Vernal freeze damage and genetic variation alter tree growth, chemistry, and insect interactions
Abstract
Anticipated consequences of climate change in temperate regions include early spring warmup punctuated by intermittent hard freezes. Warm weather accelerates leaf flush in perennial woody species, potentially exposing vulnerable young tissues to damaging frosts. We employed a 2 × 6 randomized factorial design to examine how the interplay of vernal (springtime) freeze damage and genetic variation in a hardwood species (Populus tremuloides) influences tree growth, phytochemistry, and interactions with an insect herbivore (Chaitophorus stevensis). Acute effects of freezing included defoliation and mortality. Surviving trees exhibited reduced growth and altered biomass distribution. Reflushed leaves on these trees had lower mass per area, lower lignin concentrations, and higher nitrogen concentrations, altered chemical defence profiles, and supported faster aphid population growth. Many effects varied among plant genotypes and were related with herbivore performance. This study suggests that a single damaging vernal freeze event can alter tree–insect interactions through effects on plant growth and chemistry. Differential responses of various genotypes to freeze damage suggest that more frequent vernal freeze events could also influence natural selection, favouring trees with greater freeze hardiness, and more resistance or tolerance to herbivores following damage.
1 INTRODUCTION
Effects of climate change on forests include more frequent and intense insect outbreaks, increased tree mortality (Jamieson, Trowbridge, Raffa, & Lindroth, 2012; Ryan & Vose, 2012; Weed, Ayres, & Hicke, 2013), and migration of plants and insects to higher altitudes and latitudes (Corlett & Westcott, 2013; Renwick & Rocca, 2015). These effects will likely intensify over the next 100 years, as climate models project that temperatures will increase and precipitation will become more erratic (Logan, Regniere, & Powell, 2003; Tobin, Parry, & Aukema, 2014). Environmental change will also likely drive adaptation and/or migration of long-lived plants on timescales that are similar to or faster than their generation times (Corlett & Westcott, 2013). Studies examining the interplay between environmental variation, plant trait variability, and herbivory will facilitate prediction of how climate change will affect persistence of plants in existing habitats and recruitment of plants into new habitats (Snell et al., 2014).
One probable consequence of climate change in temperate regions is more frequent damaging vernal (springtime) frosts, caused by earlier spring warmup combined with larger temperature fluctuations (Augspurger, 2013; Gu et al., 2008; Inouye, 2000). Warm weather accelerates budbreak in perennial woody plants, exposing vulnerable new growth to damaging cold temperatures (Hanninen, 2006; Inouye, 2000). Although rare, vernal freezes (with documented temperatures ranging between −7 °C [Gu et al., 2008] and −2 °C [Hufkens et al., 2012]) exert substantial impacts on trees, including increased mortality, altered morphology, decreased growth, and modified leaf chemical defences (Gu et al., 2008; Hufkens et al., 2012; Kreyling et al., 2014; Man, Lu, Colombo, Li, & Dang, 2013; St. Clair, Monson, Smith, Cahill, & Calder, 2009; Wolken, Lieffers, Landhausser, & Mulak, 2009). Of the limited studies that have examined the connection between freeze damage and insect susceptibility in trees, one (Thomson, Nicotra, & Steinbauer, 2001) revealed increased growth of two insect species on Eucalyptus, and another showed increased attraction of beetles to Fagus (LaSpina, De Cannière, Dekri, & Grégoire, 2013), suggesting that freeze damage may render trees more susceptible to insect attack. To date, studies examining the effects of freeze damage on trees have not addressed impacts on aboveground–belowground biomass allocation, or the connection between freeze-induced changes in particular plant traits (e.g., phytochemistry) and plant–insect interactions.
Trembling aspen (Populus tremuloides) provides an experimental system well suited to exploring the effects of vernal freeze damage on trees. It is a genetically diverse (Slavov & Zhelev, 2010), early successional tree species that is widespread in temperate, northern and montane forests (Sibley, 2009) of North America, where effects of climate change have been especially pronounced (IPCC, 2013). Aspen exhibits wide variation in phenotypic traits and responses to environmental factors (Stevens, Waller, & Lindroth, 2007; Couture, Meehan, & Lindroth, 2012; Lindroth & St. Clair, 2013). Following a natural frost defoliation event, reflushed leaves of wild aspen were larger and exhibited phytochemical shifts that may decrease susceptibility to herbivory (St. Clair et al., 2009), including lower concentrations of nitrogen and higher concentrations of chemical defences (condensed tannins [CTs] and phenolic glycosides [PGs]; St. Clair et al., 2009). The phytochemical impacts of other damage agents (e.g., herbivores) vary strongly among aspen genotypes (Keefover-Ring, Rubert-Nason, Bennett, & Lindroth, 2016; Rubert-Nason, Couture, Major, Constabel, & Lindroth, 2015; Stevens & Lindroth, 2005), suggesting that similar response patterns are likely for freeze damage and raising the possibility that such damage may influence natural selection. Changes in physical and chemical traits may be particularly important determinants of seedling and sapling success, because immature trees lack the nutrient reserves found in the roots and stems of mature trees.
We employed an aspen–aphid system to study how vernal freeze damage and genetic variability independently and interactively affect plant fitness and plant–insect interactions. Aphids (e.g., Chaitophorus stevensis) commonly feed on aspen; although our model system has received little attention in scientific literature, some limited evidence suggests that elevated foliar nitrogen may improve aphid performance (Mattson, 1980), and elevated CTs and PGs may be detrimental (Wong, 2013). We predicted multiple impacts of freeze damage to trees, including (a) increased mortality, (b) reduced growth, (c) decreased biomass allocation to roots relative to stems and leaves, (d) altered foliar chemistry (decreased levels of nitrogen and CTs and increased levels of PGs), and (e) improved aphid performance. We further predicted that these impacts would vary among aspen genotypes (genotype × environment [G × E] effects).
2 MATERIALS AND METHODS
2.1 Plant cultivation
Six unique aspen genotypes (demonstrated by microsatellite analysis [Cole, 2005]) were collected from southern Wisconsin, USA, and reproduced by tissue culture micropropagation at Knight Hollow Nursery (Middleton, WI, USA; Sellmer, McCown, & Haissig, 1989). Seven months later (August 2012), young trees were transplanted into Conetainers (3.8 cm × 21 cm, Stuewe and Sons, Tangent, OR, USA) containing 1:1 torpedo sand and MetroMix 366-PSC (Sun Gro Horticulture, Agawam, MA, USA), fertilized intermittently (Peters Professional water soluble fertilizer, Everris North America), and allowed to enter dormancy outdoors. Trees were overwintered in a sheltered area, grown throughout the 2013 season without fertilization, and again overwintered in a sheltered area.
The trees were prepared for our experiment in March 2014, by transplanting into 2.5-L plastic pots with a mix of 2:1 sand:silt loam, and allowed to leaf out under ambient outdoor conditions. During transplanting, tree heights, basal diameters (at 1 cm above the root collar), and numbers of primary lateral branches were recorded. A representative subset of trees from each genotype was destructively harvested, oven-dried (40 °C), and weighed to generate linear allometric regression equations for prediction of initial biomass of experimental trees. On April 14, 2014, during early budbreak, trees were fertilized (Osmocote 18:6:12 8/9 month slow-release, Everris North America) at a rate of 2.1 g/L (6 g/pot; intermediate level based on Stevens & Lindroth [2005]), and pots were arranged randomly.
2.2 Experimental design and cold treatment
We employed a 2 × 6 randomized factorial design to evaluate the effects of vernal freeze damage on six aspen genotypes. During shoot elongation, 14 replicate trees with fully expanded leaves from each genotype were placed together within a walk-in refrigerator and subjected to either a 12-hr overnight chilling cycle (8:00 p.m.–8:00 a.m.) mimicking a severe natural freeze event (causing death of all active growth) or a cold night without freezing (control). Freeze damage was imposed on May 27, 2014 (day 0), by equilibrating trees to 10 °C for 1 hr, decreasing the set temperature to 2 °C at a rate of −4 °C/hr, further decreasing the set temperature to −4 °C at a rate of −2 °C/hr, increasing the set temperature to 2 °C at a rate of 2 °C/hr, and finally increasing the set temperature to 10 °C at a rate of 4 °C/hr. Three randomly placed data loggers confirmed that trees were exposed to average temperatures <0 °C for 5.8 hr and a minimum temperature of −5.5 °C. Control trees were chilled the following evening (day 1), by equilibrating to 10 °C for 1 hr, decreasing the temperature at a rate of −4 °C/hr for 2 hr, and maintaining at 2 °C for 8 hr before returning to 10 °C at a rate of 4 °C/hr.
2.3 Observational assessment
We visually assessed tree condition before and after cold treatments (Figure 1) and documented the date of budbreak for second flush leaves on each tree recovering from damage. We also counted suckers (root sprouts) and documented the presence/absence of chlorotic leaves.

2.4 Morphological assessment
We destructively harvested approximately one half of the surviving trees from each damage × genotype combination (3–7 replicates) in early August (72 days post-treatment) to assess growth and morphology. Heights, basal diameters (mean at 1 cm above root collar), primary lateral branch counts, leaf counts, and areas (for all leaves >0.5 cm length, including petioles) were determined; leaf areas were measured on a Licor LI-3100 area metre (Lincoln, NE, USA). Leaves and stems were vacuum-dried, and roots were oven-dried (40 °C) then weighed. Dimensional and biomass measurements were used to calculate tree size (d2 × h, where d = basal diameter and h = height), leaf mass per area (LMA), and mass ratios for leaves (LMR), stems (SMR) and roots (RMR; mass of leaves, stems, or roots relative to total plant mass). Mass ratios were used to assess how freeze damage affects relative biomass allocation to leaves, stems, and roots.
2.5 Phytochemical assessment
We assessed foliar phytochemistry and LMA in all remaining trees in mid-August (84 days post-treatment), in order to evaluate impacts of vernal freeze damage in relation to insect performance (see below). Lignin, nitrogen, chlorophyll, and CTs were quantified in situ using infrared reflectance spectroscopy. Spectral measurements were recorded using a full-range spectroradiometer (Fieldspec 3, ASD, Boulder, CO, USA) applied to the adaxial surface of two or three randomly chosen leaves (>2 cm length and width) from separate lateral branches of each tree. The mean reflectance spectrum for each leaf was determined by averaging three reflectance measurements (each consisting of five averaged spectra per leaf). Nitrogen, lignin, and LMA were predicted using calibrations developed by Serbin, Singh, McNeil, Kingdon, and Townsend (2014), and CTs were predicted using calibrations reported by Couture et al. (2016); model performance was verified against laboratory measurements in a subset of the samples (following Rubert-Nason et al., 2013). Chlorophyll was computed as the relative difference between reflectance at wavelengths 750 and 705 nm (Gitelson & Merzlyak, 1994). All measurements of soluble sugars and PGs, as well as reference measurements for lignin, nitrogen, and CTs, were determined using the standard laboratory methods described below.
For laboratory analyses, 2–3 leaves per tree used for reflectance measurements were collected sans petioles. These leaves were transported on ice, vacuum-dried, pulverized by ball milling, and stored at −20 °C. Lignin was determined gravimetrically by digestion of plant material in 72% H2SO4 (aq) following the Klason method (Ankom, 2016). Nitrogen was analysed by combustion of 3–5 mg samples on a Thermo Flash EA1112 elemental analyzer (Waltham, MA, USA), as described in Sollins et al. (1999). Condensed tannins were measured by extraction of 20–30 mg leaf powder into 7:3 acetone:water (with ascorbic acid), followed by reaction with ferric ammonium sulphate in acidified butanol (Porter, Hrstich, & Chan, 1986), and spectrophotometric quantification of the chromophore standardized against purified P. tremuloides CTs. Soluble sugars were extracted from 20–30 mg leaf powder into ethanol and measured colorimetrically following reaction with dinitrosalicylic acid, with standardization against glucose, as described by Lindroth, Osier, Barnhill, and Wood (2002).
The PGs (salicin, salicortin, tremuloidin, and tremulacin) were quantified by ultra-high performance liquid chromatography with detection by negative electrospray ionization single quadrupole mass spectrometry (UPLC/MS; Acquity I-Class UPLC with 3100 SQ mass detector; Waters Corporation, Milford, MA, USA) following a modified version of the method by Abreu, Ahnlund, Moritz, and Albrechtsen (2011). Samples were sonicated for 15 min at <10°C in 1.5 ml methanol spiked with 10 mg/L β-resorcylic acid (for process control) and centrifuged for 15 min to remove large particles. Supernatant from the single extraction was diluted, spiked with salicylic acid-d6 (internal standard for quantification; Sigma-Aldrich, St. Louis, MO, USA), filtered (0.2 μm), stored at −20°C, and analysed within 5 days. Samples (2 μl) were introduced to a CSH C-18 column (2.1 × 100 mm, 1.7 μm) and separated on a gradient of water and acetonitrile (each acidified with 0.1% formic acid) at 40°C and 0.5 ml/min. We quantified the single negatively charged formate adduct ions [(M–H + HCOOH)−] with the following detector configuration: 30 V cone potential, 2500 V capillary potential, 3 V extractor potential, 0.1 V RF lens potential, 120°C source temperature, 250°C desolvation temperature, 500 L/hr N2 desolvation gas flow, 10 L/hr cone gas flow, 5 μl/min infusion rate, 0.025 s dwell time. Salicin analytical standard was purchased from Sigma-Aldrich (St. Louis, MO, USA); salicortin, tremuloidin, and tremulacin standards were extracted from aspen foliage by liquid–liquid extraction (Lindroth, Hsia, & Scriber, 1987) and purified by normal phase liquid chromatography (Still, Kahn, & Mitra, 1978).
2.6 Insect bioassays
We assessed the effects of freeze damage and tree genotype on aphid (C. stevensis) population growth rate between 77 and 90 days after cold treatment, overlapping in time with the phytochemical and LMA measurements at 84 days. Prior to the bioassay, trees were visually inspected for aphids, cleaned by wiping each leaf with a damp paper towel, and bagged with no-see-um mesh. At 77 days, we released five aphids (sourced randomly from a single lateral branch of a nonexperimental aspen tree) onto each of the bagged trees. After 13 days, we counted the total number of aphids per tree. Population growth rate on each tree was computed as by Couture, Servi, and Lindroth (2010), using (ln F − ln I)/t, where F was the total aphid population after the 13-day bioassay, I was the initial aphid population (5), and t was the time in days.
2.7 Data analysis
We evaluated effects of freeze damage and genotype on tree mortality, suckering, and chlorosis using Fisher's exact tests in JMP 11.0.0 (SAS Institute, Cary, NC, USA). We compared effects of freeze damage, genotype, and their interactions on all other variables using analysis of variance (ANOVA) in the same software package. Effects on structure and mass of trees harvested at 72 days were evaluated using a least squares ANOVA with the following covariates: initial tree size (d2 × h) for assessments of tree size, initial tree mass (determined by allometry for each genotype) for assessments of biomass (Table 2), or final tree mass for assessments of mass ratios (Table 3). Data were log10 or square root transformed when not normally distributed. Effects on aphid population growth rate were evaluated using least squares ANOVA.
We evaluated the relationship between plant traits and aphid performance using partial least squares regression following Couture et al. (2012). For the partial least squares regression, data were centred and scaled. Then, the number of latent variables was determined by minimizing the predictive residual error sum of squares statistic using k-fold cross-validation. The most influential experimental variables were classified as those having variable importance for projection scores ≥1.0 (Couture et al., 2012).
3 RESULTS
3.1 Vernal freeze damage and genotype influence mortality, growth, and biomass allocation
Acute freeze damage during late-stage leaf expansion killed all current year leaf and stem tissue, leading to complete leaf abscission and increased sapling mortality, with mortality varying between 0% and 57% among the genotypes (Figure 1a, Table 1). Surviving trees flushed new leaves from axillary buds approximately 2 weeks after damage, and leaves expanded fully within 3–4 weeks of damage. Freeze damage did not increase sprouting of suckers from the roots (Table 1). Chlorosis was common on leaves of recovering trees (Table 1).
| Mortality (%) | Trees with suckers (%) | Trees with chloroses (%) | ||||
|---|---|---|---|---|---|---|
| Genotype | Control | Freeze-damaged | Control | Freeze-damaged | Control | Freeze-damaged |
| PG2 | 7 | 14 | 7 | 14 | 7 | 43 |
| PG3 | 0 | 0 | 0 | 0 | 0 | 36 |
| PI3 | 0 | 14 | 14 | 7 | 0 | 43 |
| SAU3 | 0 | 21 | 0 | 14 | 0 | 7 |
| WAU2 | 0 | 57 | 0 | 0 | 0 | 21 |
| WAU3 | 0 | 7 | 14 | 14 | 0 | 57 |
| Freeze effecta | <0.001 | 0.380 | <0.001 | |||
| Genotype effecta | 0.013 | 0.147 | 0.160 | |||
- a Probabilities (P) of freeze damage and genotype effects on tree mortality, suckering, and chloroses, by Fisher's exact test.
Freezing generally decreased tree growth (Figure 1), with the impact varying among genotypes (Figure 2, Table 2). At 72 days after cold treatment, damaged trees were 54% shorter and 78% smaller (d2 × h) than control trees. Tree height and volume varied by approximately twofold among the genotypes and damage differentially affected these size metrics among genotypes. Damage did not increase the abundance of primary lateral branches, although branching varied among genotypes. Damaged trees averaged 74% less total dry mass and 73% less leaf mass, relative to control trees. Total and leaf mass varied by approximately twofold and threefold among the genotypes, respectively, and genotypes responded differently to freeze damage.
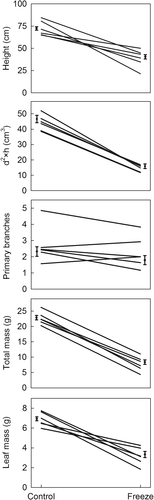
| Tree height | d2 × h | Primary lateral branches | Total mass | Leaf mass | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| df | F | p | F | p | F | p | F | p | F | p | |
| Freeze | 1 | 118 | <.001 | 144 | <.001 | 0.03 | .866 | 189 | <.001 | 78 | <.001 |
| Genotype | 5 | 2.6 | .033 | 3.7 | .006 | 3.3 | .010 | 2.0 | .099 | 3.1 | .016 |
| Freeze × genotype | 5 | 8.1 | <.001 | 6.9 | <.001 | 2.3 | .054 | 3.5 | .007 | 6.3 | <.001 |
| Initial | 1 | 6.2 | .015 | 9.8 | .003 | 1.1 | .304 | 15 | <.001 | 4.4 | .040 |
- The initial condition (i.e., height, d2 × h, branching, or mass) of the tree prior to budbreak and cold treatment was included as a covariate. Response variables were log transformed, except for primary lateral branches, which was square root transformed. Figure 2 contains complementary graphical data.
Freeze damage altered within plant biomass allocation and shifted the patterns of genetic variation in biomass allocation (Figure 3, Table 3). Damaged trees averaged 20% lower LMA at 72 days after cold treatment; variation in LMA by genotype was small (1.1-fold) but was affected by interaction with freeze damage. Leaf size (area per leaf) was unaffected by damage, although it varied 2.5-fold among genotypes. Damage generally increased mass allocation to leaves (26% increase in LMR) at the expense of roots (16% decrease in RMR). SMR and RMR each varied by 1.3-fold among the genotypes, and the responses of LMR and RMR to damage varied among the genotypes.
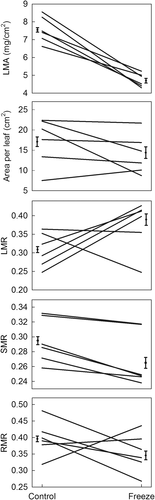
| LMA | Area per leaf | LMR | SMR | RMR | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| df | F | p | F | p | F | p | F | p | F | p | |
| Freeze | 1 | 38 | <.001 | 1.1 | .290 | 7.4 | .009 | 0.75 | .391 | 7.9 | .007 |
| Genotype | 5 | 4.4 | .002 | 7.7 | <.001 | 2.3 | .057 | 22 | <.001 | 5.5 | <.001 |
| Freeze × genotype | 5 | 10 | <.001 | 1.8 | .131 | 8.5 | <.001 | 0.82 | .538 | 10. | <.001 |
| Final mass | 1 | 53 | <.001 | 5.5 | .022 | 0.16 | .692 | 1.8 | .183 | 1.4 | .249 |
- Note. Final tree mass was included as a covariate for each variable. Figure 3 contains complementary graphical data. LMA = leaf mass per area; LMR = mass ratios for leaves; RMR = mass ratios for roots; SMR = mass ratios for stems.
3.2 Vernal freeze damage and genotype influence phytochemistry
Freeze damage influenced midsummer foliar chemistry, with effect magnitudes and directions varying among the genotypes (Figure 4, Table 4). At 84 days following cold treatment, foliar concentrations of sugars were decreased by 6%, lignin decreased by 16%, and nitrogen increased by 36% in damaged trees relative to control trees. Although concentrations of sugars, lignin, and nitrogen varied among genotypes by 1.1-fold, 1.2-fold, and 1.3-fold, respectively, there was no interaction between damage and genotype. Freezing did not alter chlorophyll concentration, although chlorophyll varied 1.3-fold among genotypes. Damage decreased CT concentrations by an average of 56% across all genotypes. CTs varied by 2.2-fold among the genotypes, and the effects of freeze damage on CT concentrations varied by genotype. PGs increased by 10% in response to freezing, and concentrations varied by 1.3-fold among the genotypes, but there was no interaction between damage and genotype.
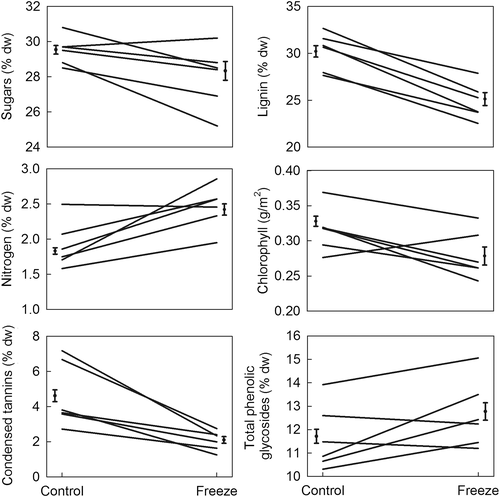
| Sugar | Lignin | Nitrogen | Chlorophyll | Tannins | Phenolic glycosides | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df | F | p | F | p | F | p | F | p | F | p | F | p | |
| Freeze | 1 | 6.7 | 0.012 | 15 | < 0.001 | 37 | < 0.001 | 1.7 | 0.193 | 39 | < 0.001 | 4.8 | 0.033 |
| Genotype | 5 | 2.6 | 0.035 | 2.7 | 0.028 | 4.7 | 0.001 | 3.4 | 0.009 | 9.3 | < 0.001 | 7.1 | < 0.001 |
| Freeze × genotype | 5 | 1.0 | 0.397 | 0.43 | 0.829 | 1.7 | 0.158 | 1.9 | 0.112 | 4.5 | 0.002 | 1.4 | 0.226 |
| Final size | 1 | 0.55 | 0.460 | 0.61 | 0.438 | 0.15 | 0.696 | 4.9 | 0.031 | 0.02 | 0.894 | 0.22 | 0.642 |
- Final size (d2 × h) was included as a covariate. Figure 4 contains complementary graphical data.
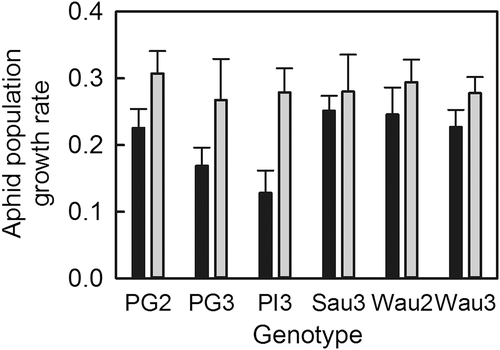
3.3 Vernal freeze damage influences aphid population growth rate
Aphid populations grew twice as rapidly on trees recovering from vernal freeze damage (Figure 5; df = 1, F = 13, p < .001). Population growth rate did not differ among genotypes (df = 5, F = 1.0, p = .405) or in response to the interaction of damage with genotype (df = 5, F = 0.49, p = .784). Partial least squares regression (Table 5) revealed a positive association of aphid population growth rate with tree size (d2 × h), and negative associations of population growth rate with LMA, lignin, chlorophyll and CTs. The strongest associations were with lignin, chlorophyll, and CTs.
| Model coefficient | VIP score | |
|---|---|---|
| Tree size (d2 × h) | 0.121 | 1.041 |
| Primary lateral branches | 0.094 | 0.631 |
| Total mass | 0.081 | 0.947 |
| Leaf mass | 0.100 | 0.951 |
| Leaves per tree | −0.058 | 0.544 |
| LMA | −0.172 | 1.393 |
| Area per leaf | 0.045 | 0.569 |
| Sugars | 0.051 | 0.423 |
| Lignin | −0.206 | 1.323 |
| Nitrogen | −0.0004 | 0.760 |
| Chlorophyll | −0.262 | 1.515 |
| Condensed tannins | −0.200 | 1.266 |
| Phenolic glycosides | 0.152 | 0.868 |
- Coefficient signs indicate directionality of predictor variable with response.
- Model fit (R2 = 0.415) based on two PLS factors (latent variables) and 61 (N) data points. Independent variables with a relatively large contribution to explaining the dependent variable (VIP > 1.0) are shown in boldface font.
4 DISCUSSION
Projected effects of climate change in temperate latitudes include warmer temperatures and larger temperature fluctuations (IPCC, 2013). These conditions hasten leaf flush in deciduous species, increasing their risk for damage by vernal frosts (Hanninen, 2006; Inouye, 2000). Predicted impacts of these frosts on plants include numerous direct and indirect effects, including increased mortality, reduced growth and apical dominance, altered biomass allocation, decreased concentrations of foliar N and secondary metabolites, and improved insect herbivore performance. This study demonstrated that a single damaging vernal freeze increased sapling mortality. Recovering saplings exhibited reduced growth, unaltered apical dominance, reduced biomass allocation to roots, modified foliar chemistry, and supported faster aphid population growth rates. Freeze damage altered foliar traits associated with changes in aphid population growth. Moreover, plant genotype influenced the responses of many tree traits to damage (G × E effects), demonstrating a role of genetic variation in determining plant responses to freezing.
4.1 Vernal freeze damage affects tree survival, growth, and mass allocation
Our findings align with previous research showing that vernal freeze damage caused defoliation and increased mortality in aspen (Egeberg, 1963; Lamontagne, Margolis, & Bigras, 1998; St. Clair et al., 2009; Man et al., 2013), and that aspen surviving freeze damage differed morphologically from undamaged trees. Damage did not affect apical dominance, in contrast to Man et al. (2013), who reported decreased apical dominance. Reduced growth, altered leaf morphology, and increased biomass allocation to leaves relative to stems and roots corresponded with results from prior research on impacts of freeze damage on aspen seedlings (Man et al., 2013) and older trees (Egeberg, 1963; St. Clair et al., 2009).
Trees in this study compensated physiologically for frost-mediated defoliation by increasing allocation of biomass to regenerating tissues for CO2 assimilation (i.e., leaves), at the expense of root and stem growth. Increased LMR in conjunction with decreased RMR and LMA, and no change in area per leaf, indicates that recovering trees sacrificed root growth to increase leaf area for photosynthesis. Increased investment in leaves relative to other tissues may serve to counteract the negative effects of freeze damage on carbohydrate concentrations in leaves following damage. Although maximizing photosynthetic gain is beneficial for trees recovering from defoliation, the concomitant decreased vertical growth and root biomass may be maladaptive in the context of competition or other stressors. For example, shorter trees with smaller root systems will have less access to light and water/nutrients than larger trees. In addition, mammalian herbivores can have a major impact on aspen fitness (Lindroth & St. Clair, 2013), and decreased growth will increase exposure time of trees to browsing damage. Thinner leaves may be more vulnerable to damage by wind (Onoda et al., 2011), desiccation, and insects (Poorter, Niinemets, Poorter, Wright, & Villar, 2009). Finally, decreased root production relative to aboveground tissue production could potentially decrease tolerance of aspen to subsequent drought and insect damage.
4.2 Vernal freeze damage induces ecologically important phytochemical changes
Despite strong links between foliar chemistry, environmental conditions, and herbivore performance (Barbehenn & Constabel, 2011; Mattson, 1980; Philippe & Bohlmann, 2007), few studies (e.g., Selig & Bohne, 2016; St. Clair et al., 2009) have evaluated the impacts of vernal freeze damage on the chemistry of tree foliage. We found that vernal freeze damage substantially altered foliar chemistry. Total soluble sugars decreased, in agreement with declines sometimes observed for mature forest aspen following a natural frost defoliation event (St. Clair et al., 2009). Lignin also decreased, an effect that is likely due to delayed foliar development in recovering trees. Damage increased nitrogen concentrations in young aspen, potentially due to increased photosynthesis during recovery. In contrast, foliar nitrogen decreased in mature, frost-damaged aspen (St. Clair et al., 2009), indicating that responses may vary with tree age or size. Effects of freezing on CTs vary according to damage severity (St. Clair et al., 2009) and tree size or age (CTs declined in our severely damaged saplings but not in mature aspen [St. Clair et al., 2009]). The PG concentrations increased in our recovering saplings as well as in mature forest aspen (St. Clair et al., 2009), indicating consistent effects across a range of plant ages and sizes. Phytochemical changes are presumably shaped by a combination of interacting factors, including physical damage caused by freezing, compensatory growth of recovering plants, leaf phenology, and plant age. Although more research is needed to disentangle these factors, our findings broadly show that vernal freeze damage induces phytochemical changes that may affect multiple ecosystem processes and by comparison with prior work (i.e., St. Clair et al., 2009) that the effects of freeze damage can vary by plant age or size.
4.3 Vernal freeze damage affects plant traits associated with herbivore fitness
Few studies (e.g., LaSpina et al., 2013; Thomson et al., 2001; Wargo, 1996) have examined the relationship between freeze damage and insect performance on trees. We showed that aphid populations on average increase twice as rapidly on trees recovering from freeze damage, a finding that agrees with the plant stress hypothesis (i.e., herbivores prefer stressed plants [White, 1984]). Under field conditions, large increases in aphid populations could amplify water and nutrient losses in trees that are already stressed by climate change factors such as vernal freezes and warmer, drier conditions.
Aphid population growth rate was related to multiple plant traits affected by freeze damage (i.e., size, LMA, and phytochemistry). A positive relationship between aphid population growth rate and tree size could arise because larger trees offer more suitable environments for aphids; however, interpretation of the relationship between aphid performance and tree size is confounded by the concomitant negative effect of freeze damage on tree size. Improved aphid performance on trees with lower LMA and lignin may be due to ease of puncturing vascular tissue by aphid stylets. Lack of a positive relationship with nitrogen (e.g., total nitrogen and chlorophyll) was unexpected because nitrogen is widely regarded as a growth-limiting resource (Mattson, 1980); however, total plant tissue nitrogen may not adequately represent the nutritional needs of phloem-feeding insects. The negative associations of CTs with aphid performance observed herein have been reported previously (e.g., Grayer, Kimmins, Padgham, Harborne, & Rao, 1992; Wong, 2013). Wong (2013) hypothesized that CT precursor compounds (i.e., catechins) dissolved in leaf mesophyll may decrease aphid fecundity and feeding preference. Negative associations of PGs with aphid performance have also been documented (Wong, 2013) but were not observed in our study. To date, the molecular mechanisms by which CTs and PGs affect aphid physiology remain poorly understood.
Our study suggests that a single damaging freeze event has the potential to alter a variety of ecological processes. Morphological changes (e.g., decreased size and LMA) may predispose recovering trees to consumption by mammalian or insect herbivores. Lower lignin and higher nitrogen concentrations in foliage may further increase susceptibility of plants to herbivory, by increasing digestibility and nutritive value (Couture et al., 2012; Ikonen, 2002; Marquis & Batzli, 1989; Mattson, 1980). Opposing effects of damage on aspen chemical defences (e.g., CTs and PGs) complicates evaluation of plant–herbivore interactions but suggest that recovering aspen may be more vulnerable to animals (e.g., Coleoptera) targeted by CTs and less vulnerable to animals (e.g., Lepidoptera) targeted by PGs. CTs also affect soil microbial processes (Hättenschwiler & Vitousek, 2000; Madritch, Greene, & Lindroth, 2009; Madritch & Lindroth, 2015), suggesting that altered CT inputs to soil through changes in leaf litter chemistry and premature abscission of freeze-damaged foliage may influence nutrient cycling in forests impacted by frost damage. That freeze events can have far-reaching and indirect ecological consequences is evidenced by the work of Mulholland, Roberts, Hill, and Smith (2009), who showed that extensive frost defoliation altered nutrient cycling across trophic levels in a forest stream.
4.4 Vernal freeze damage will likely alter the genetic architecture of aspen populations
This research confirms previous reports indicating genotypic variation in aspen responses to freeze damage in natural settings (Egeberg, 1963; Strain, 1966; St. Clair et al., 2009). By experimentally replicating genotypes, we demonstrate that putative genotypic variation is indeed a genetic, and not a spatial, effect.
Vernal freeze damage will likely shape evolutionary trajectories in tree populations. It could do so directly, via differential effects on the fitness of particular genotypes (G × E effects), as demonstrated in this study, but also indirectly, via modifications of plant responses to other environmental stressors (G × E × E effects). For example, genotypic variation in damage-induced decreases in root mass ratio may predispose certain genotypes to subsequent drought stress. Similarly, genotypic variation in damage-induced changes in foliar chemistry may compromise herbivore defence in particular genotypes. From a forest management perspective, these findings suggest that growth of aspen and other Populus genotypes with greater resistance to freeze damage should be promoted in environments that are predisposed to vernal freeze events.
In conclusion, temperate deciduous forest communities are at increasing risk for recurrent freeze events, because warmer springtime temperatures punctuated by intermittent hard freezes are anticipated to become more frequent in a warming climate. This study shows that a single damaging vernal freeze is detrimental to an early successional deciduous tree species, directly through physical damage that increases mortality, decreases growth, redistributes biomass allocation, and reduces chemical defences, and indirectly through increased susceptibility to an herbivorous insect. Moreover, vernal freezes are among many stochastic events that may influence the genetic architecture of forests through differential direct and indirect impacts on tree genotypes, especially if these events become more frequent.
ACKNOWLEDGEMENTS
We thank Nick Grout for assistance with in situ acquisition of infrared spectra using the spectroradiometer and David Voegtlin for confirming the identity of aphids as C. stevensis. We are grateful to Nicholas Keuler from the College of Agricultural and Life Sciences Statistical Consulting group (University of Wisconsin-Madison) for assistance with data analysis. This research was supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, under award number 2011-67013-30147 to RLL and award number 2012-67012-19900 to JJC, as well as U.S. Department of Agriculture McIntire-Stennis project WIS01599 to PAT and JJC, National Aeronautics and Space Administration award NNX12AQ28G to PAT, and the Purdue University Center for Plant Biology. The authors have no conflicts of interest to declare.




