Development of transgenic crops based on photo-biotechnology
Abstract
The phenotypes associated with plant photomorphogenesis such as the suppressed shade avoidance response and de-etiolation offer the potential for significant enhancement of crop yields. Of many light signal transducers and transcription factors involved in the photomorphogenic responses of plants, this review focuses on the transgenic overexpression of the photoreceptor genes at the uppermost stream of the signalling events, particularly phytochromes, crytochromes and phototropins as the transgenes for the genetic engineering of crops with improved harvest yields. In promoting the harvest yields of crops, the photoreceptors mediate the light regulation of photosynthetically important genes, and the improved yields often come with the tolerance to abiotic stresses such as drought, salinity and heavy metal ions. As a genetic engineering approach, the term photo-biotechnology has been coined to convey the idea that the greater the photosynthetic efficiency that crop plants can be engineered to possess, the stronger the resistance to biotic and abiotic stresses.
Development of GM crops based on photoreceptor transgenes (mainly phytochromes, crytochromes and phototropins) is reviewed with the proposal of photo-biotechnology that the photoreceptors mediate the light regulation of photosynthetically important genes, and the improved yields often come with the added benefits of crops' tolerance to environmental stresses.
Introduction
Plant growth and development are critically controlled by light. The process of photosynthesis involves the transduction of photons absorbed by photosynthetic pigments into chemical free energy, whereas environmental light sensed by plants through their photosensory receptors yields varied responses in their adaptive life cycle. This review focuses on the latter as it relates to the development of crop plants, specifically discussing the biotechnological importance of the photoreceptors such as phytochromes, cryptochromes and phototropins, with respect to crop improvement. Other photoreceptors not covered in this review include aureochrome (Takahashi et al. 2007), ZEITLUPE (ZTL) and its analogs that absorb near UV and blue light (300–500 nm) (Mas et al. 2003). In addition, neochrome is derived from the fusion of PHY and PHOT genes and senses both blue and red light (Kawai et al. 2003; Suetsugu et al. 2005; Kanegae et al. 2006), and the UV photoreceptor UVR8 is sensitive to UV-B (280–315 nm) (Galvão & Fankhauser, 2015). However, there are few (if any) studies reporting on the transgenic crop plants based on these photosensory receptors; thus, they remain under future review.
Phytochromes regulate many aspects of plant growth and development in response to red (R) and far-red (FR) light signals from the environment (Rockwell et al. 2006; Li et al. 2011). They are encoded in higher plants by small gene families: for example, Arabidopsis thaliana contains a phytochrome gene family with five members, with designations from phytochrome A (phyA) to phyE. Phytochromes exist in two photo-convertible isoforms: a red light-absorbing form, Pr (λmax = 660 nm), and a far-red light-absorbing form, Pfr (λmax = 730 nm). The inactive Pr form of phytochromes is assembled from the apoprotein with a chromophore, phytochromobilin, which is converted to the active Pfr form upon absorbing red light. Conversely, the Pfr reverts to the Pr by absorbing far-red light via a photochromic isomerization reaction. Due to partial overlapping of the absorption spectra, phytochromes establish the photostationary equilibrium between the Pr and Pfr forms: As an example, under canopies of green vegetation, the red to far-red light ratio is lower than under direct sunlight, in which the amount of the Pfr is reduced. In the canopy conditions, phyB plays a predominant role in mediating shade avoidance responses (SARs), while phyA functions to suppress the SARs. Thus, the Pr and Pfr photostationary ‘equilibrium’ state is important for modulating the photomorphogenic traits such as SAR/suppression in crop plants.
Phytochromes absorbing in the range of 600–800 nm mediate three types of light dose-dependent responses (Briggs & Rice, 1972): Very low fluence response (VLFR), far-red or red high irradiance reaction (FR-HIR and R-HIR) and low fluence response (LFR). The VLFR and FR-HIR are mainly regulated by phyA, and the LFR and R-HIR are primarily controlled by phyB (Shichijo et al. 2001; Schäfer & Bowler, 2002; Casal et al. 2014; Possart et al. 2014). Crop plants in their field habitats are subjected and respond to these types of light conditions, affecting the morphogenic phenotypes and harvest yields. The photoactivation of the Pr to the Pfr form is followed by translocation to the nucleus, where the Pfr form regulates the expression of genes involved in photomorphogenesis such as seed germination, seedling growth and elongation, flowering, shade avoidance and senescence (Neff et al. 2000; Smith, 2000; Sawers et al. 2005), as well as those in photosynthesis (Fig. 1 for a glimpse of the molecular events in photomorphogenesis; see Li et al. (2011) for a comprehensive review).
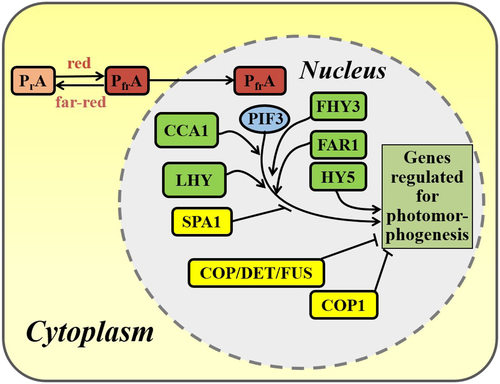
Cryptochromes are the blue light receptors that mediate the morphogenic processes including the inhibition of hypocotyl elongation and photoperiodic control of floral initiation (Ahmed & Cashmore, 1993). They are also involved in blue-light responses such as circadian rhythms, tropic growth, stomata opening, guard cell and root development, plant–pathogen interactions, abiotic stress reactions, cell cycles, programmed cell death, apical dominance, fruit and ovule development, seed dormancy and magnetoreception (Ahmad & Cashmore 1993; Cashmore et al. 1999; Yu et al. 2010; Christie et al. 2015). Cryptochromes bind two chromophores, FAD and a pterin derivative, 5,10-methenyltetrahydrofolate. FAD functions as the primary light sensor after binding to the photolyase homology region (PHR) (Hoang et al. 2008). In addition to the PHR domain, CRY1 and CRY2 also contain a distinctive cryptochrome C-terminus that is absent in CRY3. The PHR is involved in photosensing, and the cryptochrome C-terminus plays a signalling role (Yang et al. 2000; Yu et al. 2009; Liu et al. 2011; Christie et al. 2015). Cryptochromes modulate the abundance of the transcription factor (TF) Long Hypocotyl 5 (HY5) by suppressing its protein degradation in response to blue light (Osterlund et al. 2000; Liu et al. 2008; Lau & Deng, 2012).
Following the arrest of hypocotyl elongation in germinating Arabidopsis seeds, the blue light absorbed by cryptochromes elicits the greening of seedlings (de-etiolation) through the action of the Pfr form of phyB under low fluence conditions (Mohr, 1994). Such a phytochrome-dependent cryptochrome action is disrupted under prolonged blue light exposure of the seedlings, and the cryptochromes appear to function independently of phytochromes (Poppe et al. 1998). Using phytochromes-lacking Arabidopsis mutant [phyAphyBphyCphyDphyE quintuple phytochrome mutant in the flowering locus T (ft) background], it has been shown that crytochromes function independently of phytochromes in A. thaliana seedlings (Strasser et al. 2010). Because crop plants under natural light conditions are exposed to the light of both blue and red wavelengths, how cryptochrome- and phytochrome-related transgene activities are amplified in determining the crop quality and yields through their mutual interactions (and lack of thereof) is a challenging question for the plant biotechnologist.
Phototropins are blue-light receptors and named after their role in mediating phototropism of higher plants (Christie et al. 1999). Phototropins consisting of a serine/threonine kinase domain and a pair of non-identical Light-Oxygen-Voltage-sensing domains (LOV1 and LOV2) each contain non-covalently bound chromophore, FMN (Christie et al. 1999). The light-activated serine/threonine kinases undergo autophosphorylation in response to blue light irradiation. Phosphorylation occurs predominantly on multiple serine residues, at least 21 phosphorylation sites in Arabidopsis PHOT1 (Salomon et al. 2003; Inoue et al. 2008; Sullivan et al. 2008; Boex-Fontvieille et al. 2014; Deng et al. 2014) and 29 sites in PHOT2 (Inoue et al. 2011; Boex-Fontvieille et al. 2014). The autophosphorylation is important for the function of PHOTs. For example, an alanine substitution at PHOT1-Ser851 in the activation loop between the LOV2 and kinase domain causes a loss of function (Inoue et al. 2008), and impaired dephosphorylation of PHOT2 enhances the phototropic responses in hypocotyls (Tseng & Briggs, 2010). In addition, phototropins, primarily localized to the plasma membrane in darkness, translocate within the cell in response to blue light. Blue light irradiation activates PHOT1 by re-localizing it from the plasma membrane to the cytosol (Sakamoto & Briggs 2002; Han et al. 2008; Kaiserli et al. 2009), whereas PHOT2 re-localizes to the Golgi apparatus (Kong et al. 2006; Aggarwal et al. 2014). Because the kinase domain is responsible for plasma membrane and Golgi localizations, it has been suggested that light detection excludes the protein kinase domain from the inhibitory action of the amino-terminal photosensory portion of the phototropins (Fankhauser & Christie, 2015; Sakai & Haga, 2012). Recent studies have shown that phototropins are localized in the outer membrane of the chloroplast and mediate actin-based chloroplast photo-relocation movements for the regulation of the chloroplast accumulation and avoidance responses (Kong et al. 2013; Wada, 2013).
Recent advances in the field of plant biology offer several strategies for crop improvement. Among them, the selection of naturally occurring variants, artificial selection (crop breeding) and transgenic plant development are well established. However, no reports are available on SAR suppression in crop plants based on the natural variation or artificial selection procedures. Natural variation selection of Arabidopsis revealed some useful genes which are related to SAR responses (Coluccio et al. 2011; Filiault & Maloof, 2012). By overexpressing the genes necessary for SAR suppression, it is possible to develop plants with new improved traits otherwise not feasible by natural variation selection and plant breeding. Approaches for crop improvement include enhancing yield per area by manipulating photomorphogenic responses to suppress perception of nearest neighbour plants, increasing photosynthesis, leaf architecture and optimized photoperiod response, disease resistance, abiotic stress tolerance and delayed leaf senescence (Ragauskas et al. 2006). As a start, one can pick a gene encoding the light signalling component to generate the transgenic plants. However, our main focus will be on upstream components of the light signalling pathway, such as the photoreceptors (Fig. 1). There are three reasons for this: (1) the photoreceptor genes are well characterized in terms of their structure and function; (2) the light signalling starts with the photoreceptors acting as the sensor of the radiation environment in and around individual plants; and (3) more transgenic crops have been developed with the photo-sensor genes than other downstream signal transducer genes. All these photoreceptors play critical roles in responding to the changing light wavelengths and intensities to regulate the growth and development at cellular and plant levels. In addition, expression of many photoreceptor-controlled genes and pathways is essential for conferring stress tolerance to the plants (Genoud et al. 2002). Hence, the development of transgenic crops with photoreceptors is an increasingly significant goal for ‘photo-biotechnology’ (vide infra) based on plant photomorphogenesis.
Over the past three decades, innovative plant technologies resulted in a marked increase in harvest yields: for example, through the overexpression of phytochrome transgenes in crop plants (Boylan & Quail, 1989; Casal et al. 1996; Garg et al. 2006; Ganesan et al. 2012; Gururani et al. 2015a). Because the photoreceptors regulate the expression of several genes critical for plant growth and development, overexpression of the genes encoding photoreceptors can be viewed as a promising strategy to develop biotech crops with improved agronomic traits (Thiele et al. 1999; Boccalndro et al. 2003; Carvalho et al. 2011; Gupta et al. 2014). Through genetic transformation of crop plants with photoreceptors, it has been possible to develop the plants with enhanced yields and improved commercial benefits [e.g. seed productivity or harvest index (Holefors et al. 1998; Kong et al. 2004; Ganesan et al. 2012), tolerances to abiotic stresses such as cold, salt and heavy metals (Gururani et al. 2015b, 2016), and biomass production (Heyer et al. 1995; Thiele et al. 1999; Rao et al. 2013)], which will be discussed in this review. Moreover, several schematic and photographic illustrations have been included throughout the review primarily for the student readers.
Photomorphogenic Phenotypes for Crop Improvement
Plants exhibit a high plasticity in cellular, metabolic and morphological responses to a variety of biotic and abiotic factors associated with their habitat conditions. The variations in the biotic and abiotic stress factors including the quality and intensity of light are sensed by specific receptors for the plants to activate the environmental factor- or stress-responsive metabolite systems for optimal growth and development. Here, we review what photoreceptor genes have been introduced into several crop plants to improve their functional performances that led to the development of transgenic crops having commercial advantages.
Suppression of shade avoidance response and shade-tolerant crops
Of diverse photomorphogenic phenotypes of a germinating and stem growing plant, de-etiolation and SARs are perhaps the most visibly obvious examples. As the germinating seed of the plant emerges out of the ‘dark’ soil layer, it senses the light that triggers such visibly observable phenotypic transitions as the retardation of hypocotyl or sprout extension, cotyledon straightening and greening (i.e. de-etiolation). The plants exposed to light then become photosynthetically competent as photosynthetic pigments and chloroplasts are synthesized and developed at the cellular level. On the other hand, plants growing in the shade typically exhibit increased stem elongation, reduction in leaf expansion and eventual drop in harvest yields (i.e. SAR). As the plants get exposed to light, the SAR is suppressed. Under photosynthetically active visible radiation (PAR), SAR and its suppression are modulated by the interactions between the photoreceptors, prominently phytochromes and the ratio of red to far-red light (R:FR ratio) with a concomitant increase in the Pfr level. The suppression of SAR is the predominant feature of the phenotype exhibiting dwarfism, increasing number, size and greening of the leaves. Thus, one of the most important factors for the development of shade-tolerant crops is the reduced perception of nearest neighbour plants in photomorphogenic responses based on the phytochrome red/far-red light perception system.
From the above sketch of the phenotypic changes in a germinating plant, one may expect crop plants to improve their harvests by suppressing the SAR through reduction and removal of the shade from the habitats. This expectation has been demonstrated in a pioneering study of tomato plants that phyA overexpression displayed the retarded SAR trait with a marked suppression of extension growth and a greater number of dark green leaves and fruits under shade conditions relative to control plants (Boylan & Quail, 1989). A later study was conducted with the tomato using phytochrome triple mutants (phyAphyB1phyB2) to elucidate the role of different phytochrome family members. The results suggested that phyB2 functions redundantly with phyB1 in the SAR (Weller et al. 2001). From the early tomato and subsequent other crop studies, it is evident that the overexpression of photoreceptors such as phytochromes is an effective means for the crop improvement strategy.
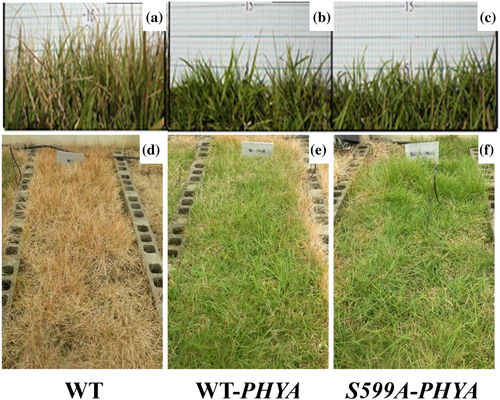
As mentioned earlier, the most visible SAR traits are the inhibition/arrest of stem extension growth and the greening in response to the changing environment from the shaded to unshaded conditions of the plants. Recently, turfgrass cultivars exhibiting the suppressed SAR have been developed (for example, Ganesan et al. 2012; Gururani et al. 2015b). Turfgrass plants germinate, grow and senesce in artificially very closely packed habitats where even in daylight the individual plant is shaded by and ‘perceives’ its surrounding neighbour plants. The mutual shade casting leads to accelerated stem extension growth (resulting in a need for frequent mowing), consuming the resources that include both endogenous and exogenous nutrients, water and agrochemicals. Figure 2 illustrates how phyA overexpression contributes to the retarded extension growth of creeping bentgrass plants under shade light (i.e. FR-enriched) conditions. Interestingly, a hyperactive mutant of oat phyA (i.e. S599A-PHYA, see below) is more effective than wild type phyA in suppressing the grass height.

Post-translational phosphorylation of phytochromes (phyA in particular) appears to play a critical role in plant light signalling during photomorphogenesis (Lapko et al. 1999; Kim et al. 2002, 2004a; Ryu et al. 2005; Phee et al. 2008; Han et al. 2010). Apparently, the phyA-mediated signalling is modulated by the Pfr-specific phosphorylation and dephosphorylation of a serine residue in the hinge region of the protein (Kim et al. 2004a; Ryu et al. 2005; Phee et al. 2008). For example, the Ser599Ala-PHYA mutant displayed a suppressed SAR phenotype in A. thaliana (Kim et al. 2004a). The mutant is also effective for the suppression of SAR in creeping bentgrass (Fig. 2) and Zoysia grass (see later for more examples). As described below, the S599A-PHYA gene provides a useful tool for developing transgenic crop plants with the suppression of the SAR (i.e. shade tolerance), especially retarding the plant heights. Sweet potato plants with the S599A-PHYA gene parallel the A. thaliana in height (Fig. 3). The similar phenotype with suppressed SAR can be seen in cassava plants with the enlarged tubers (Kim et al. 2004b).

Another hyperactive phytochrome gene of significant interest for crop engineering is the Tyr276-to-His mutant of Arabidopsis phyB (YHB) gene, the first allele for a constitutively active phytochrome (Su et al. 2007; Hu et al. 2009). Whereas a majority of phytochrome mutants reported in the literature are loss-of-function alleles (Kretsch et al. 2000; Weller et al. 2004; Dieterle et al. 2005; Rockwell et al. 2006; Mateos et al. 2006), YHB is a dominant gain-of-function allele that encodes highly fluorescent and poorly photoactive phytochrome (Fischer et al. 2005; Su et al. 2007). YHB not only complements phyB mutants as effectively as the wild-type PHYB but also confers constitutive photomorphogenesis in the absence of light. Transgenic tomato plants expressing YHB exhibit precocious seed germination and de-etiolate in darkness (Fig. 4). Recently, another constitutively active PHYB allele (i.e. the Tyr303-to-Val mutant of phyB, YVB) has been reported, including a constitutively active allele of PHYA (i.e. YVA) generated by the fusion of a nuclear localization sequence (NLS) to the Tyr-to-Val mutant (Jeong et al. 2016). Transgenic plants expressing NLS-fused YVA display a constitutive photomorphogenic development even in the dark. Collectively, YHB, YVB and NLS-fused YVA as well as the S599A-PHYA mutant represent useful tools for plant biotechnologists to develop novel transgenic crop species with improved agronomic performance.

In contrast to the long history of phytochrome research, molecular studies of cryptochromes and their transgenic applications to crop improvement date back to the pioneering work of Ahmed and Cashmore (1993). In tomato plants, phyA and CRY1 are the key photoreceptors involved in blue light-induced de-etiolation responses under low and high irradiances, respectively (Weller et al. 2001). Table 1 lists two other cryptochrome-related traits in tomato (Lopez et al. 2012; Sharma et al. 2014). However, there are fewer cases of genetic engineering of crops plants based on the cryptochrome transgenes than phytochromes (Table 2). One significant area of cryptochrome studies with biotechnological implications concerns the pigmentation of fruits, which will be reviewed later under the heading of Fruit Quality.
| Species | Allele/mutant | Traits | Remarks | Reference |
|---|---|---|---|---|
| Tomato | Aurea (phy-deficient) | Changes in phenotype and anthocyanin biosynthesis | Under R and FR light |
Kendrick et al., 1997 |
| phyA, phyB1 and phyB2 | Changes in phenotype and seedling development | Under R treatments, both phyB1 and phyB2 regulate phyA activity |
Weller et al., 2000 |
|
|
(phyAphyBphyCcry1) (Quadruple mutant) |
Changes in phenotype | phyA and cry1 together mediate photomorphogenic responses under BL |
Weller et al., 2001 |
|
| cry2 | Changes in phenotype | Altered molecular pathways in photosynthesis and flavonoids/anthocyanins |
Lopez et al., 2012 |
|
| Nps1 | Nonphototropic seedling | Low level of PHOT1 accumulation under BL and delayed fruit senescence |
Sharma et al., 2014 |
|
| phyB | Cold tolerance | Chloroplast damage and reduction in FAD and GPAT |
Yang et al., 2013 |
|
| not (ABA deficient) and spr2 (JA deficient) | Cold tolerance | phyA-dependent ABA and JA expression for cold tolerance |
Wang et al., 2016 |
|
| hp1 and au | Water stress tolerance | Stress tolerance by antioxidants and enzymes |
Alves et al., 2016 |
|
| Brassica rapa | phyB (ein) | Height and internodes increase | Internode mutant (ein) lacks phyB | Devlin et al., 1992, 1997. |
| phyB | Shade avoidance syndrome | Growth mediated by R:FR ratio | Robson et al., 1993 | |
| Cucumber | phyB (lh) | Long hypocotyls in seedlings | phyB interact with GA4 under R and FR responses | Lopez-Juez et al., 1992, 1995 |
| Pea | phyA and phyB | SAR responses (changes in phenotype) | phyA regulate flowering and phyB in light quality detection |
Weller et al. 2001 |
| Rice | phyA, phyB and phyC | Changes in phenotype |
phyA and phyB involved in de-etiolation; phyB and phyC in early flowering |
Takano et al., 2005 |
| phyAphyBphyC (triple mutant) | Disease resistance | phy-dependent SA and JA signalling pathway |
Xie et al., 2011 |
|
| phyB | Net assimilation rate of photosynthesis | phyB involved in photosynthesis regulation |
Liu et al., 2012 |
|
| phyB | Drought tolerance | Increased expression of ERECTA, EXPANSIN and osmoprotectants. |
Liu et al., 2012 |
|
| phyA, phyB and phyAphyB | Chl biosynthesis | phys regulates protochlorophyll oxidoreducatase A (PORA) |
Zhao et al., 2013 |
|
| phyB and ghd7 | Tiller and panicle branches and alteration in the photomorphogenesis responses | phyB-dependent expression of ghd7 |
Weng et al., 2014 |
|
| phyB(pale green phenotype) | Chlorophyll synthesis | phyB regulates ChlH and GUN4. |
Inagaki et al., 2015 |
|
| Sorghum | phyB (Ma3R) | Insensitive to photoperiod and internode elongation | phyB-dependent flowering |
Childs et al., 1997 |
| phyB-1 | Bud initiation and bud outgrowth | phyB-dependent bud initiation and growth |
Kebrom et al., 2006 |
|
| phyB-1 | Axillary shoot growth decreased | phyB regulates expression of SbMAX2 and SbTB1 |
Kebrom et al., 2010 |
|
| phyB-1 | Tillering ratio decreases | phyB-dependent cytokinin biosynthesis, chloroplast development and bud dormancy | Kebrom & Millet, 2016 |
| Plant | Transgene | Relevant phenotype | References |
|---|---|---|---|
| Rice | PHYA | Inhibition of the coleoptile extension under FR light |
Clough et al., 1995 |
| PHYA | No difference in phenotype | Takano et al., 2001 | |
| PHYA | Dwarfing, increased chlorophyll content, smaller tiller number and high yield | Kong et al., 2004 | |
| PHYA | Inhibition of the coleoptile extension, dwarfism and grain yield increase |
Garg et al., 2006 |
|
| PHYB* | Reduced total leaf area and reduced transpiration per unit leaf area, improved drought tolerance |
Liu et al., 2012 |
|
| PHYB* | Reduced chloroplast damage, improved photosystem II efficiency and improved chilling tolerance |
Yang et al., 2013 |
|
| Tomato | PHYA | Control of CAB expression rhythm | Kay et al., 1989 |
| PHYA | Short hypocotyls and elevated anthocyanin content |
Boylan & Quail, 1989 |
|
| PHYA, PHYB1 and PHYB2* | Distinct roles of phytochromes in de etiolation, hypocotyls elongation and anthocyanin synthesis |
Weller et al., 2000 |
|
| PHYA and PHYB * | Regulation of germination inhibition under FR light by phyA |
Shichijo et al., 2001 |
|
| PHYB1 and PHYB2 | Inhibition of hypocotyl elongation under continuous R light, inhibition of stem elongation and enhanced anthocyanin accumulation |
Husaineid et al., 2007 |
|
|
PHYA, PHYB1, PHYB2* |
Distinct roles of phytochromes in fruit development and ripening |
Gupta et al., 2014 |
|
| CRY2 | Overproduction of anthocyanins and chlorophylls in the leaves, and accumulation of flavonoids and lycopene in fruits |
Giliberto et al., 2005 |
|
| CRY2 | Increased contents of anthocyanins and lycopene in fruits and enhanced bud outgrowth |
Lopez et al., 2012 |
|
| Potato | PHYA, PHYB | Accelerated stem extension, leaf expansion and hook opening of sprouts in phyA-overexpressing lines under continuous FR light |
Heyer et al., 1995 |
| PHYB* | Increased stem length and reduced chlorophyll content | Jackson & Prat, 1996 | |
| PHYB | Reduction in internode elongation under white light, increased branching, and higher tuber numbers and yields |
Thiele et al., 1999 |
|
| PHYA, PHYB | Increased tuberization frequency in phyA-deficient plants |
Yanovsky et al., 2000 |
|
| Aspen trees | PHYA | Early inflorescence development |
Olsen et al., 1997 |
| PHYB | Early inflorescence development |
Wallerstein et al., 2002 |
|
| Apple | PHYB | Reduced stem and internode length | Holefors et al., 1998 & 2000 |
| Wheat | PHYA | Increased anthocyanins under continuous FR light, but not significant phenotypic variations |
Shlumukov et al., 2001 |
| Chrysanthemum | PHYB1 | Reduced growth, greener leaves, short branches and large branch angles | Zheng et al., 2001 |
| Cherry | PHYA | Altered morphology |
Cirvilleri et al., 2008 |
| Sweet potato | PHYA | Increased yield, short plant height, short petioles and increased chlorophyll content |
Kim et al., 2009 |
| Zoysia grass and creeping bentgrass | PHYA and S599A- PHYA |
SAR-suppressing phenotypes, short internodes, increased chlorophyll content |
Ganesan et al., 2012 |
| PHYA and S599A- PHYA | Cold tolerance |
Gururani et al., 2015b |
|
| PHYA and S599A- PHYA | Abiotic stress tolerance |
Gururani et al., 2016 |
|
| Troyer citrange | PHYB | Altered canopy growth characteristics and increased chlorophyll content |
Distefano et al., 2013 |
| Cotton | PHYB | Increase in time span and photosynthetic rate, greater yield, semi-dwarfism, decrease in apical dominance and increase in boll size | Rao et al., 2011 |
| PHYA1-RNAi | Improved fibre quality, seed cotton yield, early maturity and flowering |
Abdurakhmonov et al., 2014 |
|
| Strawberry | PHOT2 | Increased anthocyanin contents in leaves and fruits |
Kadomura-Ishikawa et al., 2013 |
| Miscanthus | PHYB | Increased chlorophyll content, shorter plant height and delayed flowering | Hwang et al., 2014 |
Increased productivity for bioenergy crops
We narrowly define the term ‘bioenergy crops’ as those plants accumulating oils, sugars and starch that can be converted to the fuel energy through chemical and biochemical processes such as alcohol fermentation. Because most cereal plants produce grains, typically rice, as staple food and raw materials for fuel, they are included in the bioenergy crop category for this review.
Photosynthetic genes regulated by photoreceptors
There are a number of research findings involving the plant photoreceptors regulating several genes related to photosynthesis. In particular, phytochrome overexpression results in an elevated production of photosynthates including starch, as described later (Thiele et al. 1999; Kim et al. 2004b; Hudson, 2007; Kim et al. 2009; Ganesan et al. 2012; Gururani et al. 2015a; Yang et al. 2016). Chlorophyll synthesis and photosynthetic gene expression in plants accompany the visually distinctive phenotypes, de-etiolation and greening under light conditions. Most of the chlorophyll a/b binding proteins and a few genes related to PSI and PSII are regulated by phytochromes. Some of the recent examples of the photosynthetic genes related to the primary photoreactions and the dark carbon dioxide fixation are listed in Table 3 (compiled from Takano et al. 2009; Chen et al. 2012). Regulation of the chlorophyll synthesis is crucial for chloroplast development during the greening process in angiosperms. With the help of phyA, phyB and phyAphyB double mutants, phyB is identified to positively mediate chlorophyll biosynthesis by regulating protochlorophyll oxidoreductase expression that determines the total chlorophyll contents in plants (Zhao et al. 2013). Red light and phyB together induce the synthesis of chloroplasts as their number increases and the thylakoid membrane architecture develops. In addition, the pale-green phenotypic phyB rice mutant grown under continuous red light (Rc) displayed repression of ChlH and GUN4 genes, which encode subunit H and an activating factor of Mg2+chelatase (Genomes Uncoupled 4, GUN4), respectively. Thus, chlorophyll synthesis is primarily mediated by phyB through its transcriptional regulation of ChlH and GUN4 genes (Inagaki et al. 2015). A recent study of a citrus plant showed that phyB elicited a faster outgrowth of axillary meristems in the PHYB1 transgenic troyer citrange and that the expression of chloroplast and nuclear genes (PSBA, CAB and RBCS) directly involved in the light and dark phases of photosynthesis is markedly enhanced in the transgenic plant (Distefano et al. 2013).
| No. | Genes | Plant species |
|---|---|---|
| 1 | Chl a/b-binding protein precursor (CAB27) | Oryza sativa |
| 2 | Type I light-harvesting Chl a/b binding protein of photosystem II (LHCPII), | Oryza sativa |
| 3 | Chl a/b-binding protein precursor (CAB26) | Oryza sativa |
| 4 | chloroplast photosystem I (PS-I subunit) (PSI-K) | Hordeum vulgare |
| 5 | Chl a/b-binding apoprotein CP26 (LHCB5-2) | Zea mays |
| 6 | Chl a/b-binding protein (RCABP69) | Oryza sativa |
| 7 | Chl a/b-binding apoprotein CP24 (LHCB6-1) | Zea mays |
| 8 | PSII-S protein of photosystem II precursor (PSII-S) | Oryza sativa |
| 9 | Type I light-harvesting chlorophyll a/b binding protein of photosystem II (LHCPII), | Oryza sativa |
| 10 | Putative cytochrome P450 (P450s) | Glycine max |
| 11 | PSI-D subunit of photosystem I (PSAD) | Barley |
| 12 | Chl a/b binding protein (RCABP89) | Oryza sativa |
| 13 | Cytochrome P450 (CYP71D4) | Solanum tuberosum |
| 14 | PsaN for photosystem I subunit N (PSAN) | H. vulgare |
| 15 | Putative cytochrome P450 (CYP87A3) | Oryza sativa |
| 16 | Photosystem I antenna protein (LHCA) | Oryza sativa |
| 17 | PsbY precursor (PSBY), nuclear gene encoding chloroplast protein | Spinacia oleracea |
| 18 | Cytochrome P450 (P450s) | Triticum aestivum |
| 19 | Precursor of the oxygen evolving complex (OEC) | Zea mays |
| 20 | Photosystem-I F subunit precursor (PSI-F) | Oryza sativa |
| 21 | Photosystem-I H subunit GOS5 (PSI-H) | Oryza sativa |
| 22 | Cytochrome P450 (CYP71D4) | Solanum tuberosum |
| 23 | Polypeptide of photosystem II (LOC4344899) | Oryza sativa |
| 24 | CL-8904 for cytochrome P450 (CL-8904) | Oryza sativa |
Rice and other cereals
In addition to being the prime staple food, rice has been an important model for molecular, genetic and biotechnological studies over the past three decades. In the biotechnology of rice, phytochrome overexpression plays a useful role in its quality and yield enhancement strategy. For example, tissue-specific expression of the PHYA gene has resulted in improved agronomic performance as the selection of a promoter determined the level of gene expression in transgenic rice cultivars. In the case of PHYA gene, the use of light-activatable ribulose 1,5-bisphosphate carboxylase/oxygenase (RBCS)-promoter led to a 10-fold increase (relative to the wild type) in PHYA accumulation in transgenic rice and a threefold increase over the 35S promoter (Clough et al. 1995). In contrast to the previous report that overexpression of PHYA displayed no significant variations and phenotypic improvement in rice and wheat plants (Clough et al. 1995; Shlumukov et al. 2001), a later work on genetically engineered rice showed that Arabidopsis PHYA under the control of the RBCS promoter displayed dwarf phenotype, with increased chlorophyll content, smaller tiller number and good grain productivity (ca. 8–9%) when compared to wild-type rice plants under daylight conditions (Kong et al. 2004). Similarly, transgenic rice (var. Pusa basmati) plants with an overexpression of Arabidopsis PHYA driven by the same RBCS promoter exhibited more tillers and an increase in grain yield (ca. 6–21%) under greenhouse conditions (Garg et al. 2006). Under field conditions, the photomorphogenic phenotypes of rice plants are determined in a density-dependent manner in their paddy habitats. For example, the interplay of phyB and Ghd7 (the seventh in a series of genes which mediate grain number, plant height and heading date) is essential to control tillers and panicle branches in a neighbour-dependent manner (Weng et al. 2014). The latter is also involved in the regulation of multiple processes including flowering time, hormonal regulations of metabolism, and biotic and abiotic stress tolerance, as briefly described in later sections.
What about other roles of phyB in rice biotechnology? Deficiency in phyB led to reduced total leaf area and retarded transpiration, which improves drought tolerance by reducing the water loss in the phyB mutant (Liu et al. 2012). Furthermore, down-regulation of PHYB displayed reduced chloroplast damage, improved photosystem II efficiency and enhanced chilling tolerance in rice plants (Yang et al. 2013). The above reports also suggest that the down-regulation of PHYB activates abiotic stress tolerance mechanisms. Ironically, it is the down-regulation of PHYB that produces the agronomically valuable traits for rice. When PHYB is absent, apparently other members of the phytochrome family, especially PHYA, take over plant's photomorphogenesis machinery. Though unrelated to cereal crop plants, suppressing PHYA alters the expression of other phytochrome genes with a substantial increase in PHYB transcript levels in cotton plants (Abdurakhmonov et al. 2014).
The suppression of SAR triggered by R light impacts on the shoot architecture by increasing the number of branches that are critical for an effective utilization of PAR by the plants. In fact, phytochromes regulate the expression of several shoot branching-related genes (Takano et al. 2009; Chen et al. 2012), and phyB plays a positive role in axillary bud outgrowth and tiller development in Sorghum bicolor (Kebrom et al. 2006, 2010; Kebrom & Mullet, 2015, 2016). The expression profile of SbTB1 (TEOSINTE BRANCHED1) and SbDRM1 (SORGHUM DORMANCY-ASSOCIATED) in phyB-1 mutant of sorghum demonstrated that phyB partially mediates axillary shoot growth by regulating SbTB1 in response to light signals. Impacts of low R:FR light and defoliation treatments in the axillary buds of sorghum led to a decreased expression of SbMAX2 (MORE AXILLARY GROWTH) and several cell cycle-related genes (Kebrom et al. 2010). Phytochrome B in sorghum plant (phyB-1) also regulates the tillering ratio through up-regulation of TFL1 (TERMINAL FLOWER1) and GA2OXIDASE (Kebrom & Mullet, 2016). However, no transgenic sorghum plants with a plant photoreceptor seem to have been developed as of this writing.
Tuber crops
In a biotechnologically significant study, it was demonstrated that two PHYB-transgenic potato lines, Dara-5 and Dara-12, produced larger and smaller potatoes with less and more number of tubers compared to the wild type, respectively (Thiele et al. 1999). In addition, photosynthetic carbon dioxide fixation was more efficient in the transgenic lines than the wild type. Moreover, the overexpression of Arabidopsis PHYB in the potato plants caused semi-dwarfism, with decreased apical dominance, increased chlorophyll accumulation, markedly elongated tubers and stronger anthocyanin pigmentation (Thiele et al. 1999; Boccalandro et al. 2003; Schittenhelm et al. 2004). Using PHYB sense and antisense transformants, it was established that overexpression of PHYB resulted in photoperiod sensitive tuber formation, while suppression of PHYA increased the frequency of tuberization (Yanovsky et al. 2000). Antisense PHYA potato lines were also substantially taller than the sense lines and wild type plants. Overall, these results suggest that both phyA and phyB play a role either additively or synergistically (or dominantly exclusive of the other) in producing higher tuber yield in potatoes.
In a preliminary study, transgenic sweet potato plants with PHYB and S599A-PHYA genes all exhibited a strong suppression of SAR, that is, a substantial increase in chlorophyll level and tuber yields, especially with the S599A-PHYA mutant line (Figs 3 & 5). The phenotypic change brought out by the mutant gene in the transgenic sweet potato is remarkably similar to that exhibited in the Arabidopsis mutant line (Fig. 3). The transgenic plants with S599A-PHYA mutant displayed approximately twofold increase in chlorophyll accumulation and about 30% reduction in petiole length relative to the control sweet potato lines, while the stem diameter was 61% thicker than the control. In particular, one of the S599A-PHYA transformant lines showed greatly enhanced tuber yields (about 8 times greater yield in fresh weight). The mutant plants had twice as many leaves per plant with 28% more branches (Fig. 5) (Kim et al.2009). Thus, it may be predicted that the transgenic expression of phytochrome genes is particularly effective in tuberization and starch accumulation for bioenergy production.

Many crop plants have the capacity to produce large volumes of biomass. For instance, overexpression of the S599A-PHYA mutant gene retarded the SAR and improved the corresponding tuberization in cassava plants (Kim et al. 2004a). As mentioned earlier, both phyA and phyB bring about a significant improvement of tuber yield in biomass producing plants (Heyer et al. 1995; Jackson et al. 1996; Boccalandro et al. 2003).
Apart from the herbaceous crop plants, here we also describe rare examples of phytochrome transgenic trees. Overexpression of PHYA in cherry trees displayed altered developmental behaviour under red-enriched light conditions (Cirvilleri et al. 2008). One particular transformant line exhibited an increased rate of phytomer formation (buds) accompanying the accelerated rate of plant growth. In aspen trees, overexpression of PHYA shortened the critical day length for inflorescence development (Olsen et al. 1997). The PHYB transgene also led to the shortened period of darkness needed to induce inflorescence development, suggesting that overexpression of either PHYA or PHYB promoted flowering shoot formation in the tree (Wallerstein et al. 2002). The implications of day/night lengths and flowering time in plant biotechnology are briefly reviewed in the next section.
Flowering time
Light quality is among the several key environmental signals that regulate the development of plants' reproductive organs (Henderson & Dean, 2004). By delaying the switch from the vegetative to the reproductive phase, a plant is forced to remain in the vegetative phase, and this results in greater resource allocation to vegetative growth. Thus, flowering time is an important trait that influences the yield of harvestable fruits and seeds (Milec et al. 2014). For fruit and cereal crops, yield is largely dependent on the proper onset of flowering. Because photoperiod or day length is known to allow plants to adjust their development to seasonal variations, light plays a very important role in regulating seasonal development by synchronizing the circadian clock with the day and night cycle (Yanovsky & Kay, 2002). The photoreceptors – mainly phyA, phyB and CRY2 – regulate flowering in response to light (Exner et al. 2010; Mockler et al. 2003). Generally, phyA and CRY2 promote flowering, while phyB plays an inhibitory role in floral initiation (Lin, 2000). It has been reported that changes in the expression patterns on photoreceptor genes in tomato, sorghum and potato resulted in changes in the timing of flowering, affecting tuberization and fruit ripening (Childs et al. 1997; Giliberto et al. 2005; Jackson et al. 1996). A recent report also showed that the overexpression of CRY2 affects the molecular pathways related to biotic and abiotic stress, photorespiration, photosynthesis, secondary metabolism, as well as flowering in tomato (Lopez et al. 2012). The low R:FR ratio elicits flowering through the expression of the floral integrator FT (FLOWERING TIME) (Halliday et al. 1994; Cerdan & Chory, 2003) and PFT1 (PHYTOCHROME AND FLOWERING TIME1) (Cerdan & Chory, 2003). Furthermore, the CO (CONSTANS) activates FT in the leaves, which moves to apical meristem to initiate flowering after interacting with FD (FLOWERING LOCUS D) (Halliday et al. 2003; Corbesier et al. 2007). In zoysia plants, the PHYA or S599A-PHYA transgene led to a significantly higher number of inflorescence axes per square foot than other lines (Ganesan et al. 2012). The S599A-PHYA zoysia plants developed their inflorescence axes earlier than the wild type plants. Thus, overexpression of the hyperactive phytochrome (S599A-PHYA) accelerates flowering time, which may be interpreted in terms of phyA playing a role in the circadian control of flowering. Moreover, RNAi-mediated down-regulation of PHYA1 led to early flowering and enhanced yield, indicating that phyA1 is involved in vegetative phase (Abdurakhmonov et al. 2014). On the other hand, PHYB overexpression induced delayed flowering in Miscanthus plants (Hwang et al. 2014). As cited earlier, phyB also functions to promote tuber formation in a photoperiod-sensitive manner (Yanovsky et al. 2000). These results suggest that both phyA and phyB are necessary for the light-dependent transition from the vegetative to reproductive phase.
Although phytochromes and cryptochromes have been described as primary red and blue light receptors, respectively, for mediating light signal transduction in the circadian clock, and phyB has been identified in many plant physiology textbooks as the main photoreceptor in controlling the clock, they do not seem to be essential for circadian control of flowering and other photomorphogenic responses to light and dark cycles. Instead, another blue light receptor, ZTL, is proposed as playing a major signalling role in the circadian clock (Sanchez et al. 2011). In spite of several studies involving phytochromes in determining the flowering time reviewed in this section, how red and blue photoreceptors affect the plant circadian clock and the transition to flowering, which impact the biotechnological traits of crop plants, remains to be elucidated.
Fruit quality
Under this heading, we shall include tomato and eggplant as fruits. The natural pigments anthocyanins and carotenoids are essential secondary metabolites present in flowers and fruits in higher plants. They attract the pollinators and also possess antioxidant capacity and nutritional values, contributing to the overall quality of fruits. Sunlight exposure has multiple effects on the phases of development from flowering to fruit maturation, as it affects the temperature of fruit tissues and the environmental light signals to which photoreceptors are sensitive, particularly those localized in the fruit itself. Apart from the normal photosynthetic and photomorphogenic responses, the fruit coloration and its enhancement are regulated by the photoreceptors (Fraser et al. 2000; Schofield & Paliyath, 2005), but the mechanisms of light action on fruit quality are not well characterized, especially in terms of the fruit-localized photoreceptors. Phytochromes-deficient mutant and PHY-overexpressing fruit plants are expected to alter the canopy growth characteristics including pigmentation. In tomato plants, phytochromes play a major role in the fruit ripening process including pigmentation (Piringet & Heinze, 1954). The photoreceptors also regulate the biosynthesis of carotenoids (Lintig et al. 1997) and are involved in affecting the fruit morphology of different phases from post-anthesis to fruit abscission (Gupta et al. 2014).
Under environmental/photomorphogenic light (R, high R:FR) conditions, phytochromes elicit anthocyanin pigmentation of plants and fruits through the light-responsive expression of phenylalanine ammonia lyase (PAL) and chalcone synthase (CHS), two of the key enzymes involved in flavonoid biosynthesis. For example, an increase in the R:FR ratio led to a fourfold increase in lycopene accumulation in the pericarp of tomatoes during ripening, as this process is apparently mediated by fruit-localized phytochromes (Alba et al. 2000). In tomato photomorphogenesis, phyB1 is the principal phytochrome involved in the de-etiolation response of seedlings and anthocyanin accumulation under white light (van Tuinen et al. 1995; Kerckhoffs et al. 1999; Weller et al. 2000). However, phyB2 plays a more prominent role in the photomorphogenesis of tomato seedlings than does phyB1 by inhibiting stem elongation and anthocyanin accumulation (Husaineid et al. 2007). In transgenic tomato plants, the PHYB2 overexpression results in a strong inhibition of hypocotyl elongation and enhanced anthocyanin accumulation under R-high irradiance conditions (Husaineid et al. 2007).
Cryptochrome-mediated photomorphogenic responses include significant effects on the pigmentation of fruits. The tomato plants overexpressing CRY2 overproduce anthocyanins and chlorophylls in the leaves, and accumulate flavonoids and lycopene in fruits (Giliberto et al. 2005). In addition, CRY2 overexpression caused an unexpected delay in flowering, a 1.7-fold higher fruit lycopene level and an increased outgrowth rate of axillary branches. The selected homozygous lines brought about a maximum threefold increase in anthocyanin content and ~60% reduction in internode length compared to the control plants. These observations are consistent with the CRY2 gene playing a regulatory role in fruit pigmentation and other SARs (Lopez et al. 2012).
Blue light responses mediated by cryptochromes include the improved production of carbohydrates (sucrose, fructose, and glucose) in postharvest Chinese bayberry fruits during storage (Shi et al. 2016). Perhaps relevantly, the transcripts of three sucrose phosphate synthase genes (MrSPS1, MrSPS2 and MrSPS3) are strongly induced by blue light treatment throughout the storage period in tomato. Interestingly, among different light sources used in the study, only the blue light raised the anthocyanin pigment level in strawberry (Fragaria x ananassa, cv. Sachinoka) fruits through the expression of the FaPHOT2 gene (Kadomura-Ishikawa et al. 2013). The FaPHOT2 knockdown and overexpression study clearly demonstrated that the FaPHOT2 is a key regulator for anthocyanin biosynthesis under blue light conditions.
One of the important fruit quality indices is fruit's shelf-life. According to the report by Sharma et al. (2013), the non-phototropic seedling 1 (nps1) tomato mutants showed a substantial delay (minimally 7 days) in fruit ripening process. The long shelf-life resulted from delayed senescence compared to the wild type, suggesting that the PHOT1-mediated fruit pigmentation is influenced by the nps1 mutation. The nps1 mutant showed very low level accumulation of PHOT1 protein relative to the wild type under dark conditions and lacked blue light-induced autophosphorylation.
Anthocyanin biosynthesis in eggplants (cultivar ‘Lanshan Hexian’) is regulated by light (Jiang et al. 2016). The blue light treatment up-regulated levels of eggplant SmCRY1, SmCRY2 and SmHY5 transcripts. In addition, the blue-light triggered the CRY1/CRY2-COP1 interaction creating the conditions for HY5 and MYB1 to co-function in regulating the anthocyanin synthesis genes (CHS and DFR) in eggplant. Generally, both red and blue light greatly impact on the quality of fruits in terms of pigmentation not only in tomato and eggplant described above, but also in many other fruits such as berries (Jiang et al. 2016).
Abiotic stress tolerance
Numerous studies have shown that the phytochrome transgenic crop plants are characterized by dwarfism, having greener green leaves, higher harvest indices and other phenotypic traits (early growth of runners, tubers and inflorescences). Significantly, they are more physically resilient than the wild type in withstanding flood and wind damages, partly due to alterations in gibberellin biosynthesis and in its signal pathways (Peng et al. 1999). As several studies have revealed, overexpression of certain phytochrome family genes, especially PHYA and PHYB, leads to the development of agronomically important traits including abiotic stress tolerance. For example, phytochromes play an important role in conferring cold stress tolerance to higher plants (Franklin & Whitelam, 2007). In abscisic acid (ABA)-deficient (not) and jasmonate (JA)-deficient (spr2) mutants of tomato plants, FR and R light detected by phyA promoted cold tolerance by controlling the levels of ABA and JA as well as C-REPEAT BINDING FACTOR (CBF) (Wang et al. 2016).
Interestingly, the phyB-deficient rice plants (phyB) are more resistant to chilling stress than the wild type under low irradiance conditions (Yang et al. 2013). Exposing the rice plants to 24 h chilling, chloroplasts are severely damaged and unsaturated fatty acid levels are markedly reduced in the wild type, but not in the mutant, the latter showing an elevated expression of fatty acid desaturases and glycerol 3-phosphate acyltransferase (GPAT) compared to the wild type. These results suggest that unsaturated fatty acids of membrane lipids play a contributing role in conferring the rice plants to better tolerate the chilling stress. Not surprisingly, the phyB-deficient rice mutant tolerates drought stress better than the wild-type plants (Liu et al. 2012). The leaves of the phyB plants shrunk in total areas and suppressed transpiration rates, accounting for the reduced water loss and thus improved drought tolerance.
The zoysiagrass with S599A-PHYA transgene remains greener than the wild-type plants under early winter conditions (Fig. 6). In fact, overexpression of the hyperactive PHYA mutant gene confers stronger tolerance to the lower temperatures, attributable partly to the lowering of hydrogen peroxide, accumulation of stress-related amino acid proline and ROS scavenging enzymes as well as through the enhanced photosynthetic efficiency (Gururani et al. 2015b, 2016) (Table 2). The mutant turf grass plants are also resistant to high salinity and heavy metal toxicity, while the levels of chlorophylls, proline and ROS scavenging enzymes are raised compared with the wild type.
Contrary to the drought conditions, flooding is a water-holding stress. The phytochrome-deficient aurea (au) and high pigment-1 (hp1) mutants (highly sensitive to light-dependent responses) have been used to elucidate the involvement of phytochromes in different light and stress conditions (Terry & Kendrick, 1996; Kendrick et al. 1997; Muramoto et al. 2005; Liu et al. 2004). The au mutant tomato under water withholding stress had higher activities of catalase and ascorbate peroxidase compared to the other genotypes (Alves et al. 2016). The stress resistance traits of the au and hp mutants are perhaps best understood in terms of ROS scavenging enzymes and anti-oxidants such as carotenoids. Last, overexpression of the S-like RNase gene (OsRNS4) in rice seedlings revealed a role of phytochromes in salinity tolerance (Zheng et al. 2014).
Pathogen resistance
Available reports on light-mediated disease resistance of plants suggest that red-light treatments or higher R:FR illumination is an important variable to elucidate the disease resistance mechanisms (Nagendran & Lee, 2015). Here, it suffices to describe a few cases of light-modulated pathogen resistance, as there are only a limited number of reports on the relationship between the photomorphogenesis and the pathogenesis in plants.
Shibuya et al. (2011) studied the effects of fluorescent illumination with a high R:FR ratio on the resistance of cucumber seedlings to powdery mildew fungus. The resistance against powdery mildew fungus and reduction of colonies in the high R:FR illuminated seedlings was probably due to the formation of thicker epidermal tissues. In sunflower plants, an increase in PAR irradiance levels and low R:FR ratio in particular raised endogenous salicylic acid (SA) content roughly by 10-fold (Kurepin et al. 2010). With the help of phyAphyBphyC rice mutants, Xie et al. (2011) showed that phytochromes regulate SA and JA signalling pathways for developmentally controlled resistance to the Magnaporthe grisea pathogen in rice, whereas photo-reversion of phyB under low R:FR ratios suppressed the JA responses (Ballaré et al. 2014).
Photo-Biotechnology: Feeding Two Birds with One Scone
Abiotic stresses may detrimentally limit plant growth and crop harvests. Genetically engineering the crop plants with a variety of specific stress-responsive TFs offers potentially beneficial traits of commercial interest. For example, the stress-responsive TFs such as MYB, bZIP and DREB are known to regulate the expression of photosynthetic genes in response to abiotic stresses. Their overexpression may well be a worthy strategy for developing stress-resistant crops with improved productivity, even though specific TF-mediated stress tolerance does not always lead to higher crop productivity. In this review, we have seen that phytochrome transgenic plants (e.g. turfgrasses and sweet potato) gain both stress tolerance traits and improved productivity through the regulation of photosynthetically relevant genes (Table 3). Thus, the phrase ‘Feeding two birds with one scone’ is appended to the heading ‘Photo-biotechnology’ where the scone is the genetic alterations and the two birds represent the major photomorphogenic traits: (1) suppressed SAR and enhanced photosynthetic efficiency for improved productivity, and (2) resistance to various abiotic stresses, as recently proposed by Gururani et al. (2015a). It seems that a healthy crop plant is not only higher yielding, but also better withstands different stresses. Figure 7 shows a schematic representation of the photo-biotechnology for the genetic engineering of a variety of crops.
However, the agronomic trade-offs when the photoreceptors are highly expressed in crops should also be noted. As an example, tobacco plants strongly overexpressing the oat PHYA gene showed a light-exaggerated phenotype under white light: internodes are shorter, leaves are darker green, smaller in size and slightly thicker, and the axillary buds are under less apical control (Keller et al. 1989). Because many genes are coordinately regulated in a coordinated fashion by phytochrome, the overexpression of PHYA might result in the consistent overexpression of other genes and increased levels of associated proteins, even though photosynthetic performance may be improved. Thus, unexpected phenotypic modifications could worsen the productivity of plants – that is, the aforementioned agronomic trade-offs – and modulation of the expression of photoreceptors may be necessary to accomplish the crop improvements. One approach would be the selection of lower-expressing lines that do not show the light-exaggerated phenotype. Another approach is to use specific promoters other than constitutive promoters such as the 35S promoter. Recently, it has been demonstrated that phyA and phyB successfully function in transgenic plants with tissue-specific expression (Kirchenbauer et al. 2016; Kim et al. 2016). So, improvement of crop plants can be achieved by controlling the photoreceptor levels and/or through the tissue-specific expression of a photoreceptor, with a desired reduction in agronomic trade-offs.
Safety of Biotech Crops and Food
Print media and the internet are rife with claims that GM crops and food are unsafe at all levels. Having read the review of the development of genetically modified crops, the reader may ask why they continue to be developed. Answers to the question are covered widely in scientific, technical and public media, and are highlighted here only in brief: Genetic engineering of crops aims to (1) feed the increasing population through improved productivity; (2) cope with climate change by addressing its agricultural impacts; (3) respond to the adverse effects of increasingly acidic and contaminated soils on crop productivity and quality; (4) meet the continuing demand for natural raw materials and resources such as rubber, wood, fish and other marine organisms by developing functional organisms; and (5) effectively address the need for environmental conservation and biodiversity preservation. Reviewing these questions/answers is beyond the scope of this review. However, to answer the anti-GMO partisans' main claim against GM food safety, we cite the fact that no conclusive evidence for any adverse health effect from biotech food has been found in the more than 600 research papers and original research studies conducted over the last 10 years summarized at the following websites, respectively:
http://chilebio.cl/documentos/Publicaciones.pdf
and
htpp://informahealthcare.com/doi/abs/10.3109/07388551.2013.823595.
In spite of the scientific evidence for the safety of genetically engineered crops and food, agricultural cultivation of GM crops is legally restricted by governmental regulatory agencies. For example, before releasing any of the GM turfgrass cultivars to the farmers discussed in this review, their environmental risks must be assessed under field conditions. One major risk factor is gene escape from the GM grass field to non-GM grasses at the surrounding locations. In fact, the transgene of the Roundup Ready creeping bentgrass was found to introgress other recipient plant species 3.8 km away from the test plot (Reichman et al. 2006). It should be noted that gene escape is a general phenomenon in nature and not unique with GM plants. The phytochrome transgenic zoysiagrass with phytochromes described in this review possesses the BAR gene for an herbicide/Basta resistance. In contrast the Roundup Ready bentgrass case, no evidence for a BAR gene escape was found within a 5.3 km radius (Bae et al. 2008; Song et al. 2013). However, the transgenic zoysiagrass has yet to gain approval for release to turfgrass growers.
For the commercialization of the photo-biotechnology-based transgenic plants that have been developed, the next step will be the de-regulation of GM crops. Although countries that allow production of genetically modified crops have benefited by improved crop productivity and food security, the lack of global consensus on the safety of transgenic crops has prevented many countries from permitting cultivation of such crops. However, the number of countries and areas are increasing with time, and recently developing GM crops having multiple traits [for example, up to 28% of GM crops with herbicide resistance (57%) and insect resistance (15%) in 2014)] offer added advantages for the farmers. Therefore, photo-biotechnology-based improvement will be an important strategy for the development of genetically engineered crops in the future.
Concluding Remarks
This review focused on the photomorphogenesis-based development of crop plants to which the photoreceptor genes, especially phytochromes, have been introduced. Both light-dependent hyperactive mutants (e.g. S599A-PHYA) and photo-inactive constitutive ‘photomorphogenesis in darkness’ gain-of-function alleles (YVA, YVB and YHB) are prime transgene candidates for the development of important crop species by means of photo-biotechnological approaches (Fig. 7). Figure 8 also lists light signalling components that are subject to the photoreceptor influences described in this review. In addition to the photoreceptors, these light-signalling components may offer potential as targets for genetic engineering in biotechnology aimed at improving crop plants. Of particular importance are the positive regulators of photomorphogenesis, for example, HY5, HFR1, LAF1 and the COP1/SPA complex that acts as a proteolytic component in modulating the light signals in photomorphogenesis. Other genes of interest for crop improvement biotechnology are the early flowering inducer genes like FyPP (flower specific, phytochrome-associated protein phosphatase), PAPP5 (phytochrome specific type 5 protein phosphatase) and senescence-related genes. Targeting these and many other photomorphogenic signal transducers and generating their indel mutants for molecular mechanistic studies and crop improvement projects are now made much more efficient (than the traditional GM approach) based on the genome editing tool, CRISPR-Cas9 (and Cas9 variants such as Cpf1, TALEN and ZFN) protocols (Sternberg et al. 2012; Pattanayak et al. 2013; Ran et al. 2013). Applications of this new GE tool to plant biotechnology are just beginning (Belhaj et al. 2015; Bortesi & Fischer, 2015; Jung & Altpeter, 2016).

Acknowledgements
We thank Professor J. Clark Lagarias for the use of unpublished data (Fig. 4) and helpful suggestions. Ms. Yeojin Hong assisted the authors with the preparation of some of the figures. The work described in parts was supported by grants (2015R1D1A3A01018983 to PSS; 2014R1A1A2057739 to JIK; 2009-0094059 to HYL) from the National Research Foundation (NRF) of Korea, and grants (PJ01104001 to JIK; PJ00949901 to HYL) from the Rural Development Administration (RDA), Republic of Korea.




