Shining a light on the Arabidopsis circadian clock
Abstract
The circadian clock provides essential timing information to ensure optimal growth to prevailing external environmental conditions. A major time-setting mechanism (zeitgeber) in clock synchronization is light. Differing light wavelengths, intensities, and photoperiodic duration are processed for the clock-setting mechanism. Many studies on light-input pathways to the clock have focused on Arabidopsis thaliana. Photoreceptors are specific chromic proteins that detect light signals and transmit this information to the central circadian oscillator through a number of different signalling mechanisms. The most well-characterized clock-mediating photoreceptors are cryptochromes and phytochromes, detecting blue, red, and far-red wavelengths of light. Ultraviolet and shaded light are also processed signals to the oscillator. Notably, the clock reciprocally generates rhythms of photoreceptor action leading to so-called gating of light responses. Intermediate proteins, such as Phytochrome interacting factors (PIFs), constitutive photomorphogenic 1 (COP1) and EARLY FLOWERING 3 (ELF3), have been established in signalling pathways downstream of photoreceptor activation. However, the precise details for these signalling mechanisms are not fully established. This review highlights both historical and recent efforts made to understand overall light input to the oscillator, first looking at how each wavelength of light is detected, this is then related to known input mechanisms and their interactions.
1 THE CIRCADIAN CLOCK
The circadian clock allows plants as sessile organisms to synchronize with diurnal changes in the environment (Dodd et al., 2005). Daily external environmental stimuli are required to initiate circadian oscillations and to maintain synchronicity with the external environment. This process is called entrainment. The environmental cues governing these processes are termed zeitgebers (from German: “time givers”). The ability to synchronize with the external environment efficiently confers enhanced fitness (Michael et al., 2003).
Diurnal changes in cellular processes controlled by the clock allow plants to anticipate, and therefore better survive, a range of stresses (Sanchez, Shin, & Davis, 2011). Diurnal changes have been shown to occur in cold/freezing tolerance (Fornara et al., 2015; Nakamichi et al., 2009), drought tolerance (Habte, Muller, Shtaya, Davis, & von Korff, 2014), pathogen response (Shin, Heidrich, Sanchez-Villarreal, Parker, & Davis, 2012; Wang et al., 2011), and photosynthesis (Pyl et al., 2012). This synchronization is the product of a large number of rhythmically regulated cellular processes (Bujdoso & Davis, 2013; Hanano et al., 2008), many of which are triggered by light perception (Wenden et al., 2011). Perception of daily zeitgebers, such as changes in light and temperature (Chow et al., 2014; Harmer, 2009), enable plants to reset the clock at dawn and feed back to the central oscillator. For this light entrainment, photoreceptors play a major role (Somers, Webb, Pearson, & Kay, 1998; Toth et al., 2001).The circadian clock was derived from the principle of interconnected, positive, and negative feedback loops (Shearman et al., 2000). For the purpose of this review, light input into the Arabidopsis thaliana (Arabidopsis) circadian clock will be the focus of discussion, with a brief examination of clock components.
In Arabidopsis, morning expressed Myb-like transcription factors CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) (Wang & Tobin, 1998) and LATE ELONGATED HYPOCOTYL (LHY) (Schaffer et al., 1998) antagonize expression of the evening expressed pseudoresponse regulator (PRR) TIMING OF CAB EXPRESSION 1 (TOC1) (Strayer et al., 2000). These three genes form the core negative feedback loop of the circadian oscillator, Figure 1 (Alabadí et al., 2001; Gendron et al., 2012). Several other genes form additional loops within this core oscillator. In day time, CCA1 and LHY repress excs PRR5, PRR7, and PRR9 (Adams, Manfield, Stockley, & Carré, 2015; Kamioka et al., 2016), as well as TOC1, GI, and the genes that generate the evening complex (Locke et al., 2006; Nakamichi et al., 2009; Pokhilko et al., 2010; Zeilinger, Farré, Taylor, Kay, & Doyle, 2006). GIGANTEA (GI) is evening expressed and is proposed to form an additional negative feedback-loop with TOC1 (Locke et al., 2006). All of these loops are connected through the action of the evening complex formed by LUX ARRHYTHMO (LUX), EARLY FLOWRING 3 (ELF3), and EARLY FLOWERING 4 (ELF4), which directly inhibits the expression of PRR9 (Helfer et al., 2011; Herrero et al., 2012), PRR7, GI, and LUX (Mizuno et al., 2014). The absence of even one component of the evening complex gives rise to plants that are photoperiod insensitive. This results in early flowering, long hypocotyl growth, and arrhythmicity of the free-running circadian period (Hazen et al., 2005; McWatters et al., 2007; Onai & Ishiura, 2005; Thines & Harmon, 2010). The importance of the three evening complex components are highlighted in maintaining a functional circadian clock, and therefore the physiological processes controlled by the clock, such as the input of diurnal photoperiod information, (Covington et al., 2001; Más, Alabadí, Yanovsky, Oyama, & Kay, 2003; Mizoguchi et al., 2005; Park et al., 1999).
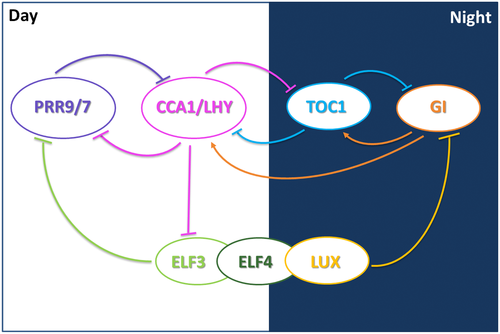
2 EFFECTS OF LIGHT ON THE CLOCK
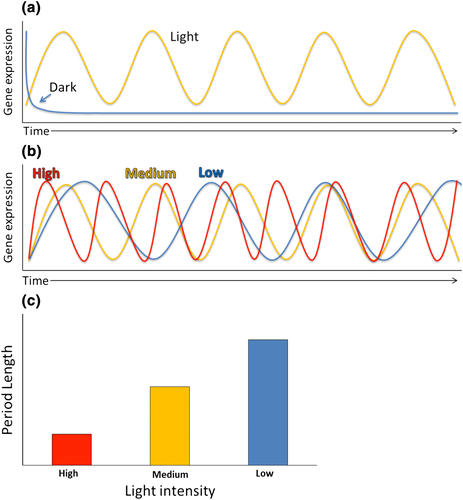
Light changes throughout a day–night cycle are pronounced and thus robustly entrain the clock. In the light phase of a daily cycle, the dark to light transition of dawn is used as a time setting checkpoint (Millar, Straume, Chory, Chua, & Kay, 1995). Prolonged darkness causes many of the core genes in the Arabidopsis central oscillator to rapidly become arrhythmic, due to the lack of essential light time setting cues (Figure 2a) (Millar et al., 1995). This dampening effect, leading to arrhythmicity, is particularly noticeable in the absence of media containing sucrose. In prolonged darkness, sucrose can act as a substitute for light in maintaining rhythmicity for a number of days (Bläsing et al., 2005). Light has two main modes to set the clock. The first is parametric entrainment, gradual entrainment of the clock, such as the acceleration of the clock induced by increased light perception, which eventually leads to a phase shift of the clock back to a correct resonance. Parametric entrainment follows Aschoff's rule, as light intensity increases, the speed of the clock increases. As intensity decreases, the speed of the clock slows (Aschoff, 1979; Figure 2b,c). Increases in light intensity lead to decreases in periodicity (Somers, Webb, et al., 1998). The second light-induced time-setting mechanism is non-parametric entrainment: rapid re-entrainment. This leads to a rapid time setting of the clock at dawn (Millar & Kay, 1996). Non-parametric entrainment requires an extended light exposure far beyond that which activates light-regulated gene expression (Millar & Kay, 1996). Metabolic entrainment is also a mechanism for non-parametric entrainment (Haydon, Mielczarek, Robertson, Hubbard, & Webb, 2013; Haydon & Webb, 2016; Sanchez-Villarreal et al., 2013; Shin et al., 2017). The different photoreceptors and photochromic proteins involved in light entrainment are described in more detail below.
3 HOW ARE DIFFERENT WAVELENGTHS OF LIGHT INPUT TO THE ARABIDOPSIS CLOCK?
Diurnal organisms, particularly plants, are subjected to Aschoff's rule: An increase in light intensity accelerates the circadian oscillator speed leading to shortening of periodicity (Aschoff, 1979). Light input to the circadian clock is presumed to occur through the action of different types of photoreceptors (Somers, Webb, et al., 1998). There are more than 10 known circadian-associated photoreceptors (Edwards, Guerineau, Devlin, & Millar, 2015). These can be split into four classes: phytochromes, cryptochromes, Zeitlupe (ZTL)/FKF1/LKP2 family, and UVR8. Each receptor contributes in the dose-dependent perception of far red, red, blue, and ultraviolet light (Cashmore, Jarillo, Wu, & Liu, 1999; Mas, Devlin, Panda, & Kay, 2000; Rizzini et al., 2011; Song, Smith, To, Millar, & Imaizumi, 2012). It is presumed that the input of this information is coordinately relayed to the central oscillator.
Both phytochromes and cryptochromes play key roles in light responsive time-setting mechanisms, in a manner that follows Aschoff's rule (Devlin & Kay, 2000a; Somers, Webb, et al., 1998). This is due to the ability of both phytochromes and cryptochromes to form photoreceptor complexes (Más et al., 2003) that are genetically interactive in clock function (Devlin & Kay, 2000a). Excitation of these photoreceptors cause the central oscillator to accelerate, changing the overall speed of the clock (Devlin & Kay, 2000b; Herrero et al., 2012; Kolmos et al., 2011; Somers, Devlin, & Kay, 1998; Somers, Webb, et al., 1998). There are a number of different known mechanisms through which light absorption by photoreceptors input environmental information to the oscillator; however, these mechanistic details are not complete. Regulation of transcription by circadian gating restricts changes in RNA levels to specific times of day. Therefore, preventing transcription of some light-regulated clock genes in response to unexpected external stimuli, for example, light pulses during the night (Millar & Kay, 1996). Light regulation of Myb transcription factors, such as CCA1 and LHY, effect the transcription and stability of other clock components, such as PRR9/7 (Carre & Kay, 1995). Messengers such as Ca2+and calmodulin signalling may also affect circadian regulation in response to light (Johnson et al., 1995; Millar & Kay, 1996). Light also directly controls the degradation of PRR5, PRR7, PRR9, TOC1, and GI proteins (Farré & Kay, 2007; Ito, Nakamichi, Kiba, Yamashino, & Mizuno, 2007; Kiba, Henriques, Hitoshi, & Chua, 2007; Más et al., 2003; Matsushika, Makino, Kojima, & Mizuno, 2000). These degradation events then act on outputs within a diurnal context, which change in duration throughout the season (Davis, 2002; Guerriero et al., 2012; Salazar et al., 2009; Song et al., 2012; Troein, Locke, Turner, & Millar, 2009). Light thus has multiple mechanistic inputs to clock processes, all of which control entrainment. How each individual wavelength of light is input to the clock will be discussed below.
4 RED LIGHT
Phytochromes are predominantly red light photoreceptors, absorbing maximally at wavelengths between 600 and 700 nm (Somers, Webb, et al., 1998). Arabidopsis has five phytochromes (Sharrock & Quail, 1989), phyA-phyE (Mathews & Sharrock, 1997). Each phytochrome acts as a light input sensor to form regulatory feedback loops within the circadian clock. Phytochromes are in turn reported to be negatively regulated by the clock through cryptochrome (CRY) signals (Devlin & Kay, 2000a; Mas et al., 2000). Phytochromes exist in two interconvertible forms; the inactive Pr form is converted by red light to the active Pfr form, which can be converted back to the inactive Pr state by far-red light (Rudiger, Thummler, Cmiel, & Schneider, 1983). These conversion events between active and inactive forms of phytochrome are essential to light input to the clock, as discussed below (see far red, phytochrome interacting factors [PIFs]). Each of the five phytochromes play distinct roles in light sensing.
phyA mediates entrainment responses to low-intensity red light and pulses of light (Quail et al., 1995; Somers, Webb, et al., 1998). A PHYA deficiency mutation results in an altered period length in dim red light (Somers, Webb, et al., 1998). It is not known how phyA signals to the clock as it has not been reported to directly bind to a clock-associated factor, in contrast to the other four phytochromes (Huang et al., 2016).
phyB is the main detector for high-intensity red light (Somers, Webb, et al., 1998). Both phyB and phyD are able to detect red and far-red wavelengths of light (Aukerman et al., 1997; Devlin et al., 1999). phyB physically interacts with ELF3 in the central oscillator to provide a direct light input to the clock (Kolmos et al., 2011; Liu, Covington, Fankhauser, Chory, & Wagner, 2001). phyb mutants show an altered response to shade avoidance (Smith, 1995), which is also a phenotype of the elf3 mutant (Huang et al., 2016). phyC to phyE also interact with ELF3 protein (Huang et al., 2016), but this has not yet been connected to the Arabidopsis clock (Liu et al., 2001). However, in barley PHYC is genetically linked to ELF3 and LUX (Campoli et al., 2013; Pankin et al., 2014). Under high-fluence red light, phyb mutants and the phyB overexpressor have a period defects and an altered phase (Anderson et al., 1997; Kolmos et al., 2011; Salomé et al., 2002; Somers, Webb, et al., 1998). Also, altered cryptochrome signalling (see blue light below), phyB, and CRY2 physically interact by translocating to the nucleus in red light (Mas et al., 2000), where phyB is then supressed by CRY2 (Mas et al., 2000). This alters clock performance under white light conditions (red and blue light together) (Devlin & Kay, 2000a).
In non-peer-reviewed work, phyC was found to play a role in white light input and red light detection. Mutations in PHYC result in a long-period phenotype, which was shown to be temperature dependant, suggesting that phyC inputs not only light information to the clock but also temperature (Edwards et al., 2015; Franklin, Davis, Stoddart, Vierstra, & Whitelam, 2003; Qin, Kuhn, Moran, & Quail, 1997). phyE along with phyD plays a role in controlling the period length of CAB gene expression; however, many of the clock effects of phyE and phyD are masked by phyB (Franklin & Quail, 2010). phyE works with phyB and phyD in the regulation of shade avoidance (Devlin, Patel, & Whitelam, 1998). Interestingly, the promoters of PHYA and PHYB are down-regulated by light, whereas the PHYC promoter is upregulated (Tóth et al., 2001), PHYD and PHYE do not show changes in expression in response to light changes. PHYB, PHYD, and PHYE mediate high-fluence red light input to the clock with PHYA, PHYB, PHYD, and PHYE acting additively to input red light information to the clock; as a result, the clock runs faster as the detected intensity of red light increases (Devlin & Kay, 2000a). The absence of all five phytochromes results in severally attenuated rhythms but not a total loss of clock function (Hu et al., 2013). Together, all five phytochromes play differing roles in mediating light-dependant changes in periodicity.
5 BLUE LIGHT
Cryptochromes are blue light (492 to 455 nm) and UVA photoreceptors present in both plants and animals (Cashmore et al., 1999). The HY4 locus was found to encode cryptochrome 1 (CRY1). It was identified due to cry1 (hy4) mutants growing with a long-hypocotyl phenotype and being unable to respond to blue light (Ahmad & Cashmore, 1993; Koornneef, Rolff, & Spruit, 1980), cry1/hy4 plants are also late flowering (Goto, Kumagai, & Koornneef, 1991; Millar et al., 1995). cry1 mutants have a long period under blue light (Somers, Webb, et al., 1998), suggesting CRY1 acts as a photoreceptor for blue light entrainment of the clock (Devlin & Kay, 2000a). Overexpression of CRY1 caused increased sensitivity to blue light and period shortening (Lin, Ahmad, & Cashmore, 1996; Somers, Webb, et al., 1998). CRY1 is a soluble protein when grown in both light and dark conditions in Arabidopsis (Lin et al., 1996), CRY1 is more stable than CRY2 and works at higher light intensities (Lin et al., 1998). Chryptochrome 2 (CRY2) can detect low intensity light and is rapidly degraded under blue light (Lin et al., 1998). In light, CRY2 promoter activity is down-regulated, whereas CRY1 is upregulated (Tóth et al., 2001). The cry2 mutation alters sensitivity to photoperiod and flowering in Arabidopsis, but does not have a detectable individual effect on circadian rhythm (Devlin & Kay, 2000a; Guo, Yang, Mockler, & Lin, 1998). Overexpression of either CRY1 or CRY2 gives rise to a higher blue light sensitivity under low light conditions than in the individual overexpression lines (Ahmad, Jarillo, Smirnova, & Cashmore, 1998). Double mutant cry1, cry2 plants are rhythmic, suggesting that although CRY1 inputs blue light into the clock CRY1 and 2 are not part of the central oscillator (Devlin & Kay, 2000a). However, CRY1 and CRY2 gene expression oscillates with a circadian rhythm under constant light (Harmer et al., 2000). CRY1 and 2 work together to input information to the clock in a similar way to phyA and B, but at differing light intensities.
Phytochromes are able to absorb low fluence blue light alongside CRY1 for period length control. phyA mutants show a period lengthening effect when free run under blue light (Somers, Webb, et al., 1998). Without phyA detection of blue light, the input relies on CRY1 alone causing the period to lengthen as the plant detects less light than the actual ambient intensity of irradiation. Conversely, PHYA overexpression has been proposed to cause period shortening under blue light, as more blue light is processed as an input than the actual ambient light intensity. Phytochromes thus also work in blue light signalling to the clock.
6 HIGHLIGHT SYNERGISM (WHITE LIGHT)
White light comprises of multiple light wavelengths. As such, interactions between phytochromes and cryptochromes are needed to input this information into the circadian clock. These interactions were found with loss of function mutants for both phytochromes and cryptochromes. In wild-type plants, CAB2 period decreases as light intensity increases, cry2 mutants were found to be deficient in a white light response as they have a CAB2 period increase in response to highlight (Mas et al., 2000). This period increase was not detected in either red or blue light alone, suggesting that to be active CRY2 needs multiple wavelengths of light simultaneously and phyB (Mas et al., 2000). CRY1 was also found to be required for phyA signalling as cry1 and cry2 mutants are unable to detect red light above the fluence range of both phyA and phyB (Devlin & Kay, 2000a). Light induces nuclear compartmentalisation of phytochromes where phyA and phyB directly interact with CRY1 and CRY2 (Mas et al., 2000), the kinase activity of phyA phosphorylates CRY1 and CRY2 (Ahmad, Jarillo, & Cashmore, 1998).
Phytochromes and cryptochromes facilitate signal integration of multiple light cues. CRY2 is activated when illuminated by multiple wavelengths of light suggesting it is needed for phytochrome activation (Mas et al., 2000). This is also highlighted as both cryptochromes reach peak RNA expression with a similar expression pattern to the corresponding phytochromes (Toth et al., 2001). It was found that the active Pfr form of phytochrome is needed for CRY2 expression, CRY2 then supresses PHYB expression. However, Pfr form of phytochrome B (PfrB) is able to override CRY2 signalling to flowering time control via pathways, such as constitutive photomorphogenic 1 (COP1; see below). PfrB binding to the intermediate SPA1 allows degradation of the COP1-SPA1 complex, which is needed as an intermediate of CRY1/2 induced inhibition of photomorphogenic factors such as hypocotyl in far red (HFR) and CO (Mas et al., 2000; Sheerin et al., 2015). Additionally, both CRY1 and CRY2 were found to be phosphorylated by the kinase activity of phyA (Ahmad et al., 1998). It could be considered that phytochromes and cryptochromes work together in the “white light” response, which is a more than the additive effect of plants grown under blue and red light. However, quadruple mutants for phya, phyb, cry1, and cry2 still showed rhythmic leaf movement in response to light–dark cycles suggesting that other photoreceptors must play a role in overall light input to the circadian clock (Yanovsky, Mazzella, & Casal, 2000). However, the exact relationship between phytochromes and cryptochromes is yet to be resolved.
7 ZTL FAMILY: BLUE LIGHT ABSORBING WITH ACTION UNDER RED LIGHT AND DARKNESS
ZTL, also reported as ADAGIO1 (ADO1), links light input by both cryptochromes and phytochromes to the clock (Jarillo et al., 2001; Kim et al., 2007). ZTL mutant lines showed altered cotyledon movement and gene expression under different light conditions (Jarillo et al., 2001). Under blue and white light, ztl plants have a long period, whereas under red light, the ztl lines were reported to be arrhythmic for CCR2 expression, cotyledon movement, and stem elongation (Jarillo et al., 2001). ZTL mutants were found to have a long-period phenotype for CAB/TOC1 under red light (Kevei et al., 2006; Kim, Hicks, & Somers, 2005). ZTL thus is required for the perception of multiple wavelengths of light into the oscillator.
ZTL encodes a protein reported to be a blue light photoreceptor, as it contains a PAS domain, F box domain linking proteins to an SCF ubiquitination complex, kelch repeats and a light, oxygen or voltage (LOV) domain allowing protein–protein interactions (Mas, Kim, Somers, & Kay, 2003). Interactions between TOC1 and ZTL were found to occur through these kelch-repeat zones (Kevei et al., 2006). The PAS/ LOV domain were identified as essential for coupling ZTL to red light (Kevei et al., 2006), which was then found to occur through ZTL binding to the C-terminus of phyB and CRY1 (Kim et al., 2007).
ZTL is constitutively expressed at the RNA level; however, oscillations in ZTL protein levels are seen (Kim et al., 2007). These are proposed to result from the binding of GI to maintain the stability of ZTL. ZTL protein folding is chaperoned by HSP90 (Kim et al., 2011), GI binds to the ZTL-HSP90 complex to ensure specificity of protein folding (Cha et al., 2017; Kim et al., 2011). Interactions between ZTL and GI are enhanced by blue light through the LOV domain in ZTL (Kim et al., 2007). ZTL controls proteomsomal degradation of TOC1 (Más et al., 2003). This ZTL-GI interaction is believed to control a central part of the circadian oscillator. ZTL and ELF3 were reported to have opposite effects on clock function. ztl mutants and ELF3 overexpression lines show a lengthened circadian period in light. Conversely, elf3 mutants and ZTL overexpression lines are reported as arrhythmic under constant light (LL) (Kim et al., 2005). However, the elf3–ztl double mutant showed that ELF3 and ZTL have additive effects on the clock (Kim et al., 2005). As GI controls the HSP90 mediated stabilisation of ZTL protein (Cha et al., 2017; Kim et al., 2011), ZTL protein then causes protein depletion of TOC1 via ubiquitination (Kim et al., 2011). ELF3 interacts as a substrate adaptor for COP1 (an E3 ubiquitin ligase) to bind to and degrade GI protein, as a light input signal and indicator of day length in response to CRY2 (Yu et al., 2008). The reduction of GI then prevents the formation of stable ZTL protein. Consequently, this prevents ZTL-mediated inhibition of TOC1 in the central oscillator and facilitates TOC1 action. ZTL also negatively regulates PRR5 by targeting PRR5 protein for degradation via the 26S proteasome (Fujiwara et al., 2008; Kiba et al., 2007). As PRR5 forms a negative regulatory feedback loop with LHY/CCA1, ZTL therefore indirectly plays a role in the regulation of LHY/CCA1 within the central oscillator (Baudry et al., 2010).
8 FAR-RED LIGHT
phyA is the presumed photoreceptor for detecting monochromatic far-red light. Mutations in PHYA resulted in loss of capacity for clock function (Wenden et al., 2011). ELF4 was proposed to restrict far-red perception in those studies. Interestingly, the active form of phyA (phyA-Pfr) is formed under far-red light (Clough & Vierstra, 1997), given that far red converts the Pfr form of phytochrome back to the inactive Pr form. In part perhaps, phyA evolved the ability to form Pfr under far red as a response to the change in light quality at the end of the day, which signals the transition from day to night and therefore the associated changes in environment. However, far red can also be a signal of shade due to far red being one of the only wavelengths of light able to pass through leaves (Federer & Tanner, 1966), suggesting that there may be different mechanisms to entrain the clock in these two circumstances, as described in the next section. Plants in constant far-red light have a faster clock and show high expression of evening genes, such as PRR1/TOC1, and low expression of the morning genes CCA1 and LHY (Wenden et al., 2011). The exact mechanism of far-red input to the clock is not fully characterised. However, far red has been shown to becon between Pfr and PIF3 (Martínez-García, Huq, & Quail, 2000). ELF4 was identified as playing a role in mediating far-red light input to the clock (Wenden et al., 2011), Far-red light was used to aid recovery of rhythmicity in the otherwise arrhythmic elf3 and elf4 mutants (Kolmos et al., 2011; Wenden et al., 2011).
9 SHADED LIGHT
White light with supplementary far-red light causes the clock to slow down (Jiménez-Gómez, Wallace, & Maloof, 2010). Under shade, far red and potentially green light are present; there is a large overlap between far-red signalling and shade. Shade, however, is a useful environmental indicator to plants for neighbour detection. phyA is thought to have the most involvement in mediating far-red signalling, but phyB also plays a key role (Kolmos et al., 2011; Wenden et al., 2011). Shading plants during the afternoon was found to have the greatest effect (Sellaro, Pacín, & Casal, 2012). Responses to shade involve the degradation of phytochrome interacting factors, namely, PIF4 and 5 (Lorrain, Allen, Duek, Whitelam, & Fankhauser, 2008). PRR5 was found to regulate the shade-avoidance response by controlling PIF4 and PIF5, as well as downstream components of the phytochrome-mediated signalling pathway. Furthermore, ZTL induces degradation of PRR5. However, this degradation was found to be repressed under blue light. It was suggested that PRR5 gates phytochrome-mediated shade responses (Takase, Mizoguchi, Kozuka, & Tsukaya, 2013). ELF3 and LUX mutants (both components of the evening complex) show a reduced response to all wavelengths of light therefore growing with elongated hypocotyls as though under shade (Jiménez-Gómez et al., 2010; Sellaro et al., 2012; Zagotta et al., 1996). This implies that ELF3 and the evening complex also play a role in the shade response to the clock (Kolmos et al., 2011).
10 ULTRAVIOLET B LIGHT
Ultraviolet B light (UVB) can be one of the more damaging wavelengths present in sunlight. UVB is a wavelength that is easily absorbed and damages both DNA and proteins (Jansen, Gaba, & Greenberg, 1998), thus making UVB a useful light signal, but at the cost of inducing a stress response. UVB is an “anti-shade” signal informing a plant it is under direct sunlight. At lower fluence rates, UVB light is able to control development, promote photomorphogenesis, and drive gene expression (Heijde & Ulm, 2012). Ultraviolet resistance locus 8 (UVR8) drives signalling for the majority of UVB responses (Favory et al., 2009; Rizzini et al., 2011). Under UVB light, COP1 promotes the induction of elongated hypocotyl 5 (HY5) and HY5 homologue (HYH), which induce stress responses such as flavonoid biosynthesis to reduce UVB induced damage (Stracke et al., 2010). UVR8 and COP1 are also crucial for UVB light entrainment of the clock (Fehér et al., 2011). Under white light supplemented with UVB light, COP1 induces HY5 and HYH, HY5, and HYH have not yet been implicated for clock entrainment by UVB (Fehér et al., 2011). UVR8 is able to mediate both parametric and non-parametric entrainment, by inducing PRR9 and GI under continuous light, alongside an increase in CCA1 and ELF3 response to UVB light pulses. UVR8 was identified as the UVB receptor that can mediate signal input to the oscillator, due to the fact that uvr8 plants cannot input UVB light into the oscillator, (Fehér et al., 2011; Heijde & Ulm, 2012). It has been proposed that UVR8 mediates UVB light input into the central oscillator by inhibiting PIF4 in the presence of UVB light. This requires COP1-mediated repression of PIF4 transcript and also through the stabilisation of HFR, which inhibits PIF4 (Hayes et al., 2017). Canonical pathways used in UVB signalling mediate entrainment in the clock, but the critical nodes in entrainment are not fully resolved (Hayes et al., 2017).
11 GREEN LIGHT
Many studies have been carried out to test the physiological effects that occur as a consequence of increased or absent green-light wavelengths. The mechanisms of sensing and input to the circadian clock are yet to be understood. It is thought that green wavelengths can operate via both a cryptochrome dependant and independent pathway (Folta & Maruhnich, 2007). Green light can reverse the effect of blue light on hypocotyl elongation (Bouly et al., 2007; Folta, 2004), potentially due to the reversal of the blue light degradation of CRY1 (Bouly et al., 2007). This could then have an effect on photoperiod and subsequently flowering time (Banerjee et al., 2007; Folta & Maruhnich, 2007). The association of green light to cryptochromes was also shown by (Lin et al., 1996) as overexpression of CRY1 causes increased sensitivity to green light. A reversible interaction between CRY and green light similar to that found for phytochrome in red and far-red light suggests that there are intermediate signalling factors similar to PIFs that are yet to be identified. It is thus plausible that green light could entrain the clock, but no definitive experiments have tested this.
12 TRANSCRIPTIONAL REGULATION OF PHOTORECEPTORS BY THE CLOCK
The circadian clock generates rhythms of RNA and/or accumulation for all photoreceptor classes. Starting with the discovery that phyB mRNA is rhythmic (Bognár et al., 1999; Toth et al., 2001; Tóth et al., 2001), subsequent findings revealed that all five phytochromes in Arabidopsis cycle. Interestingly, subnuclear accumulation of phytochrome holoprotein also appears to be under clock control. However, the implications of this are currently unclear. Similarly, CRY genes are rhythmic (Toth et al., 2001). For UVR8, as UV light induces dimer disassembly to a monomer state, a diel cycle of dimers at night and monomers during the day occur (Findlay & Jenkins, 2016). UVR8 mRNA displays robust circadian rhythms with a peak around subjective dusk (Mockler et al., 2007). For ZTL, the mRNA generated does not cycle, but robust protein cycling is readily detectable. Together, it is clear that light receptors that act as input components to the clock are themselves circadian output-regulated.
13 PHYTOCHROME INPUT TO THE CENTRAL OSCILLATOR
Light input to the central oscillator is a daily zeitgeber, but the central oscillator also acts as a feedback mechanism to phytochromes over the day. The oscillator receives a number of light queues during the light phase of each day from photoreceptors detecting the different ratio of light wavelengths across the day. In turn, the oscillator inhibits expression of phytochrome proteins at points hypothesised in Figure 3.
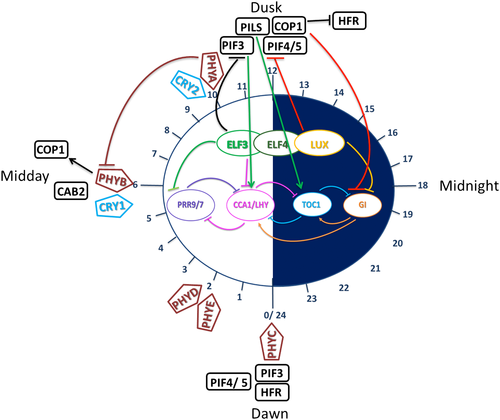
At dawn (ZT0), PHYC peaks with the return of light and changing temperature (Toth et al., 2001). HFR increases due to increased stability (Yang et al., 2005). PIF4/5 expression peaks at dawn (Nomoto, Kubozono, Yamashino, Nakamichi, & Mizuno, 2012), due to the lack of phytochromes, thus preventing phytochrome-induced degradation at dawn (Shin, Anwer, & Davis, 2013). PIF4/5 continue to be present throughout the light phase but are slowly degraded by interactions with the Pr form of phytochrome, PIFs can also interact with LHY, CCA1. PfrB interacts with PIF3, where PIF3 then binds to the G-box domain of CCA1/LHY promoters. CCA1 and LHY, as MYB transcription factors, then control other genes within the central oscillator, such as PRR5, 7, and 9, as well as noncircadian genes (Martínez-García et al., 2000; Wang & Tobin, 1998).
PHYD and E are expressed 2 hr after dawn (Toth et al., 2001). During the first half of the light phase (ZT0-6), there is an increase in light intensity up to ZT6, and alongside this, an increasing expression of phytochromes. PHYB and CRY1 reach peaks in expression around midday (ZT6), as both phyB and CRY1 work at high-light intensities (Lin et al., 1996; Lin et al., 1998; Toth et al., 2001). CAB1 expression peaks around midday as does the expression of HFR (Yang et al., 2005). HFR is thought to interact with PIF3 (Fairchild, Schumaker, & Quail, 2000), but the mechanism through which this happens is not fully understood.
Both PHYA and CRY2 peak towards the latter half of the light phase (ZT6-12) with the decreasing light and increasing far-red intensity (Toth et al., 2001). CRY2 detects lower intensity light (Lin et al., 1998), and the Pfr form of phytochrome is needed for CRY2 activation (Mas et al., 2000). CRY2 then supresses PHYB expression (Mas et al., 2000). phyA is essential in controlling the clock in low light (Quail et al., 1995; Somers, Webb, et al., 1998) and far-red conditions (Wenden et al., 2011), potentially through the Pfr form of phytochrome being unable to interact with PIF3 (Martínez-García et al., 2000).
At dusk, CAB2 expression decreases, and COP1 expression increases (Yang et al., 2005), allowing COP1 suppression of HFR throughout the dark phase of the day. COP1 accumulation along with ELF3 inhibits GI late in the afternoon (Yu et al., 2008). PIF3 like (PILs) are rapidly produced during the first hour of shade, early into the dark phase and work with TOC1 to restrict growth (Salter, Franklin, & Whitelam, 2003). PIF3 is at its highest level at dusk due to its interactions with the Pfr form of phytochrome and the highest level of Pfr being present just before dusk. Allowing information on high levels of far-red light to be input to the central oscillator (Martínez-García et al., 2000). The evening complex (ELF3, ELF4, and LUX) inhibits the transcription of PIF4/5 at dusk (Herrero et al., 2012; Nusinow et al., 2011; Raschke et al., 2015). This allows PIF protein to accumulate stably due to the lack of phytochrome inhibition overnight, which thus promotes growth, reaching a maximal level at dawn (Delker et al., 2014; Raschke et al., 2015; Shin et al., 2013).
14 CONSTITUTIVE PHOTOMORPHOGENIC 1
COP1 is an E3 ubiquitin ligase, mediating day length input to the clock and flowering time. COP1 is negatively regulated by a direct protein–protein interaction with CRYs (Jang et al., 2008; Wang, Ma, Li, Zhao, & Deng, 2001; Yang et al., 2000). phyA and B affect the nuclear abundance of COP1 (Osterlund, Ang, & Deng, 1999), as the C-terminal domain of phyB directly interacts with COP1 (Millar, McGrath, & Chua 1994). COP1 acts as an intermediate, inputting photoperiodic information from PHY and CRY into the oscillator. COP1 in turn plays a negative regulatory role targeting phyA, phyB, and HFR1 for ubiquitination (Osterlund, Hardtke, Wei, & Deng, 2000; Seo et al., 2003; Yang et al., 2005).
CRY1, CRY2, phyA, and phyB all interact with COP1 via Suppressor of Phytochrome A (SPA). SPA1 is a nuclear-localised repressor of phytochrome signalling (Hoecker, Tepperman, & Quail, 1999), which interacts with COP1 (Hoecker & Quail, 2001). SPA1 contains a coiled coil domain that enhances the E3 ligase activity of COP1 on its targets (Seo et al., 2003). The interactions between the four known SPA proteins and COP1 negatively regulate light signalling in response to certain wavelengths of light (Laubinger, Fittinghoff, & Hoecker, 2004; Zhu et al., 2008). COP1–SPA1 interaction is repressed by activated CRY1 in blue light (Lian et al., 2011); CRY2 interacts with COP1 via SPA1 to allow COP1 proteolysis of CO to control flowering time under blue light (Zuo, Liu, Liu, Liu, & Lin, 2011). The CRY1–SPA1 interaction enhances CRY2–SPA1 activity in response to blue light to supress COP1 activity resulting in a suppression of CO degradation (Ordoñez-Herrera et al., 2015; Zuo et al., 2011).
In seedlings, phyA binds to SPA1 and 2, whereas in adult plants, phyA binds to SPA3 and 4 (Laubinger et al., 2004). Binding of phyB to SPA1 is Pfr dependant allowing degradation of COP1/SPA1 in light conditions that promote nuclear accumulation of phyA and B; this enhances light responses, as the disruption of COP1/SPA1 interaction prevents degradation of photomorphogenic factors such as HFR and HY5 (Sheerin et al., 2015). The COP1/SPA complex is an important factor in repression of light responses in darkness, as the COP1/SPA complex interacts directly with photoreceptors leading to its inactivation (Huang, Ouyang, & Deng, 2014).
Within the central oscillator, COP1 interacts with ELF3 to mediate COP1 degradation of GI late in the afternoon (Yu et al., 2008), potentially using ELF3 as an adaptor for COP1 binding to GI (Liu et al., 2008). It is also possible that COP1 regulates the level of ELF3 present; in cop1 mutants, ELF3 protein accumulates to higher levels than in the wild type, but the mRNA levels remain unchanged (Liu et al., 2001).
COP1 is also involved in UVB signalling, as cop1 mutants are deficient in a UVB response (Oravecz et al., 2006). In the early stage of UVB signalling, UVR8 and COP1 directly interact in the nucleus (Favory et al., 2009); UVR8 and COP1 were found to be essential for UVB entrainment (Fehér et al., 2011). HY5 and HYH which are also important components of UVB signalling are regulated by COP1 (Brown & Jenkins, 2008). In the light, COP1 detaches from HY5 allowing stabilisation and the light responsive target genes of HY5 to be activated (Yi & Deng, 2005). COP1 plays an important mediator role in the input of light from photoreceptors to the oscillator. In turn, its regulation is dependent on photoreceptors; the short-period phenotype in mutant lines shows that COP1 plays a negative regulatory role on the clock.
15 PIFS AND PILS
PIF are a family of basic helix loop helix transcription factors. There are four well-characterised PIFs, PIF1,3,4,5 (Leivar, Monte, Cohn, & Quail, 2012; Pfeiffer et al., 2012) PIFs are unstable in the light due to their interaction with active phytochrome causing phosphorylation and subsequent degradation (Leivar et al., 2012; Soy et al., 2012). The most well-characterised PIF is PIF3, which was found to interact with the PfrB acting as a bridge between PfrB and its target gene by translocating PfrB to the nucleus. Thus allowing light induced control of gene expression, as PIF3 does not interact with the Pr form of phytochrome (Martínez-García et al., 2000; Pfeiffer et al., 2012). PIFs are also able to input information to the clock via direct interaction with clock genes that contain a G-box motif in their promoter; PIFs can interact directly with LHY, CCA1, PRR5, PRR7, PRR9, and LUX (Martínez-García et al., 2000). This is potentially one of the main mechanisms through which light/day length information is used to control or alter the clock. The central oscillator in turn regulates PIF expression. Postdusk, TOC1 peaks in expression, allowing direct interactions between TOC1 and PIF3, which results in TOC1 gating of PIF induced growth, until TOC1 levels decrease predawn (Soy et al., 2016).
PIF4 and 5 show rhythmic expression with a diurnal peak at dawn (Nomoto et al., 2012). Expression of PIF4 and 5 is controlled by the evening complex, comprising of ELF3, ELF4, and LUX (Herrero et al., 2012), which binds to the promoter region of PIF4 and 5 to inhibit transcription at dusk (Nusinow et al., 2011). PIF protein stably accumulates overnight due to the lack of phytochrome induced degradation to reach their maximum level at dawn (Shin et al., 2013). As PIFs are growth-promoting factors (Shin et al., 2013), this leads to the highest growth rate occurring at the end of the night phase.
PIFs may also input information from other environmental cues to the clock such as temperature (McClung & Davis, 2010; Raschke et al., 2015). It was shown that PIF4 expression also increases in response to temperature increases (Shin et al., 2013). As dawn induces a temperature increase, alongside the return of daylight, it would perhaps be advantageous to a plant to be able to input both of these environmental cues into the clock at the same time.
PILs are also basic helix loop helix transcription factors with large overlaps in function to PIFS but have been associated with shade avoidance (Li et al., 2014). This overlap in function has led to some ambiguous nomenclature as PIL5 is also referred to as PIF1 and likewise PIL6 as PIF5 (Li et al., 2014). PIL1 has a distinct function and was shown to work with TOC1 to restrict growth at specific times of day (Salter et al., 2003). PIL1 accumulates rapidly within the first hour of shade cover acting as part of a rapid signalling pathway to stop growth (Li et al., 2014); a secondary longer lasting shade response is then mediated by HFR and phytochrome rapidly regulated (PAR1/2) (Galstyan, Cifuentes-Esquivel, Bou-Torrent, & Martinez-Garcia, 2011). The exact mechanism through which PIL1 halts growth in shade is not known, but a number of hypothesis were presented in (Li et al., 2014). It was suggested that as PIF1 has a binding site for phyB, it is possible that in shade PIL1 may outcompete PIF for DNA binding sites on the Pfr form of phyB, therefore reducing the growth promoting function of PIF5 (Li et al., 2014). Alternatively, PIL1 may work via a PIF independent mechanism on components of downstream pathways; however, this is yet to be tested.
16 HYPOCOTYL IN FAR RED 1
Long HFR1 is a basic helix loop helix transcription factor involved in phytochrome-mediated signalling (Fairchild et al., 2000) and photomorphogenesis (Yang et al., 2005). HFR is unstable in darkness and accumulates in the light; this accumulation is due to light preventing COP1-mediated degradation of HFR (Yang et al., 2005). HFR is not able to bind phyA or B directly, instead HFR binds PIF3 forming potentially a heterodimer of PIF3/HFR, which can then bind to the Pfr form of phyA/B. This is also highlighted by the fact that HFR is more abundantly found in far-red light (Fairchild et al., 2000). Mutants deficient in HFR had defective phyA responses, such as hypocotyl elongation and induction of chlorophyll A binding protein (CAB) (Fankhauser & Chory, 2000). HFR is also thought to have a blue light response (Duek & Fankhauser, 2003) through CRY1 (Yang et al., 2005), but the exact mechanism through which this occurs is not known.
17 INTERSECTION OF THE CLOCK COMPONENTS ELF3 AND ELF4 TO LIGHT AND CLOCK SIGNALLING: MAJOR INTEGRATORS OF LIGHT TO THE CLOCK
ELF3 was first identified as a negative regulator of flowering time. In addition to the observation that elf3 mutant was shown to be early flowering, large circadian defects were identified (Hicks et al., 1996; Roden, Song, Jackson, Morris, & Carre, 2002; Undurraga et al., 2012). elf3 mutants are defective in gating of red light perception to the clock. Cloning of ELF3 allowed for interactors to be detected; phyB was revealed to be a factor that associated to the N-terminus of ELF3 (Liu et al., 2001). ELF3 was identified as playing a role in light signalling, in 12 hr light and 12 hr dark entrainment; ELF3 accumulates in the nucleus just before darkness (ZT12) (Liu et al., 2001). Increasing day length increases the nuclear accumulation of ELF3; increased darkness causes accumulation of ELF3 to decrease to an undetectable level (Liu et al., 2001) showing a direct relationship between light and ELF3.
Phase response curves are made by measuring circadian period and phase during light pulses, at times across subjective day and night. Phase response curves for wild-type Arabidopsis in both red and blue light show the greatest effect and subsequent clock resetting to be caused by a light pulse during the subjective night. ELF3 overexpression lines showed a much more gradual change in phase response with the same light pulses (Covington et al., 2001). In white light, ELF3 overexpression causes a period lengthening effect in a light-dependent manner. In darkness, the oscillator pace is not altered (Covington et al., 2001). In elf3 mutants, phase response light pulses showed a much greater effect than the wild type, suggesting that clock resetting is light dependent (Covington et al., 2001), involving ELF3 in oscillator resetting by repressing the light input to the clock (Bujdoso & Davis, 2013). However, the exact mechanism through which this occurs is unknown.
elf3 and phyB mutants were found to have similar phenotypic traits, such as hypocotyl elongation in red light and constitutive shade avoidance (Devlin et al., 1999). elf3 mutants are also defective in their response to blue and/ or red light with stronger effects showing in red light. ELF3 grown in darkness is rhythmic with a long-period phenotype, rhythmic in light/dark entrainment cycles, but arrhythmic in free run light conditions (Hicks et al., 1996). elf3 plants are also unable to inhibit hypocotyl elongation under light (Zagotta et al., 1996). The combined phenotypic characteristics of the elf3 mutants suggest that ELF3 plays a role in light perception and signalling, particularly in red light due to the interaction between ELF3 and phyB (Reed et al., 2000).
ELF4 was the first clock component interpreted as being required for the clock to cycle and it was revealed to be a component of normal light perception. Genetic loss of ELF4 resulted in plants that were markedly attenuated in the ability of a red light pulse to generate CCA1 and LHY rhythms in etiolated plants. This was concluded to be due to ELF4 being required for the phytochrome-mediated light induction of CCA1 and LHY expression (Kikis, Khanna, & Quail, 2005). Consistent with this, it was shown that elf4 mutants were hypermorphic and hypomorphic to red light cues, dependent on the assay. Notably, red light-mediated induction of CAB2 expression was elevated in elf4 (McWatters et al., 2007). This revealed that ELF4 contributes to so-called circadian gating of light responsiveness (negative photomorphogenesis), light-regulation of PIF4/5 expression, and the suppression of growth (positive photomorphogenisis; (Nozue et al., 2007).
The presence of ELF4 in the nucleus increases the accumulation of ELF3 (Herrero et al., 2012). ELF4 may function as a nuclear anchor for ELF3 but does not affect the nuclear localisation of LUX; the third component of the evening complex (Herrero et al., 2012). Nuclear import of phyB is light dependent (Kircher et al., 1999; Sakamoto & Nagatani, 1996), and it was shown that phyB does not import ELF3 into the nucleus (Bujdoso & Davis, 2013). It is possible that ELF3 plays a role in the nuclear import of phyB, as the N-terminus of ELF3 interacts with the C-terminal end of both the Pr and Pfr forms of PHYB (Liu et al., 2001). However, ELF3 and phyB have opposite roles in controlling circadian oscillations (Herrero et al., 2012). ELF3 needs the association with ELF4 to maintain circadian oscillations, counteracting the COP1 and phyB mediated repression of ELF3 (Herrero et al., 2012). ELF3 is also part of the blue light signalling pathway, through its interaction with COP1. How phyB and ELF4 coordinate the action and localisation of ELF3 seems critical for the cooperative intersection of light perception and circadian clock function.
It was originally unclear what overall effect ELF3 has on other clock genes as the elf3 loss of function mutation causes arrhythmicity. A reduction-of-function mutation in elf3-12 provided a way to explore this, as the hypomorphic elf3-12 allele is able to maintain rhythmicity (Kolmos et al., 2011). This showed elf3-12 to be light dependent but with a defective phase resetting mechanism. This elf3-12 mutant allowed the position of ELF3 within the clock to be derived as key to the regulation of PRR9 expression (Kolmos & Davis, 2007; Kolmos et al., 2011). It is known that ELF3 associates to the promoter of PRR9 to mediate its repression. In addition to clock-regulated PRR9 transcription, PRR9 expression is also light regulated. Furthermore, prr9 mutants display photomorphogenic phenotypes (Nakamichi, Kita, Ito, Yamashino, & Mizuno, 2005). This highlights the role of ELF3 in reciprocally linking light signalling to clock function.
Overexpression of PHYA in an elf3-12 background showed that the overexpressed PHYA has an additive effect with the elf3-12 mutation to give a further shortened period in red light. In a range of light conditions the elf3-12 PHYA-overexpression lines had an altered phase; however, in darkness, there was no change suggesting that light has an epistatic effect on PHYA (Kolmos et al., 2011). Overexpression of PHYB in the same elf3-12 background gave the same period shortening as the PHYA-overexpression lines; however, the PHYB-elf3-12 lines had a phase more closely linked to PHYB overexpression in a wild-type background (Kolmos et al., 2011). This result suggests that PHYB functions upstream of elf3-12 in light signalling and is able to suppress the effects of elf3-12 (Bujdoso & Davis, 2013). Together, it appears that some, but not all, inputs of phytochromes to the clock depend on ELF3.
18 DURATION AND QUALITY OF LIGHT
In regular light–dark cycles, Arabidopsis has a circadian period of approximately 24 hr in light, whereas in darkness, it has a period of 30 to 36 hr (Millar et al., 1995). Light therefore makes the clock run faster; the absence of light cues causes the clock to slow; this is in keeping with Aschoff's rule (Aschoff, 1979). The range of photoreceptors present in Arabidopsis allow a range of fluence rates to be detected, ensuring the phase of the circadian oscillator is synchronised with environmental cues (Somers, Webb, et al., 1998), at both dawn and dusk (Devlin & Kay, 2000a). Removal or even partial reduction of blue and red photoreceptors causes the clock to run slower (Millar et al., 1995), suggesting that the effects of light intensity on the speed of the clock is limited by the number of photoreceptors present. This would also suggest that it would not be possible to increase the speed of the clock with higher light intensities beyond the maximum speed obtainable by that number of photoreceptors.
Input of duration and quality of light are important in synchronising processes such as flowering time and development (Weston, Thorogood, Vinti, & López-Juez, 2000). Preceding photoperiod was shown to alter the subsequent speed of the clock (Boikoglou et al., 2011; Darrah et al., 2006). Interestingly, here is the long known role of ELF3 in processing light information to the clock (Hicks et al., 1996) and how this coordinates the capacity for a plant to perceive daily boundaries present In a day night cycle (McWatters, Bastow, Hall, & Millar, 2000). Recent work has revealed that extensive allelic variation at ELF3 contribute to alterations in photoperiodic control and this is associated to alterations in encoded nuclear abundance and in vivo turn over diurnal time (Anwer et al., 2014; Undurraga et al., 2012). Combined with temperature variation over the day, light duration gives information on the time of year or season and therefore a warning of the growth conditions to follow. It is clear that allelic variation exists in Arabidopsis to change the output of such varying entrainment processes (Anwer et al., 2014; Boikoglou et al., 2011; Darrah et al., 2006).
Plants are able to adapt to changes in light intensity, such as consistently low light intensity, by rearranging photosynthetic machinery to be more efficient at light harvesting (Weston et al., 2000). Blue light plays a major role in this. As such, it would be assumed that cryptochromes and PHYA/B play a role in directing the timing of light capture. A connection hub for this, COP1 has been identified as a signalling intermediate between these two processes (Walters, Rogers, Shephard, & Horton, 1999); however, links between photoreceptor function and organisation of the photosynthetic apparatus await further investigation (Walters et al., 1999; Weston et al., 2000). Finally, light intensity is also detected by the plastid sensing blue light, causing structural changes and elongation of the palisade to absorb more light; this process responds a lot more to blue light than red (Weston et al., 2000). It could be hypothesised that this plastid information is used as a nuclear clock and is synchronised with the phyA and phyB red light input to the central oscillator by COP1.
19 DISCUSSION
Multiple photoreceptors are essential components of light input to the clock. In this way, they play a central role in the light input to the clock. Not only with the reversible, light-mediated reactions for maximal efficiency in light or dimmer light/shade, they also directly input light into the central oscillator through clock associated factors, such as ELF3/4, COP1, ZTL, PIFs, PILs, and HFR. Transcriptional regulation and post-translational processes are all part of this complex web of interconnections between light perception and clock function. Overall, light input to the clock forms complex feedback systems that generated harmonised regulatory pathways; the mechanisms from light perception to clock function, and back again, awaits clear discoveries.
Many plant growth chambers are produced with red, blue, and far-red LED panels, but are these the optimal light regimes to measure plant gene expression under? Furthermore, most chambers have a lights-on or off function that does not represent the graded changes in intensity that would occur with a plant growing under natural sunlight (or in a greenhouse), with the gradual appearance and disappearance of light at sunrise and sunset. As white light comprises a combination of different wavelengths of light simultaneously, it may be possible that the pathways for different colours of light interact more than has been found so far. Overlapping functions have been found between blue and red light, and these are the most commonly used light wavelengths for plant LEDs. It is possible that there are essential components missing in just red and blue, although difficult to isolate in a complex web of circadian gene expression, may only be present in white light or in other light combinations not yet tested.
Light is essential for plant growth and it is therefore important to understand how plants process the daily light cues they receive. Further understanding of how each light wavelength is detected and the information fed into the central oscillator from each sensor could potentially have a large impact on plant and ultimately crop growth. With global changes in climate, knowledge on essential lighting requirements, and how this impacts on overall plant health could be used to optimise crop productivity. Indoor farming techniques using LEDs as a light source could be optimised for maximal yield and growth speed. As permafrost regions recede, suitable growth land becomes available. A detailed understanding of photoperiodicity and how it impacts fitness will help with the challenges created by growing crops in shorter growth seasons and longer daylight hours.
ACKNOWLEDGMENTS
This work was supported by the Biotechnology and Biological Sciences Research Council (BBSRC) award number BB/N018540/1.




