Integrating Antixenosis Against Helicoverpa armigera (Lepidoptera: Noctuidae) and Micronutrition in Kabuli Chickpea (Cicer arietinum L.) Genotypes
Funding: The authors received no specific funding for this work.
[Correction added on 17 September 2024, after first online publication: The affiliation of the second author has been corrected in this version.]
ABSTRACT
The leguminous chickpea is a good source of protein, but its yield potential is frequently constrained by biotic stresses, primarily Helicoverpa armigera, a major havoc for cultivation of the crop. To develop host plant resistance for minimizing the losses due to the pod borer, five kabuli parents with desired traits for pod borer tolerance were crossed in diallel mating design to produce 10 crosses which were analysed for traits related to pod borer and nutrition. Based on correlation studies, trichome density was found positively correlated with phenol content, but both the traits were negatively associated with number of damaged seeds. Therefore, the tolerant genotypes were identified on the basis of phenol content, trichome density, number of damaged seeds and field rating. Among parents ICC 12197 was found superior in terms of yield and borer tolerance features with an intermediate pest resistance susceptible rating in addition to higher Fe content. However, significant sca effects for higher phenol content and seed yield in ICC 11764 × ICC 14190 were recorded with reduced number of damaged seeds in addition to higher Fe and Zn content. It was observed that the specific combination involved good and poor combiners for each trait. The same cross also showed significant standard heterosis in desirable direction for phenol content, trichome density, number of damaged seeds and seed yield. Additionally, the ratio of σ2 GCA to σ2 SCA revealed nonadditive gene action in controlling the expression of phenol content, trichome density, number of damaged seeds and Fe and Zn content. Thus, breeder may focus efforts on desirable cross utilizing selection in further segregating generations for higher phenol content, trichome density and Fe and Zn content in addition to yield-related traits while lesser number of damaged seeds per plant to concentrate for development of pod borer resilient high yielding kabuli genotypes to combat micronutrient deficiency.
1 Introduction
Chickpea (Cicer arietinum L.) is a popular staple food legume in temperate and semi-arid tropical climates as it is high in protein, carbohydrates and minerals. About 11.20 million hectares of land in India contributes 13.98 million tons of chickpea with an average yield of 1249 kg/ha (DAC&FW 2021–22). Being a nutrition-rich leguminous crop, it is severely affected by a dozen of major pests, major diseases, nematodes and weeds. Eleven different principal harmful pests have been reported in the chickpea crop (Rahman, Mannan, and Islam 1982). Among them, Helicoverpa armigera is a pest of great economic importance and an insatiable feeder on chickpea plants. It infests the crop at seedling stage and continues to devour flowers, pods and developing seeds until crop maturity (Reed et al. 1987). The yield loss in chickpea due to the pod borer has been estimated to be 10%–60% under normal weather conditions and may elevate to 50%–100% under favourable weather conditions (Vaishampayam and Veda 1980; Upadhyaya et al. 2006; Ali, Aslam, and Nadeem 2022). Development of pod borer-tolerant varieties is an integrated approach involving antixenosis, antibiosis and tolerance (Kambrekar 2016). An understanding of different morphological and biochemical components of resistance is essential for developing strategies to breed for resistance to insect pests. Biophysical traits like trichomes density and exudates influence the borer's ovipositional behaviour and host selection process (Haralu et al. 2018). Additionally, biochemical characteristics including malic acid, phenolic compounds, cellulose, hemicellulose, lignin and free amino acids have been discovered in chickpea as potential sources of insect pest resistance (Yoshida, Cowgill, and Wightman 1995; Girija et al. 2008). These component traits in desired direction impart genotypic tolerance. There are very few reports concerning the application of molecular markers for resistance to insect pests in grain legumes (Sharma et al. 2008). In future there is a need for precise phenotyping for insect resistance in conjunction with morphological and biochemical markers to develop cultivars with resistance to insect pests. Thus, development of tolerant varieties allows plants to produce a respectable yield in the face of a pest population that would otherwise cause severe harm to vulnerable plants as tolerant cultivars do not lower pest numbers and so do not put pest populations under selection pressure. This approach also reduces cost of cultivation by managing number of raised pesticide sprays.
Chickpea seeds being rich in protein have a potential to improve nutritional status of population through biofortification of crops which has been established as a productive prolong approach to elevate the nutritional concentration in the edible portion of the crops and resolve the problem of micronutrient hunger. Their nutritional enrichment can be practiced by different ways, that is, agronomic, breeding, genetic modification and microbiological approach. Biofortification using traditional breeding methods is a cost-effective and long-term solution to micronutrient deficiency of low-income families around the world (Bouis and Welch 2010).
The examination of genetic makeup of chickpea genotypes and their subsequent usage in improvement programmes, as well as information on their mean performance and combining ability, is quite useful. As we know, both borer resistance and micronutrient enrichment are complex traits. The desired gca effect may be used to distinguish excellent parents with desirable alleles for various component qualities. For effective selection to improve these traits, it is mandatory to have an understanding of the association of various traits. Morphological and biochemical components associated with expression of resistance to pod borer in chickpea hybrids and parents were used to identify genotypes with a diverse combination of characteristics associated with resistance to this pest.
Therefore, there is a need to gain an understanding of the relative contribution of different resistance mechanisms of chickpea against H. armigera and basic knowledge about interactions between the trichome density and leaf exudates in chickpea to develop appropriate strategies. Until and unless the per se performance of the combinations is compared to that of respective mid parent, better parent and standard parent performance, the desirable sca effects may not be of practical benefit. Therefore, for development of chickpea cultivars with high levels of resistance to this pest and to gain a piece of information on the genetics of Fe and Zn biofortification, an attempt was made to comprehend the gca effects of the parents and sca effects of the combinations along with their heterosis values over three types of parents.
2 Material and Methods
To study the genotypic tolerance to pod borer, a field experiment was conducted at NEBCRC, Pantnagar, during rabi season 2018–2019 and 2019–2020. Screening of six chickpea genotypes, namely, PKG 2, ICC 11764, ICC 12197, ICC 14190, ICC 16641 and PKG 1, was carried out for desired characters against the borer and for other micronutrition traits, while L 550 was screened only for borer related traits. In the next year, based on pest incidence and yield performance, PKG 1 and L 550 were considered as resistant and susceptible checks, respectively. Thus, five parents were included in diallel crossing programme and combining ability analysis was carried out following method as described by Griffing (1956). Therefore, 15 genotypes including 10 F1s and five parental lines were evaluated in a randomized block design along with PKG 1 as standard check for heterosis analysis. At pod formation stage, the pods were taken and observed under a microscope for recording trichome density. After harvest, the data were recorded on number of damaged seeds and seed yield. Few pods were used for phenol analysis and micronutrient quantification.
The phenol content in pod walls of chickpea genotypes was estimated as per the method by Malik and Singh (1980). The trichome density was measured according to Jackai and Oghiakhe (1989). The pod was sliced into 0.20-cm2 bits, and the number of trichomes on the epidermis of the bits was counted under a binocular microscope at a magnification of 25× and expressed as the number of trichomes per 0.20 cm2. In order to facilitate a clearer examination of the length and density of trichomes on chickpea genotype pods, observations were made on fully grown, green pods. In accordance with Bozzola and Russell (1999), the plant material was preserved and photographed using a scanning electron microscope (SEM).
Based upon the formula, the disease reaction and pest incidence percentage were converted to 1–9 scale as follows:
| Scale (1–9) | Pest reaction | Pest incidence |
|---|---|---|
| 1 | No infection | No damage |
| 3 | Moderately resistant | Less than 10% of plants or pods affected |
| 5 | Average infection | 11%–20% of plants or pods affected |
| 7 | Moderately susceptible | 21%–40% of plants or pods affected |
| 9 | Highly susceptible | More than 40% of plants or pods affected |
3 Results
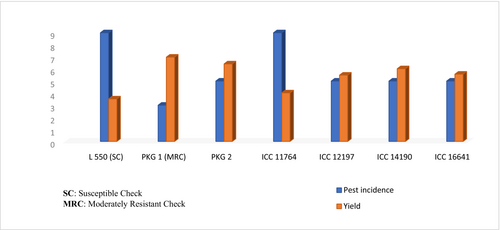
The relationship between pest incidence and yield per se performance is exhibited in Figure 1, which showcased the L 550 as a susceptible check with highest pest incidence and lowest yield and PKG 1 as a moderately resistant check with good agronomic performance. However, the genotypes, namely, PKG 2, ICC 12197, ICC 14190 and ICC 16641, showcased average infestation for borer while ICC 11764 was found to be moderately susceptible with large seed size.
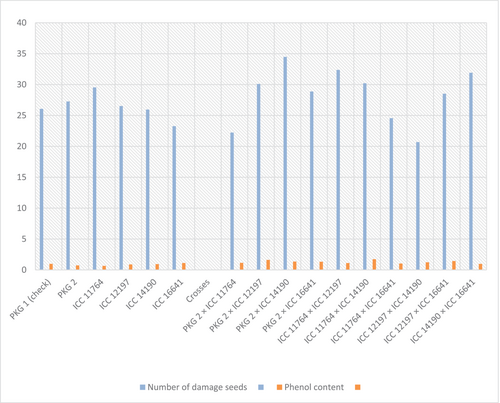
To find out genotypic response for pod borer, various traits were studied for relationship with number of damaged seeds. The correlation of phenol content as biochemical parameter and trichome density as biophysical trait with number of damaged seeds were mentioned in Table 1. It was observed that phenol content had significant positive association with trichome density (0.685) and significant negative association with number of damaged seeds (−0.724). Similarly, trichome density was also significantly and negatively correlated with number of damaged seeds (−0.807).
| Characters | Phenol | Trichome density | Number of damaged seeds |
|---|---|---|---|
| Phenol | 1.00 | 0.685** | −0.724** |
| Trichome density | 1.00 | −0.807** | |
| Number of damaged seeds | 1.00 |
- ** Significant at 1% level.
Figure 2 indicating significant negative relationship of parents and crosses between phenol content and number of damaged seeds showed that parental line ICC 16641 had high phenol content followed by ICC 14190, ICC 12197, ICC 11764 and PKG 2. The number of damaged seeds was also found less in ICC 16641. High phenol content may adversely affect the feeding of borer called as antibiosis or may cause antixenosis reaction which make the plant unfavourable for pod borer. On the combinations side, crosses ICC 12197 × ICC 14190, ICC 11764 × ICC14190 and PKG 2 × ICC 12197 showed higher phenol content and lesser borer damage, while crosses ICC 14190 × ICC 16641, ICC 11764 × ICC 12197 and PKG 2 × ICC 14190 exhibited comparatively higher number of damaged seeds with lower level of phenol content as shown in Figure 2.
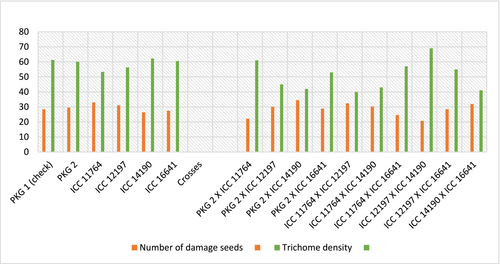
As mentioned in Figure 3, the relationship of parents and crosses between trichome density and gram pod borer infestation indicated that parental line PKG 2 had highest peak for trichome density followed by ICC 14190, ICC 16641, ICC 12197 and ICC 11764 but the number of damaged seeds by borer was least in ICC 14190 followed by ICC 16641. Parental line ICC 11764 was found to be least tolerant among all five. Among crosses, ICC 12197 × ICC 14190, PKG 2 × ICC 11764 and ICC 11764 × ICC 16641 exhibited higher peak for trichome density with lower level of pod damage. Figure 4a,b illustrated high trichome density in cross ICC 12197 × ICC 14190 while cross ICC 11764 × ICC 12197 indicated lowest trichome density (Figure 4c,d). Thus, it was very clear that greater number of trichomes act as morphological barrier that restricted the pod borer feeding, which may ultimately improve yield.

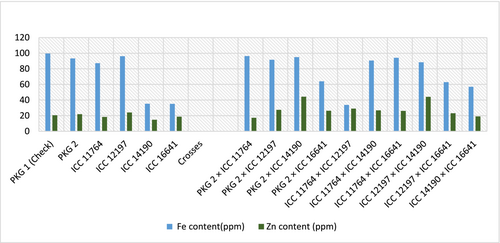
For nutritional studies, the mean performance of parental lines and their crosses for Fe and Zn content is furnished in Figure 5. On a positive note, Parental line ICC 12197 exhibited higher Fe and Zn content followed by PKG 2 and ICC 11764 for only Fe while PKG 2 and ICC 16641 for only Zn content. So, among all the five parents, ICC 12197 exhibited good mean performance for both Fe and Zn content. The different bars of per se performance of crosses displayed that cross PKG 2 × ICC 11764 showed high mean followed by PKG 2 × ICC 14190 and ICC 11764 × ICC 16641 for Fe content, while for Zn content, cross PKG 2 × ICC 14190 displayed high mean followed by ICC 12197 × ICC 14190. Cross PKG 2 × ICC 14190 manifested good mean performance for both Fe and Zn content.
The magnitude of gca effects for number of damaged seeds was found to be significant and negative in three parents, ICC 14190, ICC 16641 and PKG 2; however, in case of trichome density, the degree of gca impacts was found to be significant and positive in PKG 2, ICC 14190 and ICC 16641 (Table 2). Though for phenol content, parents ICC 16641 and ICC 11764 are significantly positive. Parents PKG 2 and ICC 14190 showcased significantly higher yield while ICC 12197 gave significant average grain yield. On nutritional side, the significant and positive gca effects were found in three parents for Fe content, namely, ICC 12197, PKG 2 and ICC 11764, whereas in ICC 14190 and ICC 11764, for Zn content. Thus, out of five parental lines, ICC 16641 showcased good general combining ability for trichome density, phenol content and number of damaged seeds but was found poor for Fe and Zn content and yield. ICC 14190 and PKG 2 were good general combiners for trichome density, lower number of damaged seeds and yield, whereas in nutritional analysis ICC 14190 was found good general combiner for Zn while PKG 2 for Fe content. Parental line ICC 12197 showed average gca for yield, borer tolerance traits and good for Fe content. Thus, this may be suggested as donor for high yield with tolerance to pod borer and biofortification breeding.
| Sl. No. | Characters | PKG 2 | ICC 11764 | ICC 12197 | ICC 14190 | ICC 16641 |
|---|---|---|---|---|---|---|
| 1. | Phenol content | (P) −0.22** | (G) 0.17** | (A) −0.01 | (P) −0.18** | (G) 0.24** |
| 2. | Trichome density | (G) 5.75** | (P) −13.24** | (A) −0.10 | (G) 4.75** | (G) 2.82** |
| 3. | Number of damaged seeds | (G) −0.74* | (P) 4.94** | (A) 0.30 | (G) −2.34** | (G) −2.15** |
| 4. | Grain yield per plant (g) | (G) 0.61** | (P) −0.15 | (A) 0.43* | (G) 0.66** | (P) −0.69** |
| 5. | Fe content | (G) 12.08** | (G) 10.74** | (G) 15.78** | (P) −9.54** | (P) −29.08** |
| 6. | Zn content | (P) −3.22** | (G) 0.70* | (P) −0.66* | (G) 6.77** | (P) −3.59** |
- Abbreviations: A = average, G = good, P = poor.
- * Significant at 5% level.
- ** Significant at 1% level.
Significant positive or negative sca effects were identified in F1 generation for pod borer tolerance-related traits (Table 3). The highest sca effect for pod borer tolerance-related traits was observed in ICC 11764 × ICC 14190 for phenol content and ICC 12197 × ICC 14190 for trichome density, and the least number of damaged seeds was observed in cross PKG 2 × ICC 11764. For Fe content, sca effects were found significantly positive in ICC 11764 × ICC 16641, PKG 2 × ICC 11764 and ICC 11764 × ICC 14190. Zn content crosses, namely, ICC 11764 × ICC 14190, ICC 11764 × ICC 16641, PKG 2 × ICC 12197 and PKG 2 × ICC 16641, showed significant positive sca.
| Sl. No. | Characters | PKG 2 × ICC11764 | PKG 2 × ICC 12197 | PKG 2 × ICC 14190 | PKG 2 × ICC 16641 | ICC 11764 × ICC 12197 | ICC 11764 × ICC 14190 | ICC 11764 × ICC 16641 | ICC 12197 × ICC 14190 | ICC 12197 × ICC 16641 | ICC 14190 × ICC 16641 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Phenol content | 0.12** | −0.23** | −0.13** | 0.21** | −0.17** | 0.67** | −0.38** | 0.34** | −0.16** | 0.17** |
| 2. | Trichome density | 39.95** | 2.31 | −26.54** | 41.38** | −27.19** | −26.04** | 25.38** | 48.31** | 10.23** | −14.61** |
| 3. | Number of damaged seeds | −12.32** | 5.81** | 8.40** | −6.40** | 7.75** | 6.91** | −3.88** | −10.52** | −5.63** | 4.35** |
| 4. | Grain yield per plant (g) | −0.36 | 1.13** | 0.32 | −1.48** | 0.13 | 0.98* | −0.76* | 1.89** | −0.08 | 0.57 |
| 5. | Fe content | 36.51** | 21.76** | 6.69** | −44.26** | −78.79** | 35.38** | 43.76** | 25.32** | −9.91** | −4.13** |
| 6. | Zn content | −2.79** | 4.25** | −12.45** | 1.78* | 0.12 | 13.01** | 5.42** | −3.06** | −1.14 | −11.19** |
- * Significant at 5% level.
- ** Significant at 1% level.
Thus, it was observed that the parents with high estimates of gca effects did not necessarily record high estimates of sca effects in the crosses, and parents with weak estimates of gca effects may also produce superior crosses, according to the ranking of crosses based on per se performance. Cross combinations that produced large sca effects involved both or at least one weak/average general combiner. In the majority of cross pairings, the result indicated the operation of dominant × dominant and/or dominant × additive gene interactions operated for trait expression.
For number of damaged seeds, phenol content, trichome density, yield, Fe content and Zn content, in different cross combinations, estimates of standard heterosis, relative heterosis and heterobeltiosis expressed as a per cent increase or decrease were computed as shown in Table 4. For number of damaged seeds, crosses, namely, PKG 2 × ICC 11764, ICC 12197 × ICC 14190 and ICC 12197 × ICC 16641, were found to be superior ones as they had significant negative heterosis over standard, mid and better parents. But for the component trait of trichome density, cross combinations PKG 2 × ICC 11764, PKG 2 × ICC 16641 and ICC 12197 × ICC 14190 exhibited significant positive heterosis over standard, mid and better parents. However, for phenol content, cross ICC 11764 × ICC 14190 was found to have significant positive heterosis over standard, mid and better parents. For grain yield, two crosses, namely, PKG 2 × ICC 12197 and ICC 11764 × ICC 14190, exhibited significant positive heterosis over check parent, that is, PKG 1. The results of heterosis for Fe content was showcased by cross PKG 2 × ICC 11764 over standard, mid and better parents. For Zn content, three crosses, namely, ICC 11764 × ICC 16641, ICC 11764 × ICC 14190 and ICC 11764 × ICC 12197, displayed significant positive heterosis over mid parent, and only one cross ICC 11764 × ICC 16641 exhibited significant positive heterosis over better parent.
| Characters | Phenol content | Trichome density | Number of damaged seeds | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Heterosis over | Heterosis over | Heterosis over | |||||||
| Standard parent | Mid parent | Better parent | Standard parent | Mid parent | Better parent | Standard parent | Mid parent | Better parent | |
| PKG 2 × ICC 11764 | −36.91** | −0.43 | −11.92** | 15.70** | 135.50** | 99.00** | −46.23** | −39.01** | −46.23** |
| PKG 2 × ICC 12197 | −66.67** | −43.98** | −47.84** | −12.79** | 50.00** | 50.00** | −13.01** | 12.43** | 10.89** |
| PKG 2 × ICC 14190 | −70.25** | −20.59** | −46.00** | −40.70** | −25.00** | −40.70** | −13.16** | 36.51** | 13.82** |
| PKG 2 × ICC 16641 | −27.55** | −6.57* | −27.55** | 36.05** | 155.74** | 134.00** | −49.13** | −33.79** | −34.25** |
| ICC 11764 × ICC 12197 | −41.87** | −2.31 | −9.05** | −69.19** | −36.90** | −47.00** | 5.78* | 17.96** | 4.83** |
| ICC 11764 × ICC 14190 | 9.41** | 155.15** | 73.50** | 58.21** | −45.83** | −62.21** | −2.82 | 28.00** | −3.70 |
| ICC 11764 × ICC 16641 | −39.12** | −21.49** | −39.12** | −4.65 | 117.22** | 97.59** | −28.92** | −20.26** | −29.56** |
| ICC 12197 × ICC 14190 | −32.51** | 80.15** | 22.50** | 39.53** | 76.47** | 39.53** | −57.16** | −33.94** | −45.61** |
| ICC 12197 × ICC 16641 | −36.91** | −18.65** | −36.91** | −6.98** | 74.86** | 60.00** | −44.65** | −29.09** | −29.72** |
| ICC 14190 × ICC 16641 | −46.56** | 6.89 | −46.56 | −30.23** | −5.88 | −30.23** | −26.61** | 13.74** | −5.14 |
| Characters | Grain yield per plant (g) | Fe content | Zn content | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Heterosis over | Heterosis over | Heterosis over | |||||||
| Standard parent | Mid parent | Better parent | Standard parent | Mid parent | Better parent | Standard parent | Mid parent | Better parent | |
| PKG 2 × ICC 11764 | −19.00* | −7.87 | −19.29* | 5.03* | 53.78** | 45.43** | −58.21** | −6.08 | −16.14** |
| PKG 2 × ICC 12197 | 19.71** | 8.29* | −1.93 | −1.99 | 13.82** | −1.99 | −45.36** | 8.79 | 7.94 |
| PKG 2 × ICC 14190 | 2.51 | 2.88 | 2.14 | −31.16** | 40.83** | −4.68* | −66.36** | −55.10** | −66.36** |
| PKG 2 × ICC 16641 | −42.65** | −33.61** | −42.86** | −82.07** | −63.28** | −75.18** | −57.59** | −8.95 | −14.89* |
| ICC 11764 × ICC 12197 | −26.88** | 1.49 | 0.99 | −75.57** | −70.26** | −75.57** | −45.81** | 21.60** | 7.05 |
| ICC 11764 × ICC 14190 | 10.86** | 7.65* | 2.17 | −11.41** | 97.28** | 37.84** | 0.23 | 44.73** | 0.23 |
| ICC 11764 × ICC 16641 | −43.30** | −21.29* | −21.68 | −19.47** | 79.57** | 25.30** | −40.43** | 45.59** | 37.48** |
| ICC 12197 × ICC 14190 | −44.09** | −34.45* | −43.48** | −15.03** | 35.73** | −14.74** | −39.30** | −18.97** | −39.30** |
| ICC 12197 × ICC 16641 | −37.63** | −13.43 | −13.86 | −54.59** | −27.40** | −54.44** | −58.44** | −10.77* | −16.59** |
| ICC 14190 × ICC 16641 | −12.54 | 1.24 | −12.86 | −68.71** | 23.45** | 23.10** | −64.35** | −50.13** | −64.23** |
- * Significant at 5% level.
- ** Significant at 1% level.
Considering both significant sca in the desired direction along with significant positive heterosis over all the standard, mid and better parents for borer tolerance-related traits, crosses PKG 2 × ICC 11764, ICC 11764 × ICC 14190 and ICC 12197 × ICC 14190 performed better for the number of damaged seeds, trichome density and phenol content. Similarly, for biofortification traits, crosses PKG 2 × ICC 11764 and ICC 11764 × ICC 16641 were considered promising for Fe and Zn contents, respectively. Therefore, above crosses can be considered as good cross combinations for selection of transgressive segregants in later generations.
It was observed that parent ICC 12197 exhibited good and average performance for the above-mentioned traits while parent ICC 16641 showcased high level of trichome density and phenol content. Parental line ICC 11764 showed good result for Fe and Zn content, ICC 12197 expressed moderate level of phenol content and ICC 14190 with less number of damaged seeds gave the best cross combination, that is, ICC 12197 × ICC 14190. Similarly, the cross of PKG 2 with less phenol content and ICC 11764 with high number of damaged seeds gave good amalgamation of both the traits in F1 and showcased best result for trichome density, phenol and Fe content.
To understand gene action for deciding future breeding programmes of the above-studied traits, the components of genetic variance were estimated) (Table 5). There was preponderance of SCA variance for all the studied traits as sepecific combining ability variance (σ2 SCA) was higher than general combining ability variance (σ2 GCA). This further clarified the GCA to SCA ratio of less than one which is attributed to non addditive gene action (dominance and epistasis) in controlling the expression of the traits. But chickpea is a cleistogamous crop hence effective strategies include breeding methods that leverage both additive and non-additive effects, followed by selection in subsequent generations to enhance the traits of interest.
| Characters | σ2 GCA | σ2 SCA | σ2 GCA/σ2 SCA |
|---|---|---|---|
| Fe content | 361.62 | 1585.80 | 0.22 |
| Zn content | 17.42 | 64.89 | 0.26 |
| Number of damaged seeds | 8.76 | 63.98 | 0.13 |
| Trichome density | 59.49 | 1090.92 | 0.05 |
| Phenol content | 0.04 | 0.11 | 0.37 |
| Grain yield per plant (g) | 0.43 | 0.85 | 0.41 |
4 Discussion
The gram pod borer, H. armigera (Hub.), infestation in chickpeas illustrated the pest's ravenous behaviour and the necessity for significant management. When identifying pest resistant genotypes, a vacuum was established in the genotype pool to meet the needs of scientists, researchers and growers. Therefore, the goal of the current study was to identify elite genotype among the available variants. Fifteen randomly chosen plants provided the data for all field experiments. The mean of those 15 plants and check variety underwent statistical analysis and was shown in all the studied parameters. Though, several studies have already been conducted for the screening of crops (Altaf 2009; Nadeem et al. 2010; Sarwar, Ahmad, and Toufiq 2011; Choudhary, Anwala, and Sharma 2014; Rehman et al. 2017; Mantesh et al. 2017; Abro et al. 2017). Their locations and variety selections, however, differed greatly from those of the current study. At the same time, no current research concentrating on the screening of elite genotypes has been published in the literature. The relationship between the quantity of damaged seeds and the biochemical parameter of phenol content and the biophysical feature of trichome density showed the profuse and nonglandular trichomes and phenols negatively hampered the feeding efficiency of borer, and the results are analogous to the findings of Ali, Aslam, and Nadeem (2022). According to Karthik and Vastrad (2022) significantly higher amount of malic acid in infested leaves indicated its role in host plant defense. Girija et al. (2008) reported that total phenol content showed a highly significant negative association with per cent pod damage by gram pod borer. Similarly, more resistance against Helicoverpa infestation in chickpea with significant trichome length, trichome density and pod wall thickness was reported by Shabbir et al. (2014) and Divija, Agnihotri, and Reddy (2021). It was observed that the combination of morphological and physiochemical characteristics that were negatively correlated with the per cent pod damage could be used as an effective and reliable selection criterion to select resistant plants, and the results were well supported by the findings of Salimath et al. (2008) and Golla et al. (2020). The chickpea cultivars listed in Figure 1 were used as the basis for the grading of resistance for each tested genotype. The modified pest susceptibility rating (PSR) (Figure 1), which is based on the scale recommended by Jackai and Oghiakhe (1989), was used to estimate the resistance or susceptibility of each tested variety of chickpea.
Malnutrition would be less of an issue if the best cultivars with desired vitamin concentrations and high protein contents were developed. Identifying micronutrient-rich chickpea types might also aid breeders in locating donors for desired features. A complex quantitative feature that is polygenic in nature is the relative concentration of Fe and Zn found in seeds. Therefore, it is clear that additional data on the micronutrients and total protein content of chickpeas are needed. Determining the genetic foundation of the variability controlling the amount of Fe and Zn in seeds is therefore absolutely necessary. Figure 4 exposed high mean performance of Fe and Zn in different crosses, but cross PKG 2 × ICC 14190 manifested good mean performance for both Fe and Zn content. Thavarajah and Thavarajah (2012) and Grewal et al. (2020) also reported high Zn and Fe content in a few genotypes of chickpea. It is worth noting that some parents (Table 2) were classified into good, average and poor categories according to their gca effects. However, the crosses made up of average × poor gca effects parents were found phenomenal for micronutrient, phenols and grain yield per plant and showed significant positive sca effects, and the expressions were supported by the findings of Jayalakshmi, Reddy, and Nagamadhuri (2019). But the high level of the phenolic compounds somehow lowers the concentration of Zn and Fe in the crosses and vice-versa (Table 4) and it has also been linked to the study of Grewal et al. (2020). The assessment of variance pertaining to general combining ability, specific combining ability and their respective ratio demonstrated the presence of nonadditive genetic mechanisms governing the manifestation of traits related to iron and zinc content, as well as the traits related to borers' resistance. Seed yield per plant showed high significant SCA variance and had fewer additive gene actions out of total genotypic variance, which is analogous to the findings of Chauhan et al. (2023). Consequently, crosses showing potential for desirable traits can be employed to select transgressive segregants in subsequent generations.
In conclusion, according to our research, ICC 12197 is superior to the other five parents in terms of borer tolerance features, yield and biofortification breeding with an intermediate pest incidence rating. Among all the 10 cross combinations, ICC 11764 × ICC 14190 showed good results for phenol content, trichome density and yield. Among all the crosses, PKG 2 × ICC 11764 combinations showcased good sca effects and significant positive heterosis over mid, better and check parents for less number of damaged seeds, Fe content and trichome density and cross ICC 11764 × ICC 14190 for phenol content and seed yield. The identification and evaluation of chickpea genotypes for pod borer resistance as well as high Zn and Fe concentration have opened the door to lowering these problems. Hence, the study and promising cross combinations could be utilized to create large genetic variability in future breeding programmes and also be found beneficial in combating micronutrient deficiency along with lowering the borer infestation which contributes to high yield.
Author Contributions
Satvinder Singh: writing – review and editing, writing – original draft, validation, supervision, software, resources, methodology, investigation, formal analysis, data curation, conceptualization. Anju Arora: writing – review and editing, validation, supervision, resources, methodology, investigation, formal analysis, data curation, conceptualization. Karthick S. Babu: software, formal analysis. S. K. Verma: field aspect. R. K. Panwar: lab aspect. Meena Agnihotri: pod borer/entomology aspect.
Acknowledgements
The authors wish to thank the Department of Genetics and Plant Breeding, Joint Director NEBCRC, and Director Experiment Station, GBPUA&T, Pantnagar, for providing the necessary resources for the present study.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




