CAM-physiology and carbon gain of the orchid Phalaenopsis in response to light intensity, light integral and CO2
Funding information: Dutch pot-orchid growers association; Dutch Program ‘Kas als Energiebron’ funded by The Ministry of LNV and Glastuinbouw Nederland; Nederlandse Organisatie voor Wetenschappelijk Onderzoek, Grant/Award Number: 14525
Abstract
The regulation of photosynthesis and carbon gain of crassulacean acid metabolism (CAM) plants has not yet been disclosed to the extent of C3-plants. In this study, the tropical epiphyte Phalaenopsis cv. “Sacramento” was subjected to different lighting regimes. Photosynthesis and biochemical measuring techniques were used to address four specific questions: (1) the response of malate decarboxylation to light intensity, (2) the malate carboxylation pathway in phase IV, (3) the response of diel carbon gain to the light integral and (4) the response of diel carbon gain to CO2. The four CAM-phases were clearly discernable. The length of phase III and the malate decarboxylation rate responded directly to light intensity. In phase IV, CO2 was initially mainly carboxylated via Rubisco. However, at daylength of 16 h, specifically beyond ±12 h, it was mainly phosphoenolpyruvate carboxylase (PEP-C) carboxylating CO2. Diel carbon gain appeared to be controlled by the light integral during phase III rather than the total daily light integral. Elevated CO2 further enhanced carbon gain both in phase IV and phase I. This establishes that neither malate storage capacity, nor availability of PEP as substrate for nocturnal CO2 carboxylation were limiting factors for carbon gain enhancement. These results advance our understanding of CAM-plants and are also of practical importance for growers.
1 INTRODUCTION
There are relatively few studies on the photosynthetic functioning of plants with crassulacean acid metabolism (CAM), compared with C4 and especially C3 plants. This is most probably because CAM-plants are less important for agriculture than C3 crops (e.g., wheat, rice, potato) and C4 crops (e.g., corn, sugar cane; (Davis et al., 2019; Yang et al., 2015)). Some examples of CAM-plants grown in agriculture are Agave spp., Opuntia spp., pineapple (Ananas comosus (L.) Merrill) and plants are grown as ornamentals in floriculture (e.g., Kalanchoe spp., Phalaenopsis orchids and several cacti and other succulent species). CAM serves to increase water use efficiency and is diverse among species (Borland et al., 2014; Cushman, 2001). In the classical model of CAM there are four phases (Osmond, 1978): CAM-phase I is at night, stomata are open and CO2 is fixed to phosphoenolpyruvate (PEP) via PEP-carboxylase (PEPC) and stored in the vacuole as malate. CAM-phase II is in the early morning, the stomata are still open (or re-open) and carboxylation shifts from PEPC to Rubisco. In CAM-phase III the stomata are closed, malate is decarboxylated and subsequently, CO2 is fixed again via Rubisco. It is CAM-phase III where normally the greatest proportion of assimilation takes place, though exceptions are possible (Griffiths et al., 2002; Lüttge, 2006). CAM-phase IV can occur in the late afternoon in a well-watered plant, the stomata re-open and CO2 is fixed mainly via Rubisco, as occurs throughout the light period in C3-plants. This pattern has been shown in detail for several species including Kalanchoe (Griffiths et al., 2002), Aechmea “Maya” (Ceusters et al., 2010) and Clusia (Lüttge, 2006).
Phalaenopsis is a genus of epiphytic orchids found mainly in tropical Asia (Tsai, 2011). It is also an important ornamental crop across the globe. Because of the variety of regions and habitats for CAM-plants (desert, mountains and epiphytic growth in tropical forests), there are strong differences in the physiological adaptation to environmental cues and the extent to which species engage in CAM relative to C3 (Winter, Holtum, & Smith, 2015). Responses of Phalaenopsis to light and CO2 have been studied in mostly horticultural studies (e.g., Guo, Lin, & Lee, 2012; Lootens & Heursel, 1998; Pollet, Steppe, Dambre, Van Labeke, & Lemeur, 2010). Both diel CO2 uptake, as well as leaf initiation rate, increased when light intensities increased (Hückstädt & Torre, 2013; Ota, Morioka, & Yamamoto, 1991). However, compared to other CAM plants, their saturation point is low (Ota et al., 1991). While the genome of Phalaenopsis equestris has been sequenced several years ago (Cai et al., 2015), the physiology of CAM in Phalaenopsis has not been studied in much detail yet. Our study provides a comprehensive analysis of the response of Phalaenopsis to different climate factors (light, CO2), using a combination of photosynthesis measurements (gas-exchange, chlorophyll fluorescence) and biochemical analyses of changes in the leaf malate and citrate concentration. In particular, this study addresses four questions on the functioning of the CAM metabolism of Phalaenopsis “Sacramento.”
Firstly, the response of malate decarboxylation in CAM-phase III to light intensity is addressed. So far, it is not known for Phalaenopsis whether the light intensity affects the rate of malate decarboxylation, the length of CAM-phase III and/or the start of CAM-phase IV. Regulation of malate decarboxylation in CAM-phase III is still the subject of debate. Several different decarboxylation pathways occur, with the involvement of different enzymes at different locations in the cell (Holtum, Smith, & Neuhaus, 2005). According to Borland, Griffiths, Hartwell, and Smith (2009), it has long been thought that the transport of malate from the vacuole to the cytosol was a passive process. Therefore, malate transport from the vacuole could be the limiting factor for the malate decarboxylation rate in CAM-phase III (Dodd, Borland, Haslam, Griffiths, & Maxwell, 2002). Alternatively, malate decarboxylation rates in the cytosol may be dependent on light intensity driven CO2 assimilation rates (Dodd et al., 2002; Lüttge, 2004). If malate decarboxylation rates are slower than CO2 assimilation rates at higher light intensities, malate decarboxylation itself could be a limiting factor for CO2 assimilation in CAM-phase III (Holtum et al., 2005). For several Kalanchoe species, it has been shown convincingly that the malate decarboxylation rate is driven, either directly or indirectly, by light intensity in CAM-phase III, which results in an earlier start of CAM-phase IV (Barrow & Cockburn, 1982; Kluge, 1968; Nishida & Hayashi, 1980; Thomas, André, & Granzin, 1987). In other CAM-plants different regulatory mechanisms may apply. The internal CO2 concentration in CAM-plant leaves during CAM-phase III may indicate if light intensity regulates the malate decarboxylation rate. When the internal CO2 concentration remains below or just slightly above saturating levels for carboxylation of Rubisco, a regulatory mechanism related to the assimilation rate seems plausible (Lüttge, 2002). Whilst CAM is considered a CO2 concentrating mechanism that reduces photorespiration via oversaturation of Rubisco in phase III, such high concentrations were not measured in most species (Lüttge, 2002). Different internal CO2 concentrations have been reported for different CAM-species, for example, 800 ppm for Hoya carnosa, 2,300 ppm for Phalaenopsis, up to 25,000 ppm for two Opuntia species, but the latter seems an exception rather than the rule for the CAM species investigated in this respect so far (Cockburn, Ting, & Sternberg, 1979; Spalding, Stumpf, Ku, Burris, & Edwards, 1979). We have made a detailed analysis of both the short-term and long-term response of malate decarboxylation to light intensity in Phalaenopsis leaves.
Secondly, the carboxylation pathway in CAM-phase IV at unnaturally long daylength (16 h) is addressed. In its natural habitat in tropical Asia, Phalaenopsis experiences daylengths close to 12 h. However, the daylength is often considerably longer when Phalaenopsis is grown as an ornamental at higher latitudes or with supplemental lighting. Diel CO2 uptake seems to increase with daylengths up to 14 h (Chen & Lin, 2012; Guo et al., 2012), but it is unclear whether this results in a more prominent carboxylation of Rubisco or PEPC. Although in CAM-phase IV CO2 is widely considered to be fixed mainly via Rubisco (e.g., Cushman, 2017; Dodd et al., 2002; Osmond & Holtum, 1981), storage of malate due to CO2-fixation via PEP-C may become more important under extended light periods, which would make CO2 uptake independent of light intensity towards the end of the day. Ceusters et al. (2010) have shown that Aechmea “Maya” grown at 16-h daylength indeed did fix CO2 via both Rubisco and PEP-C in phase IV, the ratio dependent on the growing season. We have investigated in detail how the lighting regime can affect the carboxylation-pathway in phase IV for Phalaenopsis “Sacramento.”
Thirdly, the response of diel carbon gain to the daily light integral (DLI) is analysed. The relative effects of the light integral during CAM-phase III and CAM-phase IV are disentangled. In addition, the effect of fluctuations in light conditions on malate carboxylation and decarboxylation patterns is explored. Previous studies showed that very short days (6 h) result in leakage of CO2 out of the leaf during phase III (Chen & Lin, 2012). Lee, Lee, An, Park, and Kim (2019) showed that vegetative plant biomass of a Phalaenopsis cultivar increased when DLI was kept identical (5.76 mol m−2 day−1) but photoperiod increased from 8 to 16 h (at 200 and 100 μmol m−2 s−1 PPFD, respectively), and that this was mostly due to an increase in root biomass. However, a long day with the same DLI also resulted in a faster time to flowering (Lee et al., 2019). These results indicate shifts in the carbohydrate balance, but the disentangled effects of DLI and light intensity on CO2 uptake and malate levels are not yet known. By analysing the effect of fluctuating light conditions quantitatively over both the short and long term, we show the extent to which CAM photosynthesis can adapt to changing light levels and what the consequences are for total carbon gain.
Fourthly, we studied the effect of the CO2 concentration in the growth environment on carbon gain. Despite PEPC having a low saturation point for internal CO2 at about 200 ppm, compared with Rubisco at about 600 ppm (Taiz & Zeiger, 2010), a limited stomatal conductance (gs), limited intercellular airspace (Nelson & Sage, 2008) and limited mesophyll conductance (Males & Griffiths, 2017) may still result in an internal CO2 concentration as low as 110 ppm in CAM-phases IV and I (Maxwell, von Caemmerer, & Evans, 1997). Elevated CO2 may therefore result in a higher CO2 uptake. Inconsistent effects of elevated CO2 on CAM-plants have been reported. Lootens and Heursel (1998) showed an increase in CO2 uptake in Phalaenopsis hybrids when ambient CO2 was increased, whereas for other Phalaenopsis varieties contradictory results have been reported (Matschke, Orthen, & von Willert, 1998). It was found for Kalanchoe pinnata that increased CO2 leads to a higher CO2 assimilation rate, but this hardly seemed to affect the carbohydrate pool for the provision of the nocturnal PEP substrate (Winter & Engelbrecht, 1994). Ceusters et al. (2008) measured a substantial increase in 24-h carbon gain under elevated CO2 for the CAM bromeliad Aechmea “Maya.” However, this was due to an elevated carboxylation in phases II and IV, but not in phase I, and malate accumulation was hardly affected.
Increased CO2 uptake in phase I will only result in more carbon gain if several conditions are met: Firstly, the vacuole has to be capable to store more malate. Vacuolar storage capacity is not only limited by the actual size of the vacuole but also due to the balance between active proton pumping influx and passive efflux (the difference of H+ across the tonoplast (Holtum et al., 2005; Winter, 1985)). Secondly, enough PEP needs to be available to produce more malate. CAM plants always have to precariously balance different carbohydrate pools, for nocturnal production of PEP requires a substantial amount of carbohydrates (up to 20% of daily leaf carbohydrate turnover), which competes with carbohydrates needed for respiration and export for growth (Borland, Guo, Yang, & Cushman, 2016). These pools are plastic and their size is mainly determined by direct adaptation to changes in environmental conditions (Borland, Maxwell, & Griffiths, 2000). Lastly, enough light needs to be available during the following day to assimilate the additionally stored CO2. We address the question of whether or not carbon gain of Phalaenopsis is limited by the storage capacity of the vacuoles for malate, or by the availability of PEP as a substrate for malate carboxylation in the dark period.
Together these data provide a detailed overview of the functioning of CAM in Phalaenopsis “Sacramento,” enhancing the understanding of how CAM can respond to environmental cues. The implications of our results are discussed from an eco-physiological point of view. In addition, the consequences for greenhouse growers are briefly discussed.
2 MATERIALS AND METHODS
2.1 Plant material and general growth conditions
Two experiments were conducted: a lighting experiment and an additional CO2-experiment. Both experiments were performed using Phalaenopsis “Sacramento” (further referred to as Phalaenopsis).
For the lighting experiment, young plants were potted in a bark medium, grown in a commercial greenhouse and transferred to the climate chambers of Plant Lighting (Bunnik, the Netherlands) 17 weeks after potting. The plants were divided into six climate-units of 2 m2 each, all situated within one climate chamber and 130 plants per unit (112 plants per unit after destructive measurements). The plants were grown at 29°C average diel temperature, relative humidity (RH) was ±70% and the CO2 concentration was 800 ppm. This relatively high CO2 concentration was to maximize malate storage, which increases distinctiveness between effects of lighting-treatments on malate (de)carboxylation. Every 4 to 5 days plants were watered with a nutrient solution (EC ≈ 1.2, pH ≈ 5.8). To ensure that all measurements (photosynthesis, malate and citrate samples) were under conditions without any drought stress, individual plants were sometimes watered an additional time before measuring. In the first 3 weeks, the conditions were equal in all six climate units, allowing the plants to acclimate to the new conditions.
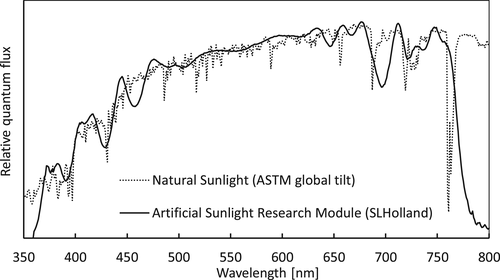
Light was provided by solar simulators emitting a spectrum nearly identical to that of natural sunlight within the range 360–780 nm (Artificial Sunlight Research Module (ASRM), SLHolland, Bunnik, the Netherlands). The spectrum is shown in Figure 1. The ASRM is dimmable with retention of the spectrum, and the UV (360–400 nm), PAR (400–700 nm) and far-red (700–780 nm) spectral ranges are also dimmable separately via three 0–10 V signals. Different lighting schedules could be programmed using a programmable DMX-interface (SLESA UE7, Nicolaudie, France) connected to a multiple-channel DMX to 0–10 V signal converter. Throughout the lighting experiment, the spectrum as shown in Figure 1 was maintained, whereas light intensity patterns were different for the different treatments (details below).
2.2 Experimental conditions used for the lighting experiments
Throughout the experiment, all plants received a 16 h photoperiod in which at certain times light intensities changed (Table 1). These settings were tuned to the expected CAM-phases based on explorative photosynthesis measurements: 6:00–7:30 (expected CAM-phase II), 7:30–17:30 (expected CAM-phase III) and 17:30–22:00 (expected CAM-phase IV). After the initial 3 weeks acclimation (exposed to MML, Table 1), the light settings were varied between all six climate-units for 1 day, to measure the impact of different light intensities on the CAM pattern (see gas-exchange and biochemical analysis methods below). The different lighting treatments are in Table 1. Expected CAM-phase III is roughly split into PAR setting I and II for, respectively, the first and the second half of CAM-phase III. Only for the expected CAM-phase III, which was determined at the end of the acclimation period, the lighting differed between treatments. Note that the lighting treatments may change the duration of phase III.
| Treatment | 22:00–06:00 | 06:00–07:30 | PAR setting I 07:30–12:30 | PAR setting II 12:30–17:30 | PAR setting III 17:30–22:00 | |
|---|---|---|---|---|---|---|
| Low/low/low | LLL | 0 | 60 | 60 | 60 | 60 |
| Med/med/low* | MML | 0 | 60 | 120 | 120 | 60 |
| High/high/low | HHL | 0 | 60 | 180 | 180 | 60 |
| Very high/very high/low | VVL | 0 | 60 | 300 | 300 | 60 |
| High/low/low | HLL | 0 | 60 | 180 | 60 | 60 |
| Low/high/low | LHL | 0 | 60 | 60 | 180 | 60 |
- * The first 3 weeks all plants were grown under this lighting regime.
After determining the short-term (1 day) effect of the changes in light regime, the plants were grown for another 10 weeks under six different lighting regimes (Table 2). The long-term effect of these treatments on the CAM-photosynthesis pattern was measured on newly developed, fully expanded leaves: photosynthesis was measured 6 to 10 weeks after starting the treatments and leaf samples for biochemical analysis were taken 9 to 10 weeks after starting the treatments. The photoperiod (16 h) and average daily light integral were equal for all six treatments (5.6 mol m−2). In treatment MML, MML/T, HLL and LHL the light integral within CAM-phase III was equal, however, according to a different pattern. This was to resolve the question of whether light intensity regulates the rate of malate decarboxylation, and if there are any consequences of different lighting patterns during CAM-phase III over the long term. LLH received only half the light intensity of the control over the time-course for CAM-phase III in the MML treatment, after which the light intensity was increased. This was to resolve the question if malate storage overnight is decreased, or if CO2 is released from the leaf during phase III and/or if CAM-phase III is extended over a longer period. The alternating light treatment received an illumination pattern of 4 days low light (LLL), and 4 days high light (HHL). Over 8 days this results in an equal average daily light integral as the MML treatment. This was to resolve the question of how photosynthesis patterns respond to fluctuating light conditions, and if such fluctuations have result in a penalty regarding the total carbon gain.
| Treatment | 22:00–06:00 | 06:00–07:00 | PAR set. I 07:00–12:00 | PAR set. II 12:00–17:00 | PAR set. III 17:00–22:00 | ||
|---|---|---|---|---|---|---|---|
| Med/med/low | MML | 0 | 60 | 120 | 120 | 60 | |
| Trapezoid* | Med/med/low | MML/T | 0 | 60 | 60–180 | 180–60 | 60 |
| High/low/low | HLL | 0 | 60 | 180 | 60 | 60 | |
| Low/high/low | LHL | 0 | 60 | 60 | 180 | 60 | |
| Low/low/high | LLH | 0 | 60 | 60 | 60 | 180 | |
| Alternating** | Low/low/low | LLL | 0 | 60 | 60 | 60 | 60 |
| High/high/low | HHL | 0 | 60 | 180 | 180 | 60 |
- * Trapezoid pattern; rising (linearly) from 60 at 07:30 towards 180 at 11:30, and decreasing from 180 at 12:30 towards 60 at 16:30.
- ** Illumination pattern; 4 days low light followed by 4 days high light (continuous cycle). Over 8 days this results in an equal average daily light integral as the other treatments.
2.3 Photosynthesis measurements
All photosynthesis measurements were performed on fully grown, unshaded leaves of well-watered plants. Measurements were performed with a Li-6400 infra-red gas-analyser with a head Fluorometer and a 2 cm2 net area leaf chamber (LI-COR, Nebraska, USA). In all measurements the light spectrum in the leaf chamber was set on 10% blue and 90% red, air flow was 200 μmol s−1, block temperature was 28°C, matching sample and reference gas was done every 20 min and the RH was similar to that in the plant growth chamber. Data were logged every 2 min for gas exchange measurements and chlorophyll fluorescence was measured every 20 minutes during daytime and not during night time. Photosystem II operating efficiency (Fq′/Fm′; where Fq′ = Fm′ − F′) (Baker, 2008)) was determined by recording F′ at steady-state and Fm′ after applying a saturating light pulse (>7,000 μmol m−2 s−1 for 0.8 s).
Initially, diel patterns of gas exchange and fluorescence were measured to determine the time periods of the four CAM-phases (n = 8). CO2 was set at 800 ppm and the leaf chamber lighting was programmed such that the light intensity pattern matched with that in the growth chamber. For all diel measurements in all experiments, each leaf was clamped in the leaf chamber for approximately 20 h. Measurements always started and ended during CAM-phase III, allowing quantitative analysis of leaf CO2 uptake during phases I, II and IV. Although leaf transpiration is measured and stomatal conductance (gs) is calculated by the Li-6400, these measurements are often below the detection limit (gs < 0.01 mol m−2 s−1) at the start of phase IV (stomata open) and end of phase II (stomata close). Therefore, we present atmospheric CO2-uptake > 0 as the moment that the stomata open.
After changing the light settings in the growth chamber (Table 1), gas-exchange was measured for ±5 min per leaf at a light intensity of 120 μmol m−2 s−1 PAR measuring light. These screening measurements were done on 29–39 leaves per treatment for the LLL, MML, HHL and VVL treatments (the treatments with a constant light intensity during PAR setting I and II) and served to determine how the growth-light intensity during CAM-phase III affects the time of malate depletion, and if that results in a lower electron transport rate (ETR) and an earlier start of CAM-phase IV (start of CO2-uptake). In addition, these measurements show if a lower light intensity (LLL) results in an extended CAM-phase III or possibly CO2 not assimilated being released from the leaf. By turning off the measuring light for 2 min per leaf in CAM-phase IV following the ±5 min light intensity of 120 μmol m−2 s−1 PAR, we could determine whether CO2 taken up by the leaf resulted in carboxylation via Rubisco (CO2-uptake drops at light off) or via PEP-C (light-independent CO2-uptake).
After 6 weeks acclimation to the long-term light treatments (Table 2), diel patterns of gas exchange and fluorescence were measured on six leaves per treatment for treatment MML, MML/T, HLL, LHL, LLH, and for the alternating light treatment six leaves on the first and on the fourth day of the alternating low and high light periods (LLL D1, LLL D4, HHL D1, HHL D4; N = 6 per measuring period). The leaf chamber lighting was programmed such that the light intensity pattern matched with that of the growth light treatment.
2.4 Malate and citrate measurements
Changes in malate and citrate concentration in the leaf during the light period were measured in the lighting experiments. On the day that the light settings in the growth chamber were changed (Table 1) and after long-term adaptation (Table 2), leaf samples were cut at approximately 2-h intervals between 06:00 and 22:00 (nine times per day). Four leaf discs (1 cm diameter) were cut from pairs of fully grown unshaded leaves from two plants (two discs per leaf) each time, and the complete time-series was made from the same pair of leaves, starting from the leaf tip towards halfway the leaf length in the direction of the leaf base (three pairs of leaves per treatment, n = 3). To exclude if the sampling position on the leaf affects the malate concentration, samples were also taken at the different positions on the leaf at the same time. No difference between the leaf tip and the middle leaf position was measured. To quantify the effect of taking samples from the same leaf, samples were taken for 4 days in a row at 08:30 from the same leaves (N = 3). The malate concentration decreased from 125 to 83 mmol/m2 (p < .05) on the second day and remained stable on the third and fourth day. This implies that the sampling method may have quantitatively affected the malate response measured in the time-series over the day. Nonetheless, it is highly unlikely that this has interacted with the clear differences in malate decarboxylation in response to light shown in the results section. The method does not affect the malate concentration measured at the start of the day as those leaves were always fully intact.
The samples were placed in Eppendorf tubes, frozen in liquid nitrogen and stored at −80°C. Subsequently, the samples were freeze-dried, weighed, powdered with a ball-mill (1 minute with two metal balls of 3 mm), after which 15 mg was weighed. The 15 mg was subsequently dissolved in 5 mL 75% ethanol, heated in a shaking water bath (80°C, 20 min) and centrifuged for 5 min (8,500 rpm, 4°C). Next, 1 mL of supernatant was dried in the Speed Vac at 55°C, evaporated ethanol was replaced by 1 mL MiliQ water and mixed for 10 min in an ultrasonic bath (50 Hz) followed by vortex and centrifuge for 10 min. Samples were diluted fivefold with MiliQ water before the malate and citrate concentrations were finally measured with a Dionex HPAEC system (Dionex Corporation, Sunnyvale, CA, USA) equipped with a GS50 pump, an ED50 detector and with an IonPac AS11-HC (250x2mm) column, and eluted with a gradient starting at 16 mM NaOH. Total malate and citrate were calculated in mmol m−2 using leaf mass area of dried leaf discs.
2.5 Experimental conditions used for the CO2 experiment
To assess the effect of elevated CO2 on carbon gain, a separate experiment was performed. Phalaenopsis “Sacramento” plants were grown for 29 weeks in four climate-controlled glass cabins situated in a greenhouse in the Netherlands. The cabins allowed accurate control of temperature, humidity and CO2. Daylight was supplemented with high-pressure sodium (HPS) lamps if necessary, to reach the required daily light integral (DLI) and a 15-h photoperiod. In the vegetative stage, the temperature was 28.5°C, relative humidity was 70% and the average DLI was 6.4 mol PAR m−2. In the flowering stage, the temperature was lowered to 19.5°C and the average DLI was 8.3 mol PAR m−2. Throughout the experiment, in two cabins the CO2 concentration was 400 ppm, and in two cabins it was 800 ppm.
After 12 to 16 weeks growth in the cabins (end vegetative stage), and again after 25 to 29 weeks (flowering stage), a selection of plants were transferred to a climate chamber with a similar climate and DLI as in the cabins, however with stable lighting conditions provided by a combination of ASRM (Figure 1) and HPS lamps. The 400 and 800 ppm grown plants were further grown in the climate chamber at 400 ppm and the 800 ppm, respectively. The plants were allowed to acclimate for 4 days and over the following days, the diel patterns of gas exchange and fluorescence were measured on fully expanded leaves of six different plants per cabin (12 leaves per CO2 treatment for both the vegetative and flowering stage; n = 12). The leaf chamber lighting was programmed according to the light intensity pattern in the growth chamber. Both in the climate chamber and in the leaf chamber the same CO2 concentration was set.
To assess if long-term growth at elevated CO2 results in acclimation of diel CO2-uptake capacity and CO2-uptake in phase I (as an indicator of the malate-pool), 400 ppm grown plants were also placed in a climate chamber with 800 ppm CO2 and photosynthesis was measured at 800 ppm CO2 (leaves of six plants per cabin, so n = 12 leaves for both the vegetative and flowering stage).
2.6 Statistics
Statistical analyses were carried out on the malate and citrate measurements via a paired t-test when comparing two timesteps within a treatment and a normal t-test when comparing for differences in-between the treatments at a certain timestep. The malate decarboxylation rate was analysed by comparing the slope of the curve within a certain time interval via a normal t-test. The data from all diel photosynthesis measurements were analysed through one-way ANOVA analyses.
3 RESULTS
3.1 CAM pattern before changing light intensity
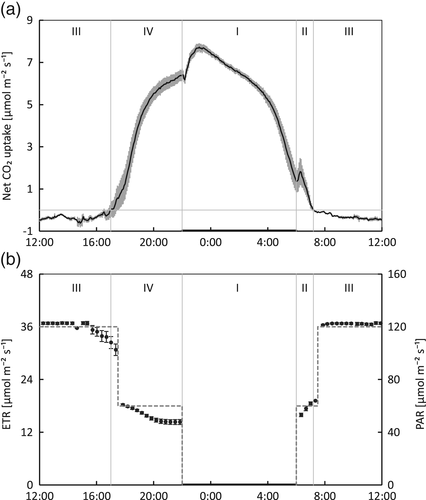
During the last 4 days of the initial 3-week acclimation period in the climate chamber, the diel gas-exchange and ETR were measured (Figure 2). During CAM-phase II (06:00–07:12) ETR was rising and during CAM-phase IV (17:00–22:00) ETR decreased gradually. A considerable part of the diel CO2-uptake was during CAM-phase IV, which lasted 5 h (17:00–22:00).
3.2 Effect short-term (1 day) change in light intensity on CAM pattern
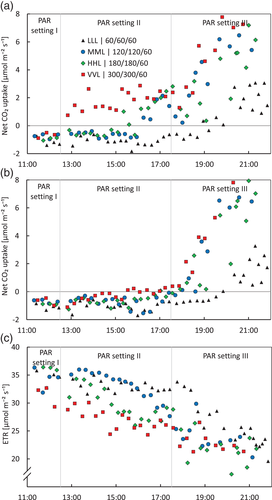
After changing the light intensity settings during 07:30–17:30 (Table 1), leaf samples were collected for malate and citrate analysis, and photosynthesis measurements were carried out on as many leaves as possible (±5 min at 120 and 2 min 0 μmol m−2 s−1 PAR measuring light) in treatments LLL, MML, HHL and VVL (i.e., the treatments with a constant light intensity of 60, 120, 180 and 300 μmol m−2 s−1 PAR during PAR setting I and II). The higher the light intensity, the earlier the opening of the stomata (CO2-uptake > 0, Figure 3a) and the earlier ETR started to decrease (Figure 3c), both indicating that the higher the light intensity, the earlier malate is depleted. There is some variation in the exact moment that net atmospheric CO2-uptake started (indication stomata started opening) between leaves, which is most visible for the MML treatment (Figure 3a). Notably, when the measuring light was switched off, CO2-uptake dropped to 0 or lower until 18:00, which points to CO2 being fixed via Rubisco until 18:00 (VVL a bit earlier). However, after 18:00 CO2-uptake became independent of light, which points at atmospheric CO2 being fixed via PEP-C later in CAM-phase IV (compare Figure 3a,b). Only the LLL treatment does not start taking up CO2 until after ±19:30. This points at a prolonged CAM-phase III and therefore delayed and shortened CAM-phase IV.
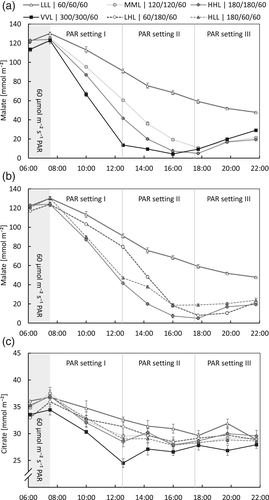
The biochemical measurements of the malate concentration are in line with the patterns measured with gas-exchange and chlorophyll fluorescence, and give more insight into the underlying processes. Figure 4a,b shows the effect of the different light intensities during CAM-phase III (Table 1) on the malate concentration in the leaves. Over the first 1.5-h interval after the start of the day (i.e., CAM-phase II, 06:00–07:30) the malate concentration still increases slightly but significantly (p < .01; analysed for all treatments together as the treatments did not differ until 7:30). This is in line with the CO2-uptake measured during that time interval (Figure 2). Apparently, at least part of the CO2 taken up during CAM-phase II is fixed via PEP-C and stored as malate. After 07:30, CAM-phase III had started and the malate concentration decreased. Clearly, the higher the light intensity, the earlier malate is depleted (Figure 4a). In addition, the rate of malate depletion changed significantly in response to a change in light intensity halfway through CAM-phase III (switch of PAR setting I and II; p < .01; Figure 4b). The time at which malate was depleted was largely in line with the time that net CO2-uptake started (stomata opened) for the MML, HHL and VVL treatments (compare Figures 3a and 4a). Hours after that moment the malate concentration started increasing again (Figure 4a; p < .01 except for the LLL treatment), which is in line with the start of the light-independent CO2-uptake (Figure 3b). In the LLL treatment, not all malate was decarboxylated during daytime: as suggested with the gas-exchange measurements, indeed CAM-phase III was prolonged and CAM-phase IV was shortened in this treatment with half the light intensity during CAM-phase III compared with the previous growth weeks. Nevertheless, despite not all malate being depleted by the end of the day, the leaves still have a CAM-phase IV with some small amount of CO2 uptake (Figure 3a,c). These results reveal that the rate of malate decarboxylation is light-regulated in Phalaenopsis. For the Δcitrate measurements, only a minor response was measured compared with Δmalate (Figure 4c). However, citrate still did decrease significantly (p < .01). There was no effect of light intensity on the decarboxylation rate of citrate (p > .25). The minor response of citrate is in line with Pollet et al. (2010) and Pollet, Vanhaecke, Dambre, Lootens, and Steppe (2011).
3.3 Effect of a long-term (>6 weeks) change in light intensity on the daily cycle of CAM
After at least 6 weeks of growth under the different lighting schedules (Table 2), leaf samples were collected for malate and citrate analysis, and CO2-uptake was measured over 24 h, starting before CAM-phase IV. In four treatments (MML, MML/T, HLL and LHL), the light integral during CAM-phase III was equal, however, according to different patterns (respectively medium = control, trapezoid, high/low, low/high). Between these treatments, no significant differences in diel CO2-uptake were measured (Table 3). As found for the short-term treatments (Figure 4b), the higher the light intensity, the faster malate was decarboxylated (p < .05, Figure 5a) and malate was depleted at approximately the same time in all four treatments. Only the difference in malate depletion rate between HLL and MML was not significant. During CAM-phase IV, the malate concentration increased again (p < .01), except for LLH, which did not deplete its malate pool. However, it may be that the moment that LLH reached the lowest malate concentration was between two sampling time points (at 18:09 phase IV started, see Table 3), and therefore, in reality, malate may have increased from this moment onwards.
| Net CO2-uptake per CAM-phase [mol m−2 day−1] | Start phase III | End phase III | Duration phase IV, I, II | ||||
|---|---|---|---|---|---|---|---|
| Treatment | Phase IV | Phase I | Phase II | Phase IV, I, II | |||
| MML | 0.09cd | 0.17b | 0.01bc | 0.27b | 07:28a | 16:32d | 14:56b |
| MML/T | 0.07de | 0.18ab | 0.01b | 0.27b | 07:07b | 17:12c | 14:18bc |
| HLL | 0.10bc | 0.16c | 0.00e | 0.27b | 06:35c | 16:39c | 13:56cd |
| LHL | 0.06ef | 0.20a | 0.02a | 0.28b | 07:45a | 18:14ab | 13:30cde |
| LLH | 0.04fg | 0.15cd | 0.01d | 0.20c | 07:30a | 18:09ab | 12:57e |
| LLL D1 | 0.03g | 0.11e | 0.00e | 0.15e | 06:39c | 18:39a | 11:59f |
| LLL D4 | 0.05ef | 0.14d | 0.01d | 0.21c | 07:08b | 17:49b | 13:19de |
| HHL D1 | 0.14a | 0.14d | 0.01d | 0.29ab | 07:35a | 12:36e | 19:07a |
| HHL D4 | 0.12ab | 0.18ab | 0.01cd | 0.31a | 07:12b | 16:31d | 14:40b |
- Note: Different letters (a, b, c,…) indicate statistically significant differences between treatments (n = 6, Fisher's LSD, α = 0.05; ns, not significant).
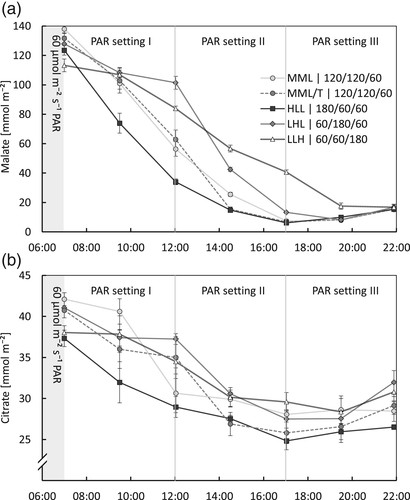
In treatment LLH, the DLI was equal to treatments MML, MML/T, HLL and LHL, however, during the first 11 h after the start of the day (i.e., approximately the natural hours for CAM-phase III in tropical Asia), the light integral was lower, and the next 5 h the light intensity was much higher. In this treatment, the diel CO2-uptake was significantly lower (−25%) compared with the other four treatments (Table 3), and the malate concentration depleted at a slower rate (p < .01; Figure 5a). The difference between the minimum and maximum daily malate concentration represents the daily amount of malate supplying the leaf with CO2 available for growth. This difference is 20% lower for treatment LLH, compared with the average difference between the minimum and maximum daily malate concentration of the other four treatments. This is approximately in line with the difference in CO2-uptake measured between treatment LLH and the other four treatments (Table 3). These results show that there are limitations in the plasticity of the time CAM-phase III can take, even for the newly developed leaves after a > 6 weeks adaptation period.
The change in citrate concentration over the day was minor with a Δcitrate in the order of 10 mmol m−2 day−1, whereas Δmalate was 10-fold (Figure 5c). As measured for malate, the citrate concentration decreased during the day (p < .01) and increased again in CAM-phase IV (p < .01).
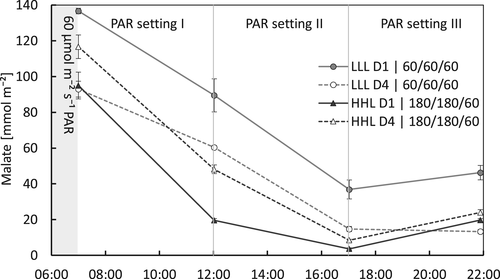
The alternating light treatment did have a DLI that was equal to the other treatments when averaged over 8 days. However, there were periods with higher and lower light during PAR setting I and II (Table 2). The first day with low light during PAR setting I and II, following 4 days with high light, malate was not depleted by the end of the day, with approximately 30% of the concentration at the start of CAM-phase III remaining (Figure 6). The CO2-uptake during the following phases IV, I and II was low (0.15 mol m−2 day−1) compared with the control (MML) treatment (0.27 mol m−2 day−1; Table 3). This lower CO2-uptake does not necessarily imply a lower malate availability on the following day due to the malate remaining from the day before. However, after 4 days low light, the malate concentration at the start of CAM-phase III was lower (p < .01) and depleted (or nearly so) by the end of the day (Figure 6), and the CO2-uptake was 0.21 mol m−2 day−1, which was similar to the LLH treatment and significantly lower than the control (MML) treatment (Table 3). During the first day of high light during CAM-phase III, following 4 days of low light, malate was depleted rapidly (Figure 6) and CO2-uptake over the following phases IV, I and II were immediately as high as after the fourth day of high light (Table 3). Alternating 4 days low and 4 days high light resulted in an average CO2-uptake of 0.24 mol m−2 day−1, which is slightly lower than the MML treatment (0.27 mol m−2 day−1), suggesting a somewhat lower growth potential under alternating light conditions compared to constant conditions at an equal DLI.
3.4 Effect of CO2 on diel carbon gain
The CO2 concentration had a significant effect on diel net CO2-uptake of Phalaenopsis (Table 4). At 800 ppm, both the diel CO2 uptake and the CO2-fixation during phase I increased 25–31% compared with 400 ppm, for both the vegetative and flowering stages. CO2-uptake also increased significantly during phase IV. During phase II, CO2-uptake was small, however, significantly higher for the 400 ppm treatment. The diel CO2-uptake was overall higher in the flowering stage than in the vegetative stage. The leaves of plants grown at 400 ppm CO2 which were subsequently exposed to and measured at 800 ppm CO2, responded with a similar increase in CO2-uptake as the 800 ppm grown leaves measured at 800 ppm. Notably, the maximum gs decreased with an increase in CO2 concentration. The effect of CO2 concentration on the start of CAM-phase III (end of net CO2-uptake, indicating stomatal closure) and CAM-phase IV (opening of stomata) was not significant or only small, resulting in a similar duration of the combined phases IV, I and II.
| Treatment | Net CO2-uptake [mol m−2 day−1] | gs max | Duration | ||||
|---|---|---|---|---|---|---|---|
| Stage | CO2 [ppm] | Phase IV | Phase I | Phase II | Phase IV, I, II | [Mol m−2 s−1] | Phase IV, I, II |
| Vegetative | 400 | 0.039b | 0.141b | 0.006a | 0.187b | 0.028a | 15:00ns |
| Vegetative | 800 | 0.047ab | 0.182a | 0.003b | 0.233a | 0.019b | 14:35ns |
| Vegetative | 400 → 800 | 0.057a | 0.174a | 0.002b | 0.233a | 0.017b | 14:33ns |
| Flowering | 400 | 0.062b | 0.146b | 0.015a | 0.223b | 0.025a | 15:59a |
| Flowering | 800 | 0.090a | 0.191a | 0.007b | 0.289a | 0.017b | 15:23b |
| Flowering | 400 → 800 | 0.098a | 0.194a | 0.005b | 0.300a | 0.016b | 15:15b |
- Note: Different letters (a, b, c) indicate statistically significant differences between treatments (analysed separately for the vegetative and flowering stage; n = 12, Fisher's LSD, α = 0.05; ns, not significant).
4 DISCUSSION
By combining gas-exchange, chlorophyll fluorescence and biochemical measurements of malate, a coherent overview of the physiology of CAM in Phalaenopsis was developed. The four CAM-phases were clearly discernible. The end of CAM-phase II in the morning was marked by net atmospheric CO2-uptake coming to a halt (Figure 1) and the point where the increasing malate concentration changed to malate decarboxylation (Figure 4). CAM-phase IV was marked by resuming CO2-uptake and a decrease in ETR at a stable light intensity. Under stable lighting conditions, malate was depleted by the start of phase IV.
4.1 Regulation of malate decarboxylation in CAM-phase III
Clearly, it is light intensity regulating malate decarboxylation in CAM-phase III, either directly or, more likely, indirectly via the CO2 assimilation rate. At a light intensity in CAM-phase III of only half of that during growth in the weeks before, no excessive CO2 leaking out of the leaf was measured, as for instance found by Chen and Lin (2012), and malate was not depleted by the end of the day (LLL in Figure 4a). In addition, the malate decarboxylation rate responded proportionately to changes in light intensity (Figures 4a,b and 5). The length of CAM-phase III and start of CAM-phase IV were adjusted to the moment malate was depleted and were therefore also influenced by the light intensity during phase III. In this respect, malate decarboxylation of Phalaenopsis responds in a very similar way to the response to light intensity reported for several Kalanchoe species (Barrow & Cockburn, 1982; Kluge, 1968; Lüttge, 2004).
4.2 Carboxylation pathway in CAM-phase IV
At higher light intensities CAM-phase III was shorter, resulting in an earlier start of CAM-phase IV (i.e., start of direct atmospheric CO2-uptake in the light period; Figure 3), which is in line with previous studies on Kalanchoe daigremontiana and Kalanchoe blossfeldiana (Barrow & Cockburn, 1982; Thomas et al., 1987). CO2 fixation was via Rubisco until about 18:00 (VVL a bit earlier), which was 12 h after lights on. This was shown by turning the measuring light off, in response to which CO2-uptake dropped to zero or below (Figure 3b). However, beyond the natural daylength for Phalaenopsis of about 12 h, CO2 was fixed mainly via PEPC: CO2-uptake did not decrease anymore when turning the measuring light off (compare Figure 3a,b), ETR dropped to lower values (Figure 3c), and the leaf malate concentration started rising again (p < .01; Figure 4a). Even in the 1-day treatment with LLL, where malate was not depleted by the end of the day (Figure 4a), direct atmospheric CO2-uptake started around 20:00 and the CO2 was taken up independent of light (Figure 3a,b). This indicates that the start of phase IV (opening stomata) is not necessarily associated with malate depletion and the subsequent decrease in internal CO2 concentration alone, which is considered an important factor in stomatal rhythms of CAM plants (Males & Griffiths, 2017). This normally occurs under stable lighting conditions (compare the control MML with LLL in Figure 4a). This is consistent with previous statements that the length of CAM-phase III, and therefore the start of CAM-phase IV, are not only regulated (indirectly) by light intensity and the drawdown of internal CO2 but also by the circadian clock imposing limitations on the plasticity of the length of CAM-phase III (Dodd et al., 2002; Hartwell, 2005; Von Caemmerer & Griffiths, 2009).
4.3 Regulation of diel carbon gain by light integral
The response of CAM-metabolism to the DLI has not been studied in much detail yet. Lüttge and co-workers have shown that Clusia responded to a higher DLI by increasing Δmalate (Franco, Ball, & Lüttge, 1991; Haag-Kerwer, Franco, & Lüttge, 1992). Nocturnal acidification was increased in several cactus species in response to an increase in DLI up to 30 mol m−2 (Nobel, 1988). In our study on Phalaenopsis, the average light integral was equal for all six long-term (>6 weeks) treatments (Table 2). The four treatments (MML, MML/T, HLL and LHL) with an equal light-sum according to different patterns during CAM-phase III, all showed a similar diel CO2-uptake (Table 3) and Δmalate during daytime (Figure 5a). The treatment with alternating periods of 4 days low and 4 days high light, elegantly shows how the Δmalate responds to the light integral (Figure 6 and Table 3): 1 day lower light following a high light period resulted in malate not being depleted by the end of the day, and a very low CO2-uptake the following night. However, after 4 days low light, malate was depleted by the end of the day, and the concentration per area of leaf at the start of the day was markedly lower (LLL D4) than after a high light period (HHL D4 and LLL D1). Only 1 day high light following 4 days low light immediately resulted in a much higher diel CO2-uptake. After the fourth day of high light, the subsequent diel CO2-uptake was higher than the control (MML; Table 3). Previous studies by Guo et al. (2012) showed that exposing a commercial Phalaenopsis cultivar to a full day of very low light conditions (25 μmol m−2 s−1) resulted in CO2 release during the day and higher pre-dusk malate levels compared to plants grown at higher light intensities. In the following night, CO2 uptake as well as pre-dawn malate levels in these plants were significantly lower, underpinning our conclusion that plasticity of these plants allows for short-term adaption to changes in light intensity.
Notably, LLH with only lower light during CAM-phase III (60 μmol m−2 s−1 PAR) followed by 180 μmol m−2 s−1 PAR, resulted in a significantly lower CO2-uptake over 24 h and a similarly smaller Δmalate, compared to the results for the MML control treatment. Apparently, it is the light integral during CAM-phase III or during the “natural photoperiod” of the first ±12 h of the day, and not the total DLI that regulates the daily carbon gain (i.e., the diel net CO2-uptake). This proposition is underpinned by comparing LLH and the alternating light treatment. After 4 days LLL the diel CO2-uptake was similar to that of LLH (Table 3), and also Δmalate over the day was similar (compare Figures 5a and 6). The light integral during CAM-phase III was also the same in LLH and the alternating light treatment (during 4 days LLL), but the light integral over the whole day was markedly higher in LLH (5.6 vs. 3.5 mol m−2 day−1 PAR for LLH and the alternating light treatment LLL respectively). From this, it can be concluded that light after the first ±12 h of the day is not used as effectively for carbon gain relative to the light in the first ±12 h.
4.4 Regulation of diel carbon gain by CO2
Despite PEP-C having a high affinity for CO2 (Lüttge, 2001), our results clearly show that a CO2 concentration well-above atmospheric levels strongly increased the diel net CO2-uptake (25–31%; Table 4). As the CO2-uptake also increased proportionally during phase I, our results support the conclusion that neither the storage capacity for malate in the vacuole nor the supply of PEP as substrate for CO2-fixation into malate via PEPC, were limiting factors for an increased carbon gain at elevated CO2 concentration under the conditions used in this study. Notably, the duration of CAM-phase III was not significantly, or only slightly longer for the plants grown at 800 ppm CO2 compared with the 400 ppm plants. This demonstrates that the rate of malate decarboxylation in the leaves grown at 800 ppm was increased. Long-term acclimation to elevated CO2 (up to 25 weeks) did not result in acclimation of CO2 storage capacity, as the leaves grown at 400 ppm CO2 exposed to 800 ppm had a similar increase in CO2-uptake as the leaves grown and measured at 800 ppm CO2, compared with the leaves grown and measured at 400 ppm CO2.
The decrease in gs in response to a higher CO2 concentration (Table 4) is seen more often in CAM plants, for example, for bromeliad Aechmea (Ceusters et al., 2008). In the same study elevated CO2 from 380 to 700 ppm did not increase CO2-uptake during phase I, possibly due to the decreased gs. It is known that stomatal behaviour of CAM plants can be sensitive to changes in internal CO2 concentrations (Wyka, Duarte, & Lüttge, 2005). When the internal CO2 concentration in the leaf rises at the start of CAM-phase III, stomatal closure serves to prevent CO2 leakage out of the leaf (Males & Griffiths, 2017). Possibly the stomatal closure response at the start of CAM-phase III is regulated by both the internal CO2 concentration and the circadian clock (Males & Griffiths, 2017). Notably, the diel CO2-uptake was higher for plants in the flowering stage than for plants in the vegetative stage (Table 4), which may be due to the higher DLI for the flowering stage plants.
4.5 Practical implications for Phalaenopsis growers
Besides the new insights into the physiological plasticity of CAM in Phalaenopsis, this study provides useful practical insights for growers, especially for those growing Phalaenopsis in high-tech production systems with control over lighting and CO2. In CAM-phase II the malate concentration continued to rise (Figure 4), and light use efficiency (ETR) gradually increased (Figure 2). This is likely to be because PEPC remained active in phase II until Rubisco was sufficiently activated (Griffiths et al., 2002; Roberts, Borland, & Griffiths, 1997). In practical terms, this implies that starting the day at full light intensity is wasting energy. Note that under natural conditions, light intensity also rises gradually after dawn. For CAM-phase III we found that malate decarboxylation responded strongly to light intensity. In addition, carbon gain was regulated by the light integral during CAM-phase III rather than the light integral over the whole day. This implies that growers need to assure that enough light is available within CAM-phase III, and have flexibility in their choice of how to reach a high enough light integral in CAM-phase III. In addition, growers should be aware of light-stress induced injury in CAM-phase IV, as the ETR decreased after malate was depleted (Figure 3). During CAM-phase IV, high light intensity was not utilized fully, so lights can be dimmed in a commercial greenhouse. To what extent phase IV then contributes to additional growth and productivity remains to be determined. CO2 fertilization is useful to increase carbon gain, but only during CAM-phase IV and I (Table 4).
In conclusion, our results show how Phalaenopsis “Sacramento” responds to light (conclusions 1 and 2) and provide novel insights into the regulation of CAM (conclusions 3 and 4):
- Light intensity drives malate decarboxylation in CAM-phase III, and thereby governs the length of CAM-phase III and starting time of CAM-phase IV. In this respect, Phalaenopsis responded in a similar way to Kalanchoe.
- Beyond the natural daylength of ±12 h, CO2 fixation in CAM-phase IV most likely occurred via PEP-C instead of Rubisco. In this respect, Phalaenopsis responded in a similar way to Aechmea “Maya.”
- Total diel CO2-uptake was regulated by the light integral in CAM-phase III, rather than the total daily light integral.
- Elevated CO2 enhanced diel CO2-uptake, via increased CO2-fixation both in CAM-phase IV and I. This shows that neither vacuolar storage capacity for malate, nor the supply of substrate for CO2 fixation (PEP) were limiting factors for carbon gain enhancement under ambient conditions.
ACKNOWLEDGMENTS
This research is supported by the Dutch Program “Kas als Energiebron” funded by The Ministry of LNV and Glastuinbouw Nederland, and by the Dutch pot-orchid growers association. EvT is supported by the Netherlands Organization for Scientific Research (NWO) under contract number 14525. We acknowledge Arjen van de Peppel (Wageningen University and Research) for assisting with the biochemical analysis and staff of Demokwekerij Westland for growing plants for the CO2 experiment.
CONFLICT OF INTEREST
The authors declare no conflict of interest.




