Pollen development in cotton (Gossypium hirsutum) is highly sensitive to heat exposure during the tetrad stage
Abstract
The development of gametes in plants is acutely susceptible to heatwaves as brief as a few days, adversely affecting pollen maturation and reproductive success. Pollen in cotton (Gossypium hirsutum) was differentially affected when tetrad and binucleate stages were exposed to heat, revealing new insights into the interaction between heat and pollen development. Squares were tagged and exposed to 36/25°C (day/night, moderate heat) or 40/30°C (day/night, extreme heat) for 5 days. Mature pollen grains and leaves were collected for physiological and proteomic responses. While photosynthetic competence was not compromised even at 40°C, leaf tissues became leakier. In contrast, pollen grains were markedly smaller after the tetrad stage was exposed to 40°C and boll production was reduced by 65%. Sugar levels in pollen grains were elevated after exposure to heat, eliminating carbohydrate deficits as a likely cause of poor reproductive capacity. Proteomic analysis of pure pollen samples revealed a particularly high abundance of 70-kDa heat shock (Hsp70s) and cytoskeletal proteins. While short-term bursts of heat had a minor impact on leaves, male gametophyte development was profoundly damaged. Cotton acclimates to maxima of 36°C at both the vegetative and reproductive stages but 5-days exposure to 40°C significantly impairs reproductive development.
1 INTRODUCTION
Susceptibility to heat stress depends on plant developmental stages; recent studies have shown that the reproductive phase is extremely sensitive to environmental stresses, especially high temperatures that dramatically reduce yields of many commercial crops (Almeida, Perez-Fons, & Fraser, 2020; Giorno, Wolters-Arts, Mariani, & Rieu, 2013; Muller & Rieu, 2016; Paupiere, van Heusden, & Bovy, 2014; Zinn, Tunc-Ozdemir, & Harper, 2010). Pollen development is composed of two sequential stages, including microsporogenesis and microgametogenesis. Microsporogenesis starts with diploid pollen mother cells that undergo meiotic divisions to proceed to post-meiotic tetrad cells and then haploid unicellular microspores. During microgametogenesis, released unicellular microspores undergo mitotic divisions to generate bicellular or tricellular pollen (Borg, Brownfield, & Twell, 2009). Depending on the stage at which plants experience stresses, the extent of damage can be different; the meiotic stage is more likely to be susceptible to various stresses (Yu, Jiang, & Guo, 2019).
Cotton is typically cultivated in hot and semi-arid regions, often suffering substantial reductions in lint yield due to episodes of extreme heat during gamete formation (Saleem et al., 2018; Song et al., 2015). A strong negative correlation has been established between cotton lint yield and above-optimal temperatures during 3 years' field research in south-eastern and mid-southern parts of the United States, with a lint yield penalty of 10% with each 1°C increase in maximum day temperature (Pettigrew, 2008). These heat-induced yield losses in cotton are strongly associated with accelerated fruit abscission (Reddy, Reddy, & Hodges, 2010), a process normally associated with the action of ethylene. However, our previous work illustrated that the primary effect of high temperature is exerted on pollen development, which in turn impairs fertilization and fruit development, resulting in premature abscission; this process appears to be independent of ethylene production (Najeeb, Sarwar, Atwell, Bange, & Tan, 2017).
Upon heat stress, a group of proteins termed as heat shock proteins (HSPs) are synthesized and accumulated in plant tissues. These HSPs are classified into five different groups according to their molecular weight, namely HSP100, HSP90, HSP70, HSP60 and small heat-shock proteins (sHSPs; Al-Whaibi, 2011). HSPs are regulated under multiple stresses, indicating their important roles in plant homeostasis (Bhattarai, Li, Liu, Dinesh-Kumar, & Kaloshian, 2007; Hu, Hu, & Han, 2009; Park & Seo, 2015; Swindell, Huebner, & Weber, 2007). These proteins are specifically involved in intracellular translocation of precursor proteins into organelles, and act as a molecular chaperone to (re)fold the imported proteins (Chirico, Waters, & Blobel, 1998; Kang et al., 1990; Miernyk, Duck, Shatters, & Folk, 1992). Recent evidence strongly supports this view; cotton plants transformed with a gene, encoding an Arabidopsis thaliana heat shock protein 101 (AtHSP101) produced pollen with a higher germination rate and larger elongation tube at elevated temperatures (Burke & Chen, 2015). This establishes a connection between molecular responses and heat sensitivity, justifying the use of proteomics as a tool to address heat-induced yield losses in cotton.
In this study, we used cotton (cultivar Sicot 71) as a moderately heat-tolerant fibre crop to investigate the response of male reproduction to moderate and extreme heat. We examined two distinct stages, including early (tetrads) and late (binucleate) pollen development, to establish physiological and molecular responses to heat. Events in leaves that supplied carbohydrates to these reproductive organs were also recorded. We performed the first proteomics on cotton pollen to identify differentially expressed proteins (DEPs) that are implicated in heat stress responses. We believe this has major implications for the identification of heat-stress markers in crops and thus novel heat-resistant cultivars.
2 MATERIALS AND METHODS
2.1 Plant materials and stress treatment
Glasshouse experiments were conducted at the Plant Growth Facility of the Department of Biological Sciences, Macquarie University, Australia. A cotton cultivar (Gossypium hirsutum cv. Sicot 71) was grown in plastic pots (25 × 20 cm; height × diameter) containing finely mixed red silt loam Ferrosol soil from Robertson, NSW, Australia. Plants were grown under similar climatic and cultural conditions as reported in our previous study (Najeeb, Atwell, Bange, & Tan, 2015). For physiological and proteomic analysis, two distinct phases of pollen development, namely tetrads and binucleate stage (Song et al., 2015) were exposed to heat stress at 36/25°C (day/night) or 40/30°C, each for 5 days. Mature pollen grains were collected at anthesis after the termination of heat that was imposed in the binucleate stage (P-BN). Plants exposed to high temperatures at the tetrad stage were returned to the optimum temperature, and the corresponding mature pollen was collected at anthesis after 9 days of recovery (P–T). Control plants were maintained at 28/20°C (day/night). Exposure of tetrads to 40/30°C resulted in failed dehiscence, so no pollen grains could be collected. However, the earliest possible stage of pollen development (1 day older than the tetrad stage) was subjected to extreme heat, and the corresponding viable mature pollen (P–T40 + 1) was collected after 8 days of recovery for analysis. Figure 1 shows the workflow of label-free quantitative shotgun proteomics and physiological measurements in pollen. All experiments were conducted in at least three biological replicates.
2.2 Gas exchange, relative cell injury and total soluble sugars
CO2 exchange parameters were measured at the termination of heat treatment, including stomatal conductance (gs), assimilation rate (Ar), the ratio of intercellular to ambient CO2 concentration (Ci/Ca) and maximal efficiency of PSII photochemistry (Fv'/Fm'). A LICOR photosynthesis system (LI-6400, LI-COR, Inc., Lincoln) with the following parameters was used to record gas exchange from the youngest fully expanded leaves. These include light intensity at 1,800 μmol m−2 s−1, a CO2 concentration of 400 μmol mol−1 and the temperature of leaf chamber set to 28, 36 or 40°C (Najeeb et al., 2017).
To measure relative cell injury (RCI), four discs (18 mm diameter) were collected from the youngest fully expanded leaves at the end of heat treatment. The samples were washed with MilliQ water and incubated in 40 ml of water. One set of samples was incubated in a water bath at 50°C, and the other was placed at 25°C for 1 hr. The tubes were then placed at 25°C to record initial electrolyte conductivity using Hanna conductivity meter (HI 98195, Hanna Instruments, Waltham). Final electrolyte conductivity was measured after boiling the samples at 100°C for 60 min and cooling to 25°C. RCI% was calculated according to Chauhan and Senboku (1996) with the following formula: RCI% = 1 - [1 - (T1/T2)/1 - (C1/C2)] × 100, where T1 and T2 are the initial and final conductance values of heat-treated vials, respectively, while C1 and C2 are the initial and final conductance values of the control vials, respectively.
Approximately 20–50 mg fresh pollen grains or leaves were collected to measure total soluble sugars according to Yemm and Willis (1954) and our previous study (Najeeb, Tan, & Bange, 2016). Briefly, the samples were extracted in boiling 80% ethanol (2.5 ml) for 5 min and twice more in boiling distilled water followed by centrifugation at 4,500 rpm for 5 min. The combined supernatants were adjusted to a final volume of 10 ml to determine soluble sugars. A reaction mixture containing 100 μl of the supernatant layered onto 2.5 ml anthrone-sulphuric acid solution (71% H2SO4), was vortexed and boiled for 10 min and immediately cooled on ice before measuring OD at 630 nm. Sugar content in leaf and pollen was extrapolated from the standard glucose curve and presented in mg g−1 tissue.
2.3 Microscopy, pollen viability and boll production
The tetrad and binucleate stages were further analysed to confirm the developmental stages (Ma et al., 2012). Briefly, Carnoy's solution (3 EtOH:1 acetic acid) was used to fix the samples for about 20–30 min, followed by maceration in distilled water and dissolution of exine in 10% HNO3:5% HCl (1:1) for 10 min. Hardening was performed using 1:1 EtOH and acetic acid for 10 min, and samples were then stained with 1% acetocarmine containing 35 μl glycerol overnight. Nuclei were visualized under an Olympus BX53 microscope. Mature pollen grains arising from 5-days heat-treated tetrads (P–T) and binucleate stage (P-BN) were observed using the Olympus BX53 microscope to record the volume of the resultant grains by ImageJ software. Pollen tube length was measured using stereomicroscope (Nikon Corporation, SMZ18, Japan). The full square was observed under a Motic SMZ-161 stereomicroscope.
A solid medium was optimized to test pollen viability consisting of (g): agar (1.5), polyethylene glycol (PEG) (2), sucrose (20), and (milligrammes) H3BO3 (10), CaCl2.H2O (18.5), MgSO4·7H2O (20), KNO3 (36.5) in 100 ml of deionized water (Jayaprakash & Sarla, 2001). Pollen grains were collected from plants in control conditions between 8:00 and 9:00 a.m. and immediately sprinkled on the medium. Pollen germination and tube length were recorded after exposure of pollen in petri dishes to moderate (36°C) and extreme heat (40°C) for 3 hr.
Yield was measured following 5 days of moderate and extreme heat to monitor whether the heat-treated plants can self-fertilize and produce normal bolls. All green bolls were counted without relating them to specific stages of pollen development. The number of green bolls was counted 0, 13 and 20 days after treatment (DAT). All plants were mapped for green bolls on individual fruiting position and branches, and pollinated flowers and bolls were assigned as green bolls.
2.4 Protein isolation, digestion and mass spectrometry
Twenty milligramme of pollen grains were ground into fine powder using a Qiagen Retsch 12090 TissueLyser II, six Zirconox beads (2.8–3.3 mm) and liquid nitrogen. A phenol-based protocol with slight modifications was used for isolation of proteins (Chaturvedi, Ischebeck, Egelhofer, Lichtscheidl, & Weckwerth, 2013). Briefly, the ground pollen grains were re-suspended in 300 μl of protein extraction buffer (100 mM Tris–HCl, pH 8.0; 5% SDS; 10% glycerol; 10 mM dithiothreitol (DTT); 1% EDTA-free protease inhibitor cocktail tablets (cOmplete, Roche Diagnostics, Mannheim, Germany), vortexed vigorously and incubated at room temperature for 30 min. The samples were then incubated and shaken at 95°C for 3 min, followed by centrifugation at 20,000g for 10 min at 4°C. Supernatants (200 μl) were transferred into a new tube, and mixed with an equal volume of 1.4M sucrose. Proteins were then extracted twice with Tris-EDTA buffer-equilibrated phenol. The phenolic phase was carefully collected each time after centrifugation at 20,000g for 10 min at 4°C, and the combined phases were extracted using an equal volume of 0.7M sucrose. Supernatants (300 μl) were transferred into a new tube and mixed with five volumes of cold 0.1M ammonium-acetate in methanol, followed by incubation at −20°C overnight. Samples were centrifuged at 20,000g for 10 min at 4°C and the pellets were washed twice with cold 0.1M ammonium-acetate in methanol and once with cold acetone. The pellets were finally air-dried and solubilized in 100 μl of 8M urea in 100 mM Tris–HCl, pH 8.0. Protein concentration was measured by the Pierce BCA protein assay (Pierce, Thermo Fisher Scientific, San Jose).
Proteins from each replicate were digested in-solution as described in (Wu, Mirzaei, Pascovici, Haynes, & Atwell, 2019). Fifty microgram protein extracts were diluted (fivefold) in 50 mM Tris–HCl, pH 8.0, followed by reduction with 10 mM DTT at room temperature for 1 hr and alkylation with 20 mM iodoacetamide (IAA) at room temperature in the dark for 45 min. Proteins were then digested using 5 μl of 0.2 μg μl−1 trypsin (Promega, Madison) and incubated at 37°C overnight. The reaction was stopped by addition of formic acid (1% final concentration).
Samples were desalted using 47-mm SDB-RPS disks (SDB-RPS, 3M, Saint Paul). Briefly, peptides were centrifuged at 2,400g for 30 min at room temperature to move through the extraction disks, followed by washing twice with 0.2% trifluoroacetic acid and finally eluting the peptides with 80% acetonitrile containing 5% ammonium hydroxide (200 μl). Samples were vacuum-dried and the resulting peptides were reconstituted with 30 μl of 0.1% formic acid. Peptides were quantified using the Micro BCA assay (Pierce, Thermo Fisher Scientific, San Jose), then analysed by nanoflow LC–MS/MS (nanoLC-MS/MS) using an Orbitrap mass spectrometer (Q Exactive, Thermo Fisher Scientific, Bremen, Germany) coupled to an Easy nLC 1,000 nanoflow liquid chromatography system (Hamzelou et al., 2020).
2.5 Protein identification, quantification and data analysis
The obtained raw data were converted to mzXML format and searched against available G. hirsutum protein sequences in UniProtKB (76,175 entries, March 2019) using the X!Tandem algorithm operating within the global proteome machine (GPM, version 3.0; Craig & Beavis, 2004). Peptide to spectrum matching parameters included a mass tolerance of ±10 ppm for the parent ion and 0.4 Da for the fragment ion, log (e) values less than −1 for peptides and proteins, tolerance of one missed tryptic cleavage, complete modification of cysteine by carbamidomethylation, and potential modification of methionine by oxidation. Mass spectrometry data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository (Perez-Riverol et al., 2019) with the dataset identifier PXD015721.
To create a single merged list of reproducibly identified proteins, three biological replicates were combined using the Scrappy program. The criteria for a protein to be retained in the merged data set were that it was identified in all three biological replicates of at least one condition, and the total spectral count across the three replicates was at least five (George, Fennell, & Haynes, 2018). As a result, 15 merged output files were generated, and false discovery rates were calculated at the protein and peptide levels for each. Protein abundances were calculated using Normalized Spectral Abundance Factors (NSAF) with addition of a spectral fraction of 0.5 to all spectral counts to compensate for null values and allow log transformation for additional statistical analyses (Neilson, Keighley, Pascovici, Cooke, & Haynes, 2013).
The resulting data set of significantly differentially expressed proteins were then functionally annotated. Gene ontology (GO) was extracted from the Nr database using the Blast2GO software (Conesa et al., 2005) and matched to the list of reproducibly identified proteins. PloGO was used to process the gene ontology annotation (Pascovici, Keighley, Mirzaei, Haynes, & Cooke, 2012). Functional categories of interest for G. hirsutum were obtained from the AmiGO browser and were enriched with the available GO annotations for differentially regulated proteins at each condition. Proteins were further classified into specific pathways using available information based on biological processes in the KEGG database (Zhang, Jiang, Chen, Niu, & Cai, 2013).
2.6 Parallel reaction monitoring analysis
Six candidate proteins associated with heat stress were selected for targeted parallel reaction monitoring (PRM) quantification. An inclusion list consisting of unique peptides with their mass to charge ratio was created using Skyline-daily (version 19.1.9.338; MacLean et al., 2010). The same Q-Exactive was operated in PRM mode using the acquisition method including a single full MS scan combined with targeted MS/MS scans. For the PRM method, a resolution of 17,500, a target AGC value of 2 × 105, a maximum injection time (IT) of 55 ms, and a normalized collision energy of 30% were used. The sum of areas of all transition peaks for each peptide was calculated and used for quantification using Skyline-daily. Actin, as a standard, was selected to normalize the summed ion peak areas within every three replicates. The normalized peak areas were log2 transformed and the replicates were averaged, followed by t tests analysis to compare stress versus control condition.
2.7 Statistical analysis
A series of student t tests were performed to identify proteins that were significantly up-and down-regulated between control and heat stressed samples. The log-transformed NSAF values were used for two-sample unpaired t tests, and the proteins with a p-value <.05 were considered to be differentially regulated. Ratios less than one are represented as negative values of the inverse ratio. Additional filtering of fold change values was performed on Z-score transformed data. Z-score transformation has previously been demonstrated to be of utility in minimizing distortion in quantitative datasets caused by occasional experimental or mathematical artefacts (Cheadle, Vawter, Freed, & Becker, 2003). For a normally distributed dataset, Z-scores are clustered around the mean, so retaining only those values with a Z-score less than plus or minus one standard deviation from the mean value can be used to minimize the effect of statistical outliers (George, Pascovici, Mirzaei, & Haynes, 2015). In this study, fold change values with a Z-score >1 are represented as 50 and −50 for up- and down-regulated proteins, respectively.
Data for different growth parameters including gas exchange parameters, RCI, total soluble sugar concentration, pollen volume, number of green bolls, pollen germination, and pollen tube length were processed using R (R Core Team, 2019, version 3.6.1), with a one way ANOVA to assess temperature-dependent differences. Differences with p-value <.05 and p-value <.01 were considered significant, according to Tukey HSD post hoc test.
3 RESULTS
3.1 Extreme heat altered multiple photosynthetic variables of leaves
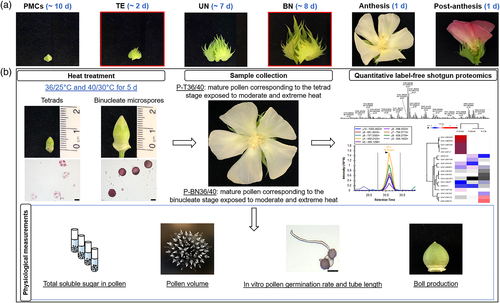
Extreme heat had significant effects on leaf gas exchange variables, with stomatal conductance, Ci/Ca and PSII efficiency significantly higher at 40°C than at 28°C. Ar did not change significantly, even at 40°C (Figure 2a). Despite a slightly lower Fv'/Fm' under moderate heat (36°C), the ratio rose by 15% after exposure to extreme heat (Figure 2b). Moderate heat did not significantly change gs, while extreme heat increased gs by 122% above the control (Figure 2c). Moderate heat also increased Ci/Ca slightly, whereas the ratio was significantly higher at 40°C (16% higher than control; Figure 2d).
3.2 Electrolyte leakage increased in leaves under extreme heat
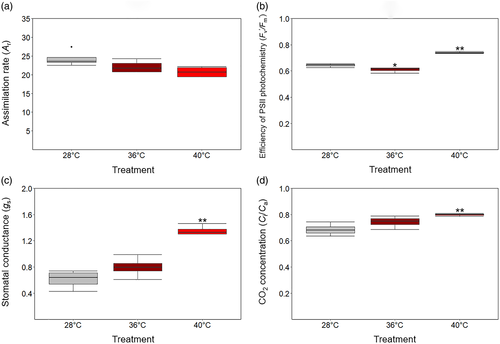
Cotton plants exposed to 40°C had significantly higher electrolyte conductivity than those at 36°C. No significant effect was observed when plants were exposed to moderate heat. However, extreme heat significantly increased electrolyte conductivity that is, up to 48% compared to control plants (Figure 3a).
3.3 Soluble sugars increased in pollen and leaves under moderate and extreme heat
Total soluble sugars in leaf and P-BN did not change significantly after exposure of plants to 36 or 40°C for 5 days. Likewise, moderate heat did not significantly increase the level of soluble sugars in P-T. The level of sugar could not be measured in P-T exposed to extreme heat due to its lethal impact on the tetrad stage (14 days prior to anthesis) and anther dehiscence, resulting in damaged pollen 9 days after the termination of heat stress. To address lethality at the tetrad stage, extreme heat was started 1 day later (13 days prior to anthesis), resulting in mature pollen being collected 8 days after the termination of extreme heat (termed P-T40 + 1) to estimate the sugar concentration; this enabled assessment of the impact of heat at the earliest possible stage of pollen development. Moderate and extreme heat increased total soluble sugars in P-BN only by 5 and 10%, respectively. The concentration in P–T rose by 31% at moderate heat, but dehiscence failed in extreme heat. Notably, the samples corresponding to P-T40 + 1 dehisced successfully and had a 72% increase in total soluble sugar concentration (Figure 3b).
3.4 Extreme heat adversely affected pollen volume, anthesis, dehiscence and boll production
To assess reproductive potential, the two-dimensional projected volume of mature pollen was measured to monitor its development after exposure to high temperatures (Figure 3c). The projected volume of P-BN was not reduced, even after exposure of binucleate stage to 40°C, whereas heat stress at the tetrad stage, caused the mature pollen grains (P-T) to be 11% smaller at 36°C and 36% smaller at 40°C (p < .05).
The consequence of impaired reproductive capacity was also investigated by assessing boll production in Sicot 71. The number of green bolls reported in Figure 3d reflected the effect of moderate and extreme heat on pollen developmental stages, particularly the tetrad stage where the pollen was damaged and the corresponding anthers failed to dehisce. Following heat stress at anthesis (0-DAT), only 2–3 green bolls were observed in plants. In the subsequent 13- or 20-days recovery periods (13-DAT and 20-DAT), there was no significant effect of 36°C on boll development, where the number of bolls increased from 2 to 20. By contrast, only two new bolls formed after exposure to 40°C during the 13-days following heat treatment and a further three in the next 7 days. That is, extreme heat reduced yield by two-thirds. The adverse effect of extreme heat on P–T and the corresponding flower size, dehiscence and boll development can be seen in Figure 4a–c, indicating the vulnerability of tetrads to 40°C. Importantly, the plant reproductive structures could not recover even after a long period of recovery, signifying that early stages of pollen development were very sensitive to heat shock.
3.5 Pollen viability was negatively affected by heat stress
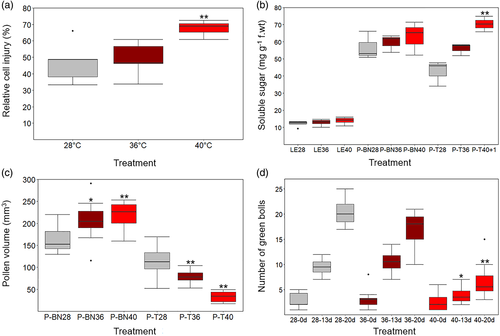
High temperature significantly affected the germination percentage and tube length compared with control (Figure 4d–e). Pollen germination declined by 27% at moderate heat and by 55% at extreme heat. Likewise, the elongation of pollen tube underwent a substantial change, with 14 and 50% decline when exposed to 36 and 40°C, respectively. Pollen tube length diminished from an average of 242 μm at 28°C to 209 and 122 μm at 36 and 40°C, respectively. The results demonstrated an adverse impact of high temperature on pollen viability and subsequent germination and tube elongation of pollen.
3.6 Distinctive proteome responses of two pollen developmental stages to high temperatures
A total of 868 non-redundant proteins were reproducibly identified from all treatments analysed (Table S1). A subset of 253 reproducibly identified proteins was identified only under stress conditions (P-T36, P-BN36 and P-BN40), and not found in control samples. The number of proteins uniquely present in each of the seven possible permutations of these three condition is shown in Figure 5a, Table S2. The largest number of proteins (86 proteins) was identified only in P-T36, indicating the importance of these proteins in the early stage of tetrad developmental for synthesis of amino acids and secondary metabolites required for cellular divisions.
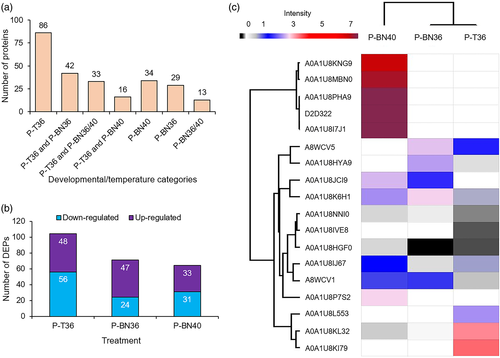
The total identified proteins in each condition (P-T28, P-T36, P-BN28, P-BN36 and P-BN40; Table S3) were analysed to determine significantly changed protein abundances. Differential expression analysis demonstrated that 48 proteins were up-regulated in pollen grains arising from exposure of the tetrad stage to 36/25°C (P-T36), while 56 proteins were down-regulated. Significantly smaller pollen volume was observed after exposure of the tetrad stage to 40/30°C, resulting in failed dehiscence (P-T40; Figures 3c and 4a–c). Consequently, no mature pollen grains were collected for proteome analysis. Moderate heat at the binucleate stage resulted in up-regulation and down-regulation of 47 and 24 proteins in the mature pollen grains (P-BN36), respectively, while extreme heat during binucleate development did not affect dehiscence and enabled the identification of 64 differentially expressed proteins in mature pollen, consisting of 33 up-regulated and 31 down-regulated (P-BN40; Figure 5b). Details of the significantly differentially expressed proteins can be found in Table S4. The 10 most up-and down-regulated proteins in P-T36, P-BN36 and P-BN40 are shown in Table 1, sorted by relative fold change.
| Condition | Up-regulated | Fold change | Down-regulated | Fold change |
|---|---|---|---|---|
| Uniprot ID | Uniprot ID | |||
| P-T36 | Actin-like | 50.0 | V-type proton ATPase subunit G | −17.3 |
| Glycine-rich RNA-binding protein GRP1A-like | 50.0 | Elicitor-responsive protein 3-like | −11.0 | |
| Elicitor-responsive protein 3-like isoform X3 | 23.8 | Protein DJ-1 homolog B-like | −9.6 | |
| Calreticulin-like | 20.9 | Tubulin beta-6 chain | −9.6 | |
| Eukaryotic initiation factor 4A-8-like | 19.0 | 26S protease regulatory subunit 8 homolog A-like | −7.3 | |
| RBR-type E3 ubiquitin transferase | 17.9 | NADH–ubiquinone oxidoreductase chain 3 | −5.7 | |
| D-3-phosphoglycerate dehydrogenase | 15.4 | Uncharacterized protein | −5.4 | |
| Protein EXORDIUM-like 6 | 15.2 | UDP-L-rhamnose synthase | −5.3 | |
| Tubulin beta chain | 14.9 | GDSL esterase/lipase At1g29660-like | −5.0 | |
| Uncharacterized protein | 12.5 | Late embryogenesis abundant protein 2-like | −4.4 | |
| P-BN36 | Polygalacturonase-like | 50.0 | Pectate lyase | −50.0 |
| UDP-arabinose 4-epimerase 1 | 28.7 | Alpha-1,4-glucan-protein synthase 2-like | −36.4 | |
| Acetyltransferase component of pyruvate dehydrogenase complex | 22.3 | Probable mitochondrial-processing peptidase subunit beta, mitochondrial | −13.8 | |
| Late embryogenesis abundant protein 47-like | 21.1 | Probable calcium-binding protein CML27 | −6.0 | |
| Probable fructokinase-4 | 16.8 | Probable fructokinase-4 | −5.4 | |
| Elongation factor 1-alpha | 16.8 | Acyl-CoA-binding protein; ACBP | −4.6 | |
| Guanine nucleotide-binding protein subunit beta-like protein | 10.8 | Probable calcium-binding protein CML27 | −4.4 | |
| Ribonuclease | 9.7 | Uncharacterized protein | −3.2 | |
| Ras GTPase-activating protein-binding protein 1-like isoform X2 | 7.9 | Gamma carbonic anhydrase-like 1, mitochondrial | −2.9 | |
| Serine–threonine kinase receptor-associated protein-like isoform X2 | 7.9 | Late embryogenesis abundant protein D-7-like | −2.6 | |
| P-BN40 | Luminal-binding protein 5-like | 50.0 | Phospholipase D | −50.0 |
| Heat shock protein 70 | 50.0 | 60S acidic ribosomal protein P1-like | −11.8 | |
| Heat shock cognate 70 kDa protein 2-like | 50.0 | Late embryogenesis abundant protein D-34-like | −9.2 | |
| Heat shock 70 kDa protein | 50.0 | Late embryogenesis abundant protein D-7-like | −8.7 | |
| Luminal-binding protein 5-like | 50.0 | Glycine-rich RNA-binding protein | −8.0 | |
| UDP-arabinose 4-epimerase 1 | 28.2 | D-3-phosphoglycerate dehydrogenase | −7.9 | |
| Acetyltransferase component of pyruvate dehydrogenase complex | 21.2 | Uncharacterized protein | −7.1 | |
| Probable fructokinase-4 | 17.6 | Glycine, alanine and asparagine-rich protein-like | −7.0 | |
| Glutathione reductase, GRase | 8.6 | Probable fructokinase-4 | −6.2 | |
| 23.6 kDa heat shock protein, mitochondrial-like isoform X2 | 7.1 | Pectinesterase | −6.1 |
- Note: The log-transformed NSAF values were used for two-sample unpaired t tests, and proteins with a p-value <.05 were considered to be differentially regulated.
3.7 HSPs were up-regulated in pollen in response to heat
Several HSPs were identified in this study, ranging from small to large molecular masses. Five proteins including two isoforms of heat shock 70 kDa protein 17-like (A0A1U8KI79 and A0A1U8L553), HSP 90b (A0A1U8KL32), 17.5 kDa class I heat shock protein-like (A8WCV1) and 17.6 kDa Class I heat shock protein 2-like (A8WCV5) were up-regulated in P-T36. Similarly, three proteins were up-regulated in P-BN36, namely heat shock 70 kDa protein 14-like (A0A1U8K6H1), heat shock 70 kDa protein 15-like (A0A1U8HYA9) and 17.6 kDa Class I heat shock protein 2-like. On the other hand, extreme heat up-regulated 10 proteins in P-BN40, including two isoforms of luminal-binding protein 5-like (A0A1U8I7J1 and A0A1U8KNG9), two isoforms of heat shock protein 70 (D2D322 and A0A1U8MBN0), heat shock 70 kDa protein 14-like, two isoforms of heat shock cognate 70 kDa protein 2-like (A0A1U8PHA9 and A0A1U8NNI0), heat shock cognate protein 80-like (A0A1U8IJ67), HSP70-HSP90 organizing protein 3-like (A0A1U8JCI9) and 23.6 kDa heat shock protein mitochondrial-like isoform X2 (A0A1U8P7S2). Importantly, among all the HSPs identified in this study, only heat shock cognate 70 kDa protein 2-like was down-regulated in P-T36 (Figure 5c).
3.8 Late embryogenesis abundant proteins were mainly down-regulated in pollen under heat
Six late embryogenesis abundant (LEA) proteins identified in this study including LEA 47-like (A0A1U8JBP6), LEA 3-like (A0A1U8KQ89), LEA D-7-like (A0A1U8JFE9), LEA 2-like (A0A1U8JWM1), LEA D-34 like (A0A1U8IXG2) and LEA 2 (A0A1U8MJD9) were differentially regulated in response to the elevated temperatures. Proteomics revealed that LEA 47-like protein was the only LEA up-regulated in P-T and P-BN at 36°C (up to 4.3- and 21-fold, respectively). On the other hand, the other five identified LEA proteins were down-regulated significantly. LEA D-7-like protein abundance decreased in P-T36 and P-BN36/40. LEA 2 protein was down-regulated in P-T36 and P-BN40. Moderate heat (36°C) decreased the abundance of LEA 3-like and LEA 2-like proteins in P-T, while extreme heat (40°C) down-regulated LEA D-34 like protein in P-BN. High diversity of the LEA protein family in cotton reflects their vital roles in plant growth and development, particularly in protecting cells from environmental stresses.
3.9 At least 80% of the proteins involved in starch and sucrose metabolism became more abundant in pollen after heat treatment
Glucose-1-phosphate adenylyltransferase (A0A1U8P2K6), phosphotransferase (A0A1U8I774) and starch synthase, chloroplastic/amyloplastic (A0A1U8NE25) were up-regulated in P-T36, up to 6.16-, 8.18- and 12.25-fold, respectively, compared with control. We found another isoform of phosphotransferase (A0A1U8HSB5) that was up-regulated in P-BN36 up to 6.31-fold. Surprisingly, two isoforms of probable fructokinase-4 including A0A1U8I467 and A0A1U8PA88 were identified to be differentially regulated in P-BN at moderate and extreme heat. To accurately determine the regulation of probable fructokinase-4 at high temperatures, its response should be further analysed at the biochemical level. Overall, the average fold change of proteins involved in starch and sucrose metabolism increased when pollen at distinct developmental stages was exposed to high temperatures, compared with controls. This result is consistent with the increased level of soluble sugars in pollen exposed to transient heat.
3.10 Functional distribution of differentially expressed proteins in pollen under heat
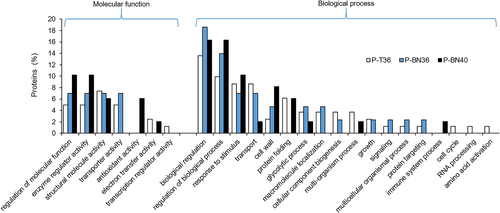
Gene ontology enrichment analysis of all 239 DEPs shown in Table S5, was performed in order to better understand the functional categories affected by moderate and extreme heat preceding mature pollen grain formation. The DEPs were allocated to functional categories in the cellular component, molecular function and biological process ontologies, by comparing control pollen with P-T36 and P-BN at 36 and 40°C. To investigate the functions of these proteins, both up-and down-regulated proteins were classified into narrower functional categories based on their GO (Figure 6). The data indicated that the percentage of proteins involved in different functional categories including ‘regulation of molecular function’, ‘enzyme regular activity’, ‘antioxidant activity’, ‘regulation of biological process’, ‘response to stimulus’, ‘cell wall’ and ‘protein folding’ increased in response to extreme heat; however, ‘structural molecular activity’, ‘transporter activity’, ‘transport’ and ‘glycolytic process’ were inhibited in response to 40°C.
3.11 Key pathways induced in pollen in response to high temperatures
For a deeper analysis of the metabolic pathways linked to heat stress, we focussed on proteins with ≥3-fold difference in abundance (p < .05) between control and treatments, and annotated these using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database and BRITE (Table S6). Table 2 represents the differentially regulated pathways in P-T36, P-BN36 and P-BN40 according to the average fold-change in each category.
| KEGG and BRITE pathways | P-T36 | P-BN36 | P-BN40 | |||
|---|---|---|---|---|---|---|
| Up | Down | Up | Down | Up | Down | |
| Protein processing in endoplasmic reticulum | 3.2 | 0 | 2.8 | 0 | 5.6 | 0 |
| Chaperones and folding catalysts | 3 | 0 | 2.7 | 0 | 5.2 | 0 |
| Glycolysis/gluconeogenesis | 3 | 0 | 3.8 | 0 | 4.4 | 0 |
| Pyruvate metabolism | 1.8 | 0 | 4.5 | 0 | 4.4 | 0 |
| RNA transport | 4.2 | 0 | 4.1 | 0 | 2 | 0 |
| Translation factors | 4.2 | 0 | 4.1 | 0 | 2 | 0 |
| Exosome | 3.6 | −1.7 | 2.9 | 0 | 5.6 | −3 |
| Messenger RNA biogenesis | 3.5 | 0 | 3.5 | 0 | 2 | 0 |
| Protein phosphatases and associated proteins | 1.7 | 0 | 0 | 0 | 5.3 | 0 |
| Carbon metabolism | 3.3 | 0 | 3.8 | 0 | 3 | −2.5 |
| Spliceosome | 6 | 0 | 0 | 0 | 5.6 | 0 |
| Ribosome biogenesis | 0 | 0 | 0 | 0 | 5.6 | 0 |
| Membrane trafficking | 2.6 | 0 | 3.0 | 0 | 5.6 | −5.6 |
| Protein export | 0 | 0 | 0 | 0 | 5.6 | 0 |
| Citrate cycle (TCA cycle) | 0 | 0 | 4.5 | 0 | 4.4 | −1.8 |
| Cytoskeleton proteins | 4.3 | 0 | 0 | 0 | 0 | 0 |
| Transfer RNA biogenesis | 1.9 | 0 | 4.1 | 0 | 0 | 0 |
| Alanine, aspartate and glutamate metabolism | 2.7 | −1.7 | 2.7 | 0 | 2.2 | 0 |
| Starch and sucrose metabolism | 3.2 | 0 | 3.5 | −2.4 | 4.1 | −2.6 |
| Galactose metabolism | 3 | 0 | 2.7 | 0 | 0 | 0 |
| Fructose and mannose metabolism | 3 | 0 | 3.5 | −2.4 | 4.1 | −2.6 |
| Thiamine metabolism | 2.2 | 0 | 0 | 0 | 2.8 | 0 |
| Biosynthesis of secondary metabolites | 3.2 | −2 | 3.5 | −2 | 4 | −4.1 |
| Proteasome | 0 | −2.9 | 0 | 0 | 5.6 | 0 |
| Endocytosis | 0 | 0 | 2.7 | 0 | 5.6 | −5.6 |
| Selenocompound metabolism | 1.9 | 0 | 0 | 0 | 0 | 0 |
| Ubiquitin system | 4.2 | 0 | 0 | 0 | 0 | 0 |
| Biosynthesis of amino acids | 3.0 | −1.7 | 2.7 | 0 | 2.2 | −2.5 |
| Transcription factors | 1.7 | 0 | 0 | 0 | 0 | 0 |
| Chromosome and associated proteins | 3.8 | 0 | 0 | 0 | 0 | 0 |
| Lectins | 3.6 | 0 | 0 | 0 | 0 | 0 |
| Glycine, serine and threonine metabolism | 3.9 | 0 | 0 | 0 | 2.6 | −3 |
| Ribosome | 2.6 | 0 | 2.1 | 0 | 2.3 | −3.6 |
| Nitrogen metabolism | 0 | −1.7 | 2.7 | 0 | 2.2 | 0 |
| Glutathione metabolism | 0 | 0 | 0 | 0 | 3.1 | 0 |
| Mitochondrial biogenesis | 2 | −2.2 | 0 | −3.8 | 5.6 | 0 |
| N-glycan biosynthesis | 0 | 0 | 2.8 | 0 | 0 | 0 |
| Pentose and glucuronate interconversions | 0 | 0 | 5.6 | −5.6 | 0 | 0 |
| Transporters | 2.5 | 0 | 0 | 0 | 0 | 0 |
| Protein kinases | 2.2 | 0 | 0 | 0 | 0 | 0 |
| Aminoacyl-tRNA biosynthesis | 1.9 | 0 | 0 | 0 | 0 | 0 |
| Lysine biosynthesis | 1.6 | 0 | 0 | 0 | 0 | 0 |
| Glyoxylate and dicarboxylate metabolism | 0 | −1.7 | 0 | 0 | 2.6 | 0 |
| Cysteine and methionine metabolism | 3.9 | 0 | 0 | 0 | 0 | −3 |
| 2-Oxocarboxylic acid metabolism | 2.7 | 0 | 0 | 0 | 0 | −1.8 |
| Oxidative phosphorylation | 3 | −3.1 | 0 | 0 | 0 | 0 |
- Note: The represented key differentially regulated pathways are based on the average fold-change of all proteins in each category. The relative fold-change is shown as log2.
The majority of pathways (Table 2) were up-regulated in P-T36, with the greatest increase in cytoskeleton proteins, compared with P-BN36/40. In this study, several cytoskeletal proteins were identified including actin-like protein (A0A1U8NR56), myosin-9 (A0A1U8HT19), tubulin beta-5 chain (A0A1U8ISX7) and tubulin beta chain (B1PBW0), that were up-regulated above 50-, 4-, 13- and 15-fold, respectively. These proteins were not up-regulated in P-BN at either moderate or extreme heat but their abundance was significantly up-regulated in P-T under moderate heat. Up-regulation of the cytoskeletal components seems to be essential for tetrads to maintain cell shape and homeostasis, implying their higher sensitivity to short-term heat.
Proteins involved in the TCA cycle, pyruvate and carbon metabolism and glycolysis/gluconeogenesis were highly up-regulated in pollen when plants had been exposed to 36 and 40°C. Acetyltransferase component of pyruvate dehydrogenase complex (A0A1U8PLQ0) was up-regulated in P-BN36 and P-BN40 (21- and 22-fold, respectively) in the TCA cycle, while the protein was not significantly changed in P-T36. In the carbon metabolism pathway, D-3-phosphoglycerate dehydrogenase (A0A1U8N291) was highly up-regulated in P-T36 (15-fold), whereas A0A1U8PLQ0 became more abundant in P-BN36 and P-BN40, compared with control. With regard to the pyruvate metabolism, protein DJ-1 homolog D-like (A0A1U8P1A3) was the only protein up-regulated in P-T36 (threefold). On the other hand, A0A1U8PLQ0 was up-regulated in P-BN under the elevated temperatures. Two isoforms of phosphotransferase (A0A1U8I774 and A0A1U8HSB5) were enriched in the glycolysis/gluconeogenesis pathway, being up-regulated in P-T36 and P-BN36, respectively, while extreme heat increased the abundance of A0A1U8PLQ0 only in P-BN36/40. Acetyltransferase component of pyruvate dehydrogenase complex was up-regulated in all pathways in P-BN at 36 and 40°C, indicating its important role in a key branch point of carbon metabolism under heat stress.
Exposing P-BN to extreme heat highly activated the key pathways involved in response to heat, including protein export, protein processing in endoplasmic reticulum and chaperones and folding catalysts. The high fold change of these pathways indicates the necessity of the corresponding proteins to protect pollen at 40°C. Heat shock 70 kDa protein (A0A1U8MBN0), heat shock cognate 70 kDa protein 2-like (A0A1U8PHA9), luminal-binding protein 5-like (A0A1U8KNG9) and heat shock protein 70 (D2D322) were highly induced in these pathways.
3.12 Parallel reaction monitoring validation
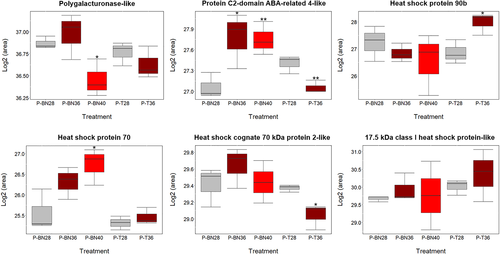
Parallel reaction monitoring (PRM) analysis was carried out on six heat-responsive proteins to validate the differentially expressed changes in proteins reported by label-free shotgun proteomics. Unique peptides were selected up to two different sequences and included in the PRM run. Data analysis obtained from Skyline-daily demonstrated that the differentially changes in abundance of A0A1U8KL32 completely agree with the label-free data in P-T36, P-BN36 and P-BN40. PRM results also confirmed the similar expression patterns in A0A1U8MXF7, A0A1U8HY33, A8WCV1 and A0A1U8NNI0 at least in two out of three conditions. The up-regulation of D2D322 was more pronounced in P-BN40 compared with P-BN28, which is in agreement with the label-free shotgun data analysis (Figure 7). Even though D2D322 had not been detected in P-T36 and P-BN36 using the label-free data, PRM could detect the protein in both conditions (no significant difference compared with PT28 and P-BN28), showing the higher sensitivity of PRM for MS/MS acquisition. PRM generally confirmed the expression patterns of the six proteins measured using label-free proteomics, giving us confidence in the reliability of label-free shotgun proteomics. However, any claims of heat-tolerance mechanisms built on a single protein peaks derived from shotgun proteomics would need corroborating using PRM.
4 DISCUSSION
Several mechanisms could explain the effect of short periods of extreme heat on ultimate boll yield. While pollen viability is, a priori, the most likely, we first explored the impact of high temperature on leaf-level processes to test the claim that a severe carbohydrate restriction through damage to photosynthetic integrity could indirectly reduce pollen viability and impair its function (Pressman, Peet, & Pharr, 2002). Moderate levels of heat (36°C) had negligible or non-significant effects on the major variables of photosynthesis, including assimilation rate, electron transport, intercellular CO2 concentrations and stomatal conductance. This demonstrates the resilience of photoassimilation in cotton under maximum air temperatures that damage photosynthesis in temperate species. Even in more extreme heat conditions (40°C), CO2 assimilation was not significantly affected, and Fv'/Fm', Ci/Ca and gs were significantly increased compared with controls. Essentially, heat stress impairs the photosynthetic machinery in plants in part by lowering Fv'/Fm'; however, the ratio remains unchanged or increases in tolerant species as reported in previous studies (Eustis, Murphy, & Barrios-Masias, 2020; Sharma, Andersen, Ottosen, & Rosenqvist, 2012). Stable gas exchange variables and efficient electron transport in Sicot 71 is strong evidence of the cultivar being resistant to extreme heat (40°C) at the vegetative stage. By contrast, the reproductive stage, and particularly the tetrad stage, is very vulnerable to extreme heat leading to failure in dehiscence. Our finding in Sicot 71 is in contrast to Perry, Krieg, and Hutmacher (1983) who had studied 10 different cotton cultivars and discovered 33°C as an optimum air temperature for photosynthesis in cotton. Presumably, cotton cultivars have adapted to different temperatures depending on their origin. Conaty, Burke, Mahan, Neilsen, and Sutton (2012) also suggested that the threshold temperature for a closely related variety (Sicot 70BRF) is about 28–30°C, which is again below than the optimum temperature for Sicot 71 in this study.
RCI% is considered as a powerful indicator of heat tolerance in cotton, having a strong negative correlation with yield and fibre quality (Azhar, Ali, Akhtar, Khan, & Trethowan, 2009; Najeeb et al., 2017). In the present study, 36°C did not cause significant cellular damage in leaves; however, 40°C significantly increased RCI, reflecting damage to cell membranes under extreme heat. Likewise, boll production was adversely affected when plants were treated at 40°C. In addition to causing impaired tetrad development (P-T40), extreme heat can negatively affect later pollen developmental stages, including uninucleate and binucleate stages, as evidenced by the dramatic reduction in boll development at 13-DAT or 20-DAT. Despite flowering and releasing pollen, heat-treated plants did not successfully produce many bolls even up to 20 days after heat stress ceased. This implies that pollen grains might be damaged even at the binucleate stage (P-BN40).
Yield failure can also be associated with the viability of mature pollen, which we showed to be significantly decreased under high temperatures, particularly 40°C. The rate of pollen germination and pollen tube length can indicate thermotolerance in cotton and tomato (Kakani et al., 2005; Zhou et al., 2015). Similarly, our data obtained from an in vitro experiment demonstrated the highest vulnerability of pollen grains to 40°C, at which the percentage of pollen germination and pollen tube length declined significantly. This is supported by Parrotta, Faleri, Cresti, and Cai (2016) where pollen germination and tube length decreased in tobacco under extreme heat. Poor boll production can also be attributed to a significant reduction in pollen viability, adversely affecting fertilisation in cotton.
Pressman et al. (2002) concluded that pollen infertility in tomato might be associated with the adverse effect of heat (32°C) on specific developmental stages, which consequently reduced sugar concentration in mature pollen. We do not support their findings, as 40°C in the present study increased the total soluble sugars in mature pollen. In cotton, there was a slight increase in the level of sugars in leaves and pollen exposed to moderate and extreme heat; moreover, a significant increase was observed in P-T40 + 1 exposed to extreme heat. Our finding is supported by previous studies on rice (Chung, Hsiao, Chen, Chang, & Wang, 2014) and bell pepper (Aloni, Peet, Pharr, & Karni, 2001), reporting the increasing level of sucrose and starch in pollen grains under heat stress. Reduction in the metabolic rate of pollen grains under heat as well as increased photoassimilate supply is speculated to lead to an increase in the level of sugar in pollen under heat. We conclude that failure in dehiscence in P-T40 was not related to sugar supply. Instead, the failure can be strongly associated with the arrested development of P-T40 due to high susceptibility to extreme heat. The significantly reduced volume of individual pollen grains in P-T40 compared with P-T36 and P-BN at 36 and 40°C supports claims of the specific sensitivity of pollen grains to stresses (Mercado, Mar Trigo, Reid, Valpuesta, & Quesada, 1997). A study on cotton showed that H05 line (heat-sensitive) was indehiscent after exposure to a temperature up to 39°C for 7 days, whereas line 84021 (heat-tolerant) showed a successful dehiscence, indicating that anther dehiscence can be a good representative of thermotolerance (Min et al., 2013). Porch and Jahn (2001) indicated that heat stress (32°C) prior to anthesis can lead to abnormal pollen and anther development in Phaseolus vulgaris, and therefore reduction in yield. This agrees with our data that boll production was significantly affected when cotton exposed to 40°C for 5 days. The complexity of the developmental responses to heat stress indicate subtle patterns of gene expression that were explored using proteomics.
Proteomics revealed that cytoskeletal proteins were only identified in P-T36. This reflects the vulnerability of the tetrad stage even to moderate heat, activating these proteins to support cell division and homeostasis. The observation agrees with previous studies in Arabidopsis roots, where both cytoskeletal elements including microtubules and actin microfilament underwent a dramatic change after exposure to heat stress (Muller, Menzel, & Samaj, 2007). This presents the possibility of developmentally differential sensitivity to heat because each phase is associated with a specific pattern of expressed genes (Borg et al., 2009). Importantly, the majority of pathways were up-regulated in P-T36, whereas many pathways were down-regulated in P-BN40. This might indicate that tetrad cells responded more acutely to moderate heat, by synthesising cellular components that enable subsequent mitoses and structural changes to the haploid germ cells. Failure to activate heat-acclimation pathways might have led to lethal effects on tetrads at 40°C.
Proteomics also indicated a major impact on carbohydrate metabolism in pollen under the elevated temperatures. Importantly, all proteins associated with glycolysis/gluconeogenesis and pyruvate metabolism were up-regulated in P-T and P-BN exposed to 36 or 40°C. Moreover, at least 80% of the proteins involved in starch and sucrose metabolism were up-regulated in pollen after exposure to heat stress. This is consistent with the increased level of total soluble sugars measured in P-T and P-BN under high temperatures, even though some proteins involved in TCA cycle, carbon metabolism and starch and sucrose metabolism were specifically down-regulated in P-BN under heat stress. Carbohydrate deficits do not inevitably result from abiotic stresses; Chen et al. (2016) reported suppressed glycolysis and pyruvate metabolism in cotton leaves by salt stress. This emphasises the specific nature of the response of reproductive development to heat in cotton plants.
Furthermore, proteomic analyses revealed that the unfolded protein response (UPR) was activated in heat-treated pollen, particularly at 40°C. This is a cellular response to accumulation of unfolded and misfolded proteins in the endoplasmic reticulum (ER) driven by exposure to stress (Bao & Howell, 2017; Deng, Srivastava, & Howell, 2013). The response was associated with pathways of protein processing in endoplasmic reticulum, chaperones and folding catalysts, spliceosome and protein export. Accordingly, heat shock proteins were a dominant family of heat-responsive proteins, as illustrated by their abundance after exposure to high temperatures. For example, 10 out of 33 up-regulated proteins were accounted for by HSPs in P-BN40. A microarray study on cotton demonstrated high expression levels of GhHSP90 and GhHSP70 at different fibre developmental stages, indicating the importance of this family of proteins in fibre production (Sable et al., 2018). Suppression of these genes led to impairment in fibre initiation and elongation, adversely affecting the fibre development.
It is important to note that the spliceosome was only triggered in P-T36 and P-BN40, whereas there was no evidence of the pathway after exposure of binucleate-stage pollen to 36°C. It can be concluded that the splicing machinery was highly activated in P-T36 and P-BN40 to splice genes, such as HSPs and BiP5 required to mitigate the stress. On the other hand, the spliceosome was not significantly changed in P-BN under moderate heat, demonstrating that 36°C did not trigger the pathway in the late pollen developmental stage (P-BN). Hopf, Plesofsky-Vig, and Brambl (1992) studied two different classes of HSP70, namely premature (with intron) and mature (without intron) HSP70 in maize pollen, and found that high levels of mature HSP70 (2.2 kb) transcripts were processed at 40°C. However, unprocessed HSP70 (2.9 kb) transcripts were accumulated at 45°C, showing that high temperature interferes with RNA splicing. In the current study, HSP70s were mainly induced after exposure of the binucleate stage to extreme heat (P-BN40), compared with P-BN36. The sensitivity of tetrads to extreme heat can be attributed to lack of HSP70s, which are essential in protein (re)folding under stress. As a result, tetrads might have experienced lethal cell damage under 40°C.
Even though HSPs specifically enriched in the spliceosome pathway were not identified in tetrads, we found that two isoforms of heat shock 70 kDa protein 17-like (A0A1U8KI79 and A0A1U8L553), heat shock protein 90b (A0A1U8KL32), 17.5 kDa Class I heat shock protein-like (A8WCV1), and 17.6 kDa Class I heat shock protein 2-like (A8WCV5) were up-regulated in response to moderate stress. Using RNAseq analysis to study the transcriptome of maize pollen after a brief period of moderate heat (35°C), Begcy et al. (2019) also reported an increase in expression of HSPs including HSP70, HSP18C and HSP1 genes. Moreover, the accumulation of BiPs can lead to stress tolerance in plants by alleviating the ER stress. For instance, overexpression of BiP in tobacco resulted in increased tolerance to drought stress, while drought tolerance was disrupted by knocking out the gene (Alvim et al., 2001; Leborgne-Castel, Jelitto-Van Dooren, Crofts, & Denecke, 1999). The high abundance of BiPs at the binucleate stage might play a role in suppressing cell damage resulting from extreme heat.
5 CONCLUSION
Cotton is an important fibre crop but few studies have been carried out to unravel the responses of pollen development to heat stress when water supply was unlimited. When developing male gametophytes were exposed transiently to 40°C during either tetrad or binucleate stages formation, pollen was found to be acutely sensitive while there was little impact on photosynthetic performance. Subsequent yield impacts were reflected in failure of anther dehiscence and boll development. Proteomic analysis showed that ER stress was induced by the elevated temperature, especially at 40°C. The UPR was initiated in response to stress, activating key pathways to control protein folding; these pathways appear to be less active in tetrads exposed to high temperatures. Further studies are required to identify all stress-responsive proteins and the molecular mechanisms that are specific to each pollen developmental stage.
ACKNOWLEDGMENTS
F.M.-A. acknowledges support from Macquarie University in the form of the iMQRES scholarship. UN acknowledges the University of Sydney Alumni and Development Research Grant. F.M.-A., U.N. and B.J.A. acknowledge use of the Plant Growth Facility (PGF), Australian Proteome Analysis Facility (APAF) and Macquarie University Faculty of Science and Engineering Microscope Facility (MQFoSE MF) and their valuable support. We acknowledge the assistance of Yasmin Asar and Samira Badri with R scripting and the collection of pollen samples for proteomics, respectively. We would especially like to thank the two referees for the high calibre reviews which have undoubtedly greatly improved the quality of the manuscript.
CONFLICT OF INTEREST
The authors declare they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Farhad Masoomi-Aladizgeh, Ullah Najeeb, Daniel K. Y. Tan, Brian J. Atwell: Designed and developed the research. Farhad Masoomi-Aladizgeh, Ullah Najeeb: Cultivated and treated the plants, collected pollen, recorded the physiological data and interpreted the results. Farhad Masoomi-Aladizgeh: Performed proteomic experiments and prepared the original draft of the manuscript. Sara Hamzelou: Helped with peptide preparation, PRM and data analysis. Ardeshir Amirkhani: Assisted with LC–MS/MS and PRM analysis. Dana Pascovici, Mehdi Mirzaei, Paul A. Haynes: Contributed to data analysis and interpretation. Brian J. Atwell: Contributed to data interpretation and preparation of the final manuscript. All contributed to revising the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings will be available publicly in [PRIDE] at [https://www.ebi.ac.uk/pride] after the date of publication. The data is accessible for reviewers through the following details: Project accession: PXD015721; Username: [email protected]; Password: VXSJTxtX.




