coupled with solar radiative absorption, leaf temperature can exceed air temperature by more than 10°C (Blonder & Michaletz, 2018). As a result, under both hot and dry conditions, the temperature experienced at the site of mitochondria could approach, and potentially exceed, Tmax even if the ambient air is substantially cooler. Conversely, if stomata remain open, a leaf can remain substantially cooler than the ambient air. The importance of leaf cooling is evident in eucalypt trees (Eucalyptus parramattensis E.C. Hall) during a simulated heatwave, in which transpirational cooling reduced leaf temperatures by as much a 7°C, enough to keep them below the point at which assimilation fell by half (Drake et al., 2018). As trees have deep tap roots, it becomes apparent that increased VPD may be beneficial in terms of speeding up transpiration cooling and latent heat transfer, but only if water is plentiful at the root source and stoma remain open.
If a leaf does experience water shortage in conjunction with heat stress, there can be dramatic implications on leaf R. Severe water stress, as with heat stress, can lead to a burst in leaf R activity, including an increase in R with temperature in herbaceous leaves that are wilted (Slot, Zaragoza-Castells, & Atkin, 2008). This is presumably related to the energetic costs associated with preserving cellular activities that are collapsing, such as maintaining membrane stability (Atkin & Macherel, 2009). Over sustained periods of severe drought leaf R rate declines. For example, R significantly declined in Quercus ilex L. when exposed to severe drought, related to changes in TCA cycle intermediates rather than sugar substrate limitation (Rodríguez-Calcerrada et al., 2018). Exploration of in situ leaf temperature under varying VPD and soil moisture contents is required to more accurately assess the role of water relations in the high temperature response of leaf R, as well as to accurately determine the frequency at which leaves will surpass Tmax over the coming years. Based on projections of future climate, as many as 60% of plant species – specifically those that grow in inland-temperate, semi-arid and arid sites prone to periodic drought and heating events – will exceed their Tmax with a 10°C rise in leaf temperature (O'Sullivan, Heskel, et al., 2017). This highlights the profound implications that heating and drought have on the viability of entire ecosystems.
4 CONCLUSIONS
The sensitivity of leaf R to changes in air and leaf temperature over the course of a hot day most notably reflects the effects of high temperatures on protein and membrane structural integrity. Enzymes have faster reaction rates but also begin to denature with heat stress, leading to increased rates of leaf R and an increased demand for respiratory energy associated with protein maintenance; together, these factors drive faster rates of leaf R with rising air and leaf temperature. Conversely, the greater sensitivity of photosynthesis to heat stress limits assimilate acquisition, growth and metabolite transport rates, all of which contribute to suppressing leaf R rate. Enzymes directly involved in leaf R pathways also deactivate under heat stress. The combined influence of these competing mechanistic and regulatory processes is responsible for the near exponential rise in leaf R rate with short-term heating in the low-moderate thermal range and decreasing acceleration of leaf R rate as temperatures approach the high-temperature threshold (i.e. Tmax). With more severe heat stress, membranes leak and ROS are produced, which then promotes further membrane damage and ROS production. The loss of membrane integrity and ROS production likely sets the Tmax, and thus the point at which heat stress becomes lethal. Whether leaves can survive heatwaves of greater severity and duration over the coming years will in large part depend on whether leaf temperatures approach or exceed the respiratory Tmax. Other plant organs, such as roots and reproductive tissue, require greater study to determine the whole plant response to heatwaves. Even if hot days do not exceed this upper thermal limit, merely approaching the Tmax is likely to cause extreme duress to cellular integrity, rendering plants more susceptible to other concomitant biotic and abiotic stresses. This will have profound implications on the productivity of both agricultural crops and biodiversity in natural ecosystems.
ACKNOWLEDGMENTS
This work was supported by grants from the Grains Research Development Corporation (GRDC) (US00080, and 2016.02.01G) and the ARC Centre of Excellence in Plant Energy Biology (CE140100008). Onoriode Coast was supported by a GRDC Fellowship: ‘Photosynthetic Acclimation to High Temperature in Wheat’ (US1904-003RTX – 9177346) aligned with the GRDC National Wheat Heat Tolerance Project (US00080). Bradley C. Posch was also supported by the Australian Government Research Training Program. Yuzhen Fan was supported by an ANU PhD Scholarship (International) Full-Time (737/2018) and Higher Degree Research Fee Remission Merit Scholarship.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Andrew P. Scafaro drafted the manuscript with input from all other authors.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.




