Extracellular ATP: an emerging multifaceted regulator of plant fitness
Summary
Adenosine 5′-triphosphate (ATP) is the energy currency of living organisms and the primary form of organic phosphate (Po) involved in cellular metabolism. In plants, some ATP is released into the extracellular matrix (ECM) in response to various stimuli, where it functions as extracellular ATP (eATP), a key signalling molecule. Recent advances have shed light on the mechanisms of eATP signalling in plants. This review consolidates these findings, beginning with the role of eATP in regulating plant growth, development and responses to biotic and abiotic stresses. It further summarizes the pathways of eATP accumulation and degradation in the ECM and introduces the cellular signalling pathways mediating eATP responses, as reported in key studies. Finally, perspectives on future research directions in this field are presented.
Introduction
Adenosine 5′-triphosphate (ATP) is ubiquitous in all organisms and plays essential roles in various biological processes. While ATP primarily participates in intricate intracellular activities, a portion is released into the extracellular matrix (ECM) as extracellular ATP (eATP). Unlike in mammals, where eATP is well established as a signalling molecule regulating numerous cellular activities, the functional roles and signalling mechanisms of eATP in plants have remained unclear for a long time. Early studies have suggested the presence of ATP in the ECM of plants and highlighted its physiological effects (Jaffe, 1973; Lew and Dearnaley, 2000; Tang et al., 2003; Udvardy and Farkas, 1973). However, direct evidence confirming the existence of eATP has been provided in several key studies. Kim et al. developed a cellulose-binding domain (CBD)–luciferase fusion protein targeting the plant cell wall, and luminescence from exogenously applied CBD–luciferase to root hair tips demonstrated the widespread presence of eATP in various plant species (Kim et al., 2006). Additionally, eATP concentrations were measured in extracellular fluids extracted from Arabidopsis thaliana leaves at wound sites (Jeter et al., 2004; Song et al., 2006). Using a suspension cell culture system, researchers have monitored ATP release into the medium under different stimuli (Ramachandran et al., 2019). Collectively, these findings provide solid evidence of the presence of eATP in plants.
The in vivo imaging and electrophysiological experiments have revealed that eATP can induce the cytosolic free calcium oscillations and depolarize the plasma membrane (PM) potentials, indicating its role as the signalling molecule and the potential existence of a putative eATP receptor in plants (Jeter et al., 2004; Lew and Dearnaley, 2000; Tanaka et al., 2010). Genetic and biochemical evidence further supports the signalling function of eATP (Jeter et al., 2004). This review summarized recent progress in eATP, including its biological functions, mechanisms of accumulation and degradation, molecular signalling pathways and measurement methods. Finally, several perspectives were proposed to guide future research in this field.
eATP in plant growth and development
In mammals, eATP regulates various biological processes including exocrine and endocrine secretion, immune responses, inflammation, pain, platelet aggregation, cell proliferation, differentiation and cell death (Abbracchio and Burnstock, 1998; Burnstock, 2002; Burnstock and Knight, 2004; Gordon, 1986). Similarly, growing evidence has demonstrated that eATP plays a significant role in regulating plant growth, development and stress responses. This discussion highlights the developmental phenotypes influenced by exogenous ATP treatment and eATP over- or under-accumulation. Arabidopsis seeds release ATP during germination and eATP can inhibit germination in a receptor-dependent manner (Wang et al., 2022a). eATP regulates pollen germination and tube growth in A. thaliana and Nicotiana tabacum (Wu et al., 2007, 2018). Exogenously applied ATP inhibits Arabidopsis root growth, as the addition of 1 mM ATP to solid 1/2 Murashige and Skoog (MS) medium disrupts auxin distribution and attenuates root gravitropism (Tang et al., 2003). Similarly, in liquid 1/2 MS medium, 0.5 mM ATP suppresses root length by inducing a burst of reactive oxygen species (ROS) and altering cell wall integrity (Shi et al., 2022; Sowders et al., 2023). Moreover, this eATP-mediated root growth inhibition extends to Nicotiana benthamiana, a model species of the Solanaceae family, suggesting a conserved response across plant species (Sowders et al., 2023). Additionally, eATP plays a critical role in legume nodulation (Tanaka et al., 2011) and regulates stomatal closure and ROS production in leaves, contributing to pathogen defence (Clark et al., 2011; Song et al., 2006). Furthermore, eATP acts as an extracellular stress signal, influencing cell wall development and integrity, where its over-accumulation triggers stress-like responses and impairs cell wall structure (Lim et al., 2014; Zhang et al., 2024). In the following sections, we explore the critical role of eATP in defence against various stresses (Figure 1 and Table 1).
| Plant species | Organ/tissue | Function | Effect |
|---|---|---|---|
| Arabidopsis thaliana | Seed | Germination | Inhibition (Wang et al., 2022a) |
| Arabidopsis thaliana | Pollen | Germination and growth | Promotion (Wu et al., 2018) |
| Arabidopsis thaliana | Cell wall | Development and integrity | Promotion (Lim et al., 2014; Zhang et al., 2024) |
| Nicotiana tabacum | Pollen | Germination and growth | Promotion (Wu et al., 2018) |
| Arabidopsis thaliana | Root | Growth and gravitropism | Inhibition (Tang et al., 2003) |
| Nicotiana benthamiana | Root | Growth | Inhibition (Sowders et al., 2023) |
| Glycine max | Root nodule | Nodulation | Inhibition (Tanaka et al., 2011) |
| Gossypium hirsutum | Fibre | Length | Double effects (Clark et al., 2010) |
| Arabidopsis thaliana | Stomata | Aperture | Double effects (Chen et al., 2017; Clark et al., 2011) |
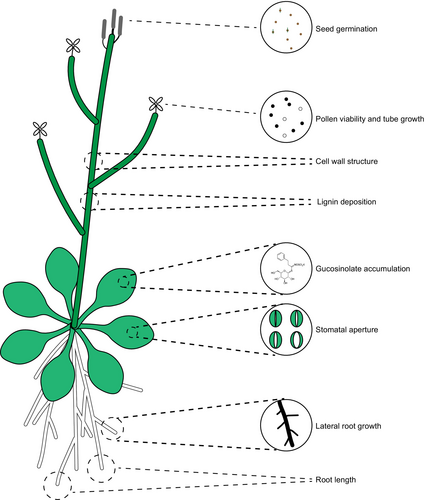
Functional roles of eATP in plant–biotic stress interaction
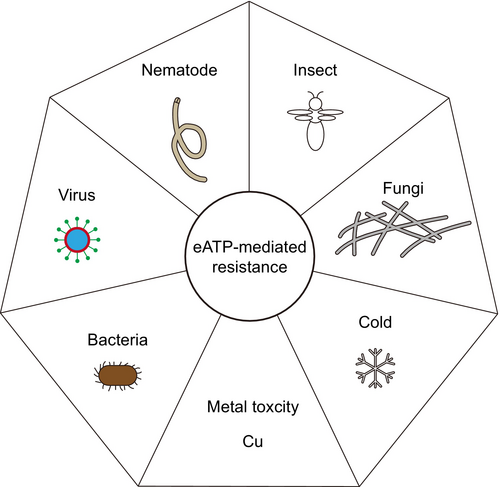
eATP is generally recognized as a danger-associated molecular pattern (DAMP) that activates plant defence responses (Tanaka et al., 2014). Upon pathogen challenge, plants release ATP into ECM to initiate eATP signalling (Cao et al., 2014). Arabidopsis eATP receptor P2-type purinoreceptor kinase 1 (P2K1, also known as DORN1 or LecRK-I.9) is essential for resistance to oomycetes and bacterial pathogens (Balague et al., 2017; Bouwmeester et al., 2011). Overexpression of P2K1 or treatment with exogenous ATP enhances resistance to the soil-borne fungus Rhizoctonia solani, whereas the p2k1–3 knockout mutant exhibits increased susceptibility (Kumar et al., 2020). In addition, P2K1-mediated disease resistance has also been observed in solanaceous plants (Bouwmeester et al., 2014). Another soil-borne fungus Fusarium oxysporum (Fo), the causal agent of Fusarium wilt disease, causing great yield losses across the world. Kesten et al. (2023) demonstrated that Fo could secrete adenosine (Ado), an ATP metabolic product, into ECM to counteract the eATP-mediated plant defences. An imbalance in the eAdo/eATP ratio has been identified to alter plant resistance levels (Daumann et al., 2015; Kesten et al., 2023). Additionally, eATP enhances the tobacco resistance to tobacco mosaic virus (TMV) and Pseudomonas syringae pv. tabaci (Chivasa et al., 2009). In summary, eATP plays a pivotal role in conferring plant resistance to multiple pathogens (Jewell et al., 2019).
Mechanistically, P2K1 directly interacts with and phosphorylates NADPH oxidase respiratory burst oxidase homologue D (RBOHD), facilitating the ROS production and regulating the stomatal aperture (Chen et al., 2017; Clark et al., 2011; Song et al., 2006). P2K1 also induces phosphorylation of mitogen-activated protein kinase 3 (MPK3) and MPK6 in response to eATP (Choi et al., 2014; Kim et al., 2023a). These findings align with the notion that eATP enhances pathogen-associated molecular pattern-triggered immunity (PTI) responses (Cao et al., 2014; Chen et al., 2021). Additionally, eATP modulates defence-related hormone pathways, including those involving jasmonic acid (JA), salicylic acid (SA) and ethylene (ETH), to combat invading pathogens. eATP treatment activates JA signalling, conferring resistance to the necrotrophic fungus Botrytis cinerea (Tripathi et al., 2018). The induction of P2K1 expression by disease inoculation is dependent on JA signalling components, coronatine insensitive 1 (COI1) and jasmonate resistant 1 (JAR1) (Balague et al., 2017). To systematically investigate the interplay between eATP signalling and defence-related hormones, a comprehensive transcriptome analysis was performed using P2K1 mutants and receptor genes associated with JA, SA and ETH pathways (Jewell et al., 2019). Transcriptome assays have revealed that the most enriched gene ontology (GO) terms for ATP-responsive genes included responses to wounding, chitin, JA, protein phosphorylation, innate immune regulation, SA biosynthesis and the abscisic acid (ABA)-activated signalling pathway, all in a P2K1-dependent manner. Notably, approximately 67% of ATP-responsive genes were less sensitive in the coi1-30 mutant, with 154 out of 543 genes solely dependent on COI, indicating a strong convergence between eATP and JA signalling pathways (Jewell et al., 2019). In contrast, only 6 and 21 genes have been observed to depend only on ethylene insensitive 2 (EIN2) and non-expressor of pathogen-related gene 1 (NPR1), respectively (Jewell et al., 2019). These findings have demonstrated the intricate interplay between eATP signalling and defence-related hormones, providing a physiological basis for how eATP enhances defence against biotrophic, hemi-biotrophic and necrotrophic pathogens (Jewell et al., 2019). Furthermore, eATP can mitigate cell death induced by the mycotoxin fumonisin B1 (FB1). Using this system, ribonuclease 1 (RNS1) can be identified as a component of eATP signalling through proteomics. RNS1 also participates in the SA pathway, suggesting a convergence between eATP and SA signalling (Goodman et al., 2022). Mevalonate kinase (MVK) is a core regulator of the mevalonate pathway and plays a critical role in plant metabolite biosynthesis. Recent findings by Cho et al. identified MVK as an upstream regulator of eATP signalling and a direct target of the eATP receptor P2K1, with impaired eATP responses observed in mvk mutants. These mutants exhibited significantly reduced accumulation of SA and cytokinin (CTK) along with diminished expression of SA-induced genes, rendering them more susceptible to biotrophic pathogens (Cho et al., 2022). Interestingly, kinase-impaired mutants of the secondary eATP receptor P2-type purinoreceptor kinase 2 (P2K2) display autoimmune phenotypes, including spontaneous lesion formation, activation of the SA pathway and heightened resistance to P. syringae pv. tomato DC3000 (Zhao et al., 2023). Additionally, evidence has indicated that exogenous ATP not only enhances plant defence responses, but also directly inhibits fungal growth (Kesten et al., 2023; Kumar et al., 2020). Herbivore feeding activates the JA signalling response, suggesting that eATP may regulate the plant resistance to insects. Supporting this, eATP activates the indolic glucosinolate biosynthesis pathway, enhancing resistance to Pieris rapae, Spodoptera exigua, Meloidogyne javanica and turnip mosaic virus (TuMV) (Jewell et al., 2022). Thus, eATP functions as a DAMP, conferring resistance to various biotic stresses in plants (Table 2).
| Plant species | Pathogens | Effect on resistance |
|---|---|---|
| Arabidopsis thaliana | Phytophthora brassicae | Positive (Bouwmeester et al., 2011) |
| Arabidopsis thaliana | Pseudomonas syringae | Positive (Balague et al., 2017) |
| Solanum tuberosum | Phytophthora infestans | Positive (Bouwmeester et al., 2014) |
| Nicotiana benthamiana | Phytophthora infestans | Positive (Bouwmeester et al., 2014) |
| Arabidopsis thaliana | Rhizoctonia solani | Positive (Kumar et al., 2020) |
| Arabidopsis thaliana | Fusarium oxysporum | Positive (Kesten et al., 2023) |
| Nicotiana benthamiana | Tobacco Mosaic Virus | Positive (Chivasa et al., 2009) |
| Nicotiana benthamiana | Pseudomonas syringae | Positive (Chivasa et al., 2009) |
| Arabidopsis thaliana | Pieris rapae | Positive (Jewell et al., 2022) |
| Arabidopsis thaliana | Spodoptera exigua | Positive (Jewell et al., 2022) |
| Arabidopsis thaliana | Meloidogyne javanica | Positive (Jewell et al., 2022) |
| Arabidopsis thaliana | Turnip mosaic virus | Positive (Jewell et al., 2022) |
| Arabidopsis thaliana | Botrytis cinerea | Positive (Tripathi et al., 2018) |
Regulation roles of eATP in plants abiotic stress tolerance
Touching promotes ATP release from the Arabidopsis root (Weerasinghe et al., 2009). Real-time luciferase imaging suggested that eATP accumulated within 5 min at the wound site in Arabidopsis leaves. However, it is ROS, not eATP, which acts as a systemic wounding signal, indicating that eATP does not have mobile capacity but accumulates locally at the wound site. Interestingly, both wounding and ATP treatment can trigger systemic ROS waves in a P2K1-dependent manner, suggesting that local eATP signalling is essential for initiating long-distance ROS waves and the systemic wounding response (Myers et al., 2022). Moreover, Shi et al. reported that repeated wounding stunted the growth of Arabidopsis; however, this phenotype was alleviated in the p2k1–3 mutant or by the application of ibuprofen, an inhibitor of JA biosynthesis (Shi et al., 2022). These findings can highlight the connection between eATP and JA signalling in the context of the wounding response.
Luciferase-mediated real-time whole-plant imaging revealed that Arabidopsis can increase eATP concentrations under salt stress (Kim et al., 2023b). P2K1 overexpression plants exhibited more severe growth inhibition, whereas the knockout mutants were less sensitive to salt stress, suggesting that eATP signalling can mediate growth inhibition under such conditions (Kim et al., 2023b). Plants also produce eATP in response to copper stress, which helps to alleviate copper stress-induced cell death (Jia et al., 2019). Transgenic Arabidopsis overexpressing APY2, a nucleoside triphosphate-diphosphohydrolase, from Populus euphratica can display enhanced cold tolerance with lower eATP concentrations than wild-type plants (Deng et al., 2015). Overall, eATP regulates plant tolerance to abiotic stress, but the mechanisms of crosstalk between eATP signalling and abiotic stress pathways remain to be further explored (Figure 2 and Table 3).
| Plant species | Organ/tissue | Abiotic stresses | Effects |
|---|---|---|---|
| Arabidopsis thaliana | Root | Touching | eATP release (Weerasinghe et al., 2009) |
| Arabidopsis thaliana | Whole plant | Salt | Promoting whole plant growth inhibition (Kim et al., 2023b) |
| Arabidopsis thaliana | Suspension cells | Osmosis | eATP release (Kim et al., 2009) |
| Arabidopsis thaliana | Leaf | Wounding | eATP release (Myers et al., 2022) |
| Arabidopsis thaliana | Leaf | Repeated wounding | Promoting whole plant growth inhibition (Shi et al., 2022) |
| Nicotiana tabacum | Suspension cells | Metal toxicity | Alleviate cell death (Jia et al., 2019) |
| Triticum aestivum | Root | Metal toxicity | Alleviate cell death (Jia et al., 2019) |
| Populus euphratica | Whole plant | Cold | Negative effect on cold tolerance (Deng et al., 2015) |
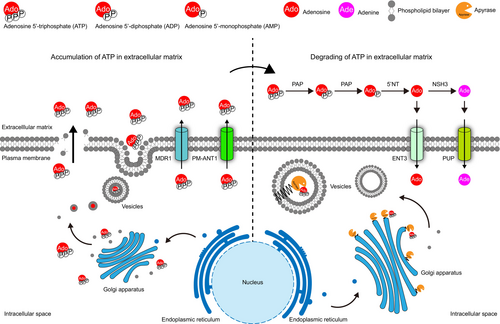
Mechanisms of ATP accumulating at ECM
Plants release ATP into the ECM during various growth phases and in response to various stimuli (Clark and Roux, 2011; Kim et al., 2006). Due to its high charge, ATP cannot freely diffuse across the PM, indicating the existence of ATP export mechanisms. Mechanical wounding disrupts the integrity of the PM and facilitates ATP release (Myers et al., 2022; Song et al., 2006). Additionally, the use of vesicle-trafficking inhibitors reduces eATP-mediated cytosolic calcium oscillations, suggesting that vesicle secretion serves as a major pathway for eATP release (Tanaka et al., 2010). ATP exporters, such as members of the multidrug resistance (MDR) transporter family, utilize ATP hydrolysis to pump out drugs and have been reported to mediate ATP release in mammalian and yeast cells (Boyum and Guidotti, 1997a, 1997b; Roman et al., 1997). Similarly, the Arabidopsis MDR1 homologue also facilitates ATP efflux (Thomas et al., 2000). Rieder and Neuhaus (2011) identified the PM-targeted mitochondrial carrier family (MCF)-type protein PM-ANT1 as being responsible for ATP export to the ECM, a process critical for anther dehiscence. Together, these mechanisms mediate ATP efflux from the plant cells.
Mechanisms of ATP degrading at ECM
As eATP acts as a signalling molecule in ECM and is released from cells under both normal growth conditions and stress stimuli (Clark and Roux, 2011), it is essential to regulate its concentration to maintain cellular homeostasis. In mammals, cell surface-anchored ecto-apyrases (nucleoside triphosphate diphosphohydrolases, NTPDases) are known to degrade eATP (Enjyoji et al., 1999), making plant apyrases the primary candidates for eATP hydrolysis. In Arabidopsis, two mammalian ecto-apyrase homologues, APY1 and APY2, have been studied. Although single knockout mutants exhibited no significant phenotypic differences from wild-type plants, double mutants (apy1-1/apy1-1, apy2-1/apy2-1 and apy1-1/apy2-1) demonstrated over-accumulation of eATP and male sterility due to pollen germination failure (Steinebrunner et al., 2003). Additionally, silencing of both APY1 and APY2 via RNAi can elevate eATP levels and trigger defence-like responses (Lim et al., 2014). Several studies have also confirmed that apyrases can hydrolyse ATP and that exogenous application of apyrases effectively eliminates eATP (Chivasa et al., 2005, 2009, 2010; Shi et al., 2023; Tanaka et al., 2011). These findings suggest that, similar to mammals, ecto-apyrases may be involved in eATP degradation in plants. However, emerging evidence has indicated that neither APY1 nor APY2 are associated with the PM; instead, both are localized in the Golgi apparatus (Chiu et al., 2012; Parsons et al., 2012; Zhang et al., 2024). A comprehensive investigation of the Arabidopsis apyrase family (APY1-7) revealed that all seven members are endo-apyrases located in the endomembrane system with distinct substrate preferences (Chiu et al., 2015). These results indicate that plant apyrases function within the endomembrane system, including the endoplasmic reticulum, Golgi apparatus and vesicles, to indirectly regulate eATP concentration.
As ecto-apyrases may not exist in plants, the true degraders of eATP in ECM remain unknown. Interestingly, a family of plant-secreted acid phosphatases (APases; EC 3.1.3.2), known as purple acid phosphatases (PAPs), have attracted attention owing to their secreted nature and ATP hydrolysis activity. To adapt to phosphorus (P) starvation, plants secrete PAPs into the ECM or rhizosphere to degrade organic phosphate (Po) and release inorganic phosphate (Pi) (Bhadouria and Giri, 2022). Different PAPs exhibit tissue-specific expression patterns, and studies have indicated that PAPs play a role in P recycling, remobilization and reallocation within cells and organs (Veljanovski et al., 2006; Wang et al., 2014). Several studies have demonstrated that ATP is a substrate for PAPs and that secreted PAPs can effectively hydrolyse eATP in the ECM, making them potential candidates for eATP degradation (Liang et al., 2010; Tian et al., 2012; Zhu et al., 2020) (Figure 3).
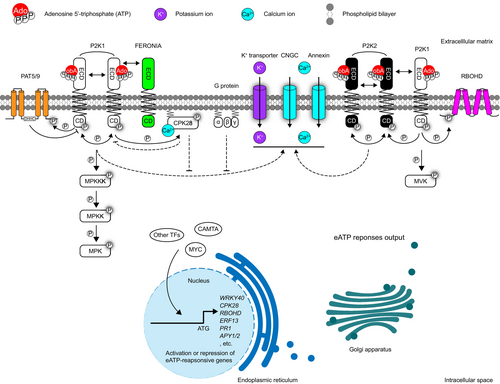
Signalling transduction events inside cells
As a regulatory signalling molecule, eATP is believed to be perceived by the corresponding receptors that initiate signal transduction. Growing evidence has shown that plants sense eATP in the PM (Demidchik et al., 2009; Jeter et al., 2004; Lew and Dearnaley, 2000). In mammalian cells, two types of eATP receptors embedded in the PM have been characterized: the P2X family consisting of ligand-gated ion channels with two transmembrane (TM) regions and a large extracellular loop responsible for ATP binding and the P2Y family comprising G protein-coupled receptors with seven TMs (Burnstock, 2007). Inspired by these findings, extensive efforts have been made to identify the eATP receptors in plants. However, no homologues of P2X and P2Y have been identified in plants, based on their protein sequences and structural similarities. Although the existence of an eATP receptor in plants has been suggested by various clues, it was not identified until the discoveries made by Choi et al. (2014) who revealed the first purinergic receptor in plants. Using a forward genetic approach and aequorin (AEQ)-based calcium influx assays, they identified the ethyl methanesulfonate (EMS)-mutagenized Arabidopsis mutants, which were insensitive to eATP and designated as does not respond to nucleotides 1 (dorn1). Map-based cloning revealed that DORN1 encodes the L-type lectin receptor kinase-I.9 (LecRK-I.9). Biochemical data confirmed that DORN1 binds ATP through its ecto-domain (ECD) and maintains its autophosphorylation activity in its cytoplasmic kinase domain (CD) (Choi et al., 2014). Following this discovery, DORN1 was renamed P2K1 (P2-type purinoreceptor kinase 1) to align with the P2-type purinoreceptor nomenclature used for similar receptors in mammals.
As the second eATP receptor, P2K2 partially restores the impaired response to eATP in the p2k1–3 mutant background. P2K2 is a functional kinase with ATP binding capacity in its ECD (Pham et al., 2020). It self-associates and interacts with P2K1, with P2K1 phosphorylating P2K2, but not the other way around (Pham et al., 2020). Unlike the ubiquitous expression pattern of P2K1, P2K2 is primarily expressed at the root tip and expressed at the lower levels in the root and mesophyll cells (Zhao et al., 2023). Although P2K2 exhibits a stricter nucleotide preference than P2K1, genetic evidence indicates partial functional redundancy between P2K1 and P2K2 in plant immunity (Pham et al., 2020).
Starting with the eATP receptor P2K1 in the PM, downstream signalling components have been identified using various approaches. Given the kinase activity of P2K1, a kinase client assay (KiC) was adopted to identify the substrates of its kinase domain (Chen et al., 2017). One of the identified phosphorylated peptide corresponds to RBOHD, a PM-localized protein responsible for ROS production. RBOHD can associate with P2K1 in planta, and this interaction is enhanced by eATP, revealing a direct regulatory mechanism linking eATP signalling to ROS accumulation (Chen et al., 2017). Another substrate identified using the KiC method is Raf-like MPK kinase kinase (MPKKK) integrin-linked kinase 5 (ILK5). Kim et al. demonstrated that P2K1 interacts with and phosphorylates ILK5, which, in turn, phosphorylates the downstream target MPK kinase 5 (MKK5), an upstream regulator of MPK3/6 (Kim et al., 2023a). This elucidates a missing link between P2K1 activation and MPK3/6 phosphorylation. Additionally, another receptor-like kinase, FERONIA (FER), has been identified as a P2K1 interactor in the PM. Although eATP signalling was disrupted in the fer-4 mutant, the precise molecular mechanisms remain unclear (Sowders et al., 2024b).
Cytosolic Ca2+ ([Ca2+]cyt) spiking is an early event in the eATP response (Tanaka et al., 2010) mediated by Ca2+ influx from the extracellular space (Wang et al., 2018b). Recent studies have identified PM-localized Ca2+ channels, including cyclic nucleotide-gated channels (CNGC) 2, 4 and 6, as key mediators of eATP-induced Ca2+ influx (Duong et al., 2022; Wang et al., 2022b; Wu et al., 2021). Furthermore, Annexin 1 can contribute to the eATP-induced increase in [Ca2+]cyt (Mohammad-Sidik et al., 2021). Impaired eATP perception inhibits the [Ca2+]cyt spike (Choi et al., 2014; Pham et al., 2020), highlighting a direct link between eATP signalling and Ca2+ channel regulation. However, the key components of eATP signalling responsible for modulating these channels remain unknown. Furthermore, electrophysiological analyses have revealed P2K1-dependent K+ influx in response to eATP treatment, though the significance of this K+ influx and the specific transporters involved are yet to be clarified (Wang et al., 2018b).
Negative regulators of eATP signalling have been identified in P2K1 interactors. Calcium-dependent protein kinase 28 (CPK28) is a direct substrate of P2K1 that can be upregulated upon eATP treatment and functions as an inhibitor of eATP responses (Choi et al., 2014; Sowders et al., 2024a), indicating the presence of a negative regulatory loop in eATP signalling. Interestingly, previous studies identified CPK28 as a negative regulator of plant immunity (Monaghan et al., 2014). Botrytis-induced kinase 1 (BIK1) phosphorylates and activates CPK28, which in turn phosphorylates BIK1 and the E3 ligases plant U-box 25 (PUB25) and PUB26. These E3 ligases target BIK1 for ubiquitin-mediated degradation, thereby suppressing the plant immune responses (Bredow et al., 2021; Monaghan et al., 2014; Wang et al., 2018a). Additionally, P2K1 undergoes S-acylation mediated by its interaction with S-acyltransferases (PATs), and PAT5/9 can negatively regulate eATP-mediated immune responses by inhibiting P2K1 kinase activity (Chen et al., 2021). These findings suggest a link between eATP signalling and PTI. Although there is evidence implicating G proteins in eATP signalling, the detailed molecular mechanisms remain unclear (Hao et al., 2012; Weerasinghe et al., 2009; Wu et al., 2021; Zhu et al., 2017) (Figure 4).
Methods of eATP measurement
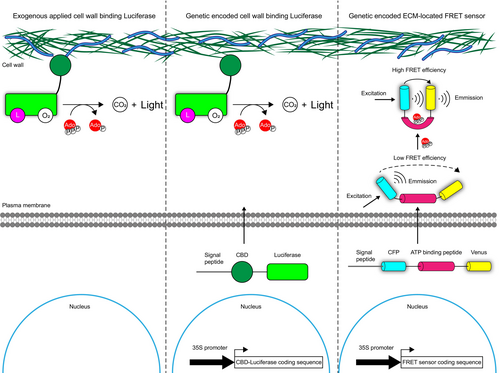
Traditional methods for determining eATP concentrations involve extracting extracellular fluid from plant tissues or collecting secreted eATP in a liquid medium (Ramachandran et al., 2019; Song et al., 2006). However, these approaches often fail to capture real-time eATP dynamics and pose a risk of contamination by intracellular ATP. The luciferase–luciferin system, in which luciferase catalyses luminescence in a reaction dependent on ATP concentration, serves as a powerful indicator of eATP levels. Cell wall-localized luciferase effectively hydrolyses exogenous luciferin, enabling the visualization and quantification of eATP. Studies have demonstrated the successful application of luciferase systems to both visualize and quantify eATP concentrations (Kim et al., 2006, 2023b; Myers et al., 2022). Clark et al. (2011) developed a modified luciferase-based method using signal peptides to guide luciferase to the ECM, creating transgenic reporter plants to monitor eATP dynamics during ABA-induced stomatal movement. More recently, a genetically encoded Förster resonance energy transfer (FRET)-based technology has been introduced to trace eATP changes (Shi et al., 2023). These advanced methods enable visualization, quantification and monitoring of eATP and offer robust tools for eATP research. At present, luciferase-based in vivo imaging is the most widely used method for quantification and visualization of eATP. Compared with extracellular fluid extraction, luciferase-based in vivo imaging reflects the concentration and dynamic of eATP more accurately and efficiently. In contrast, FRET sensor technology is a non-invasive method that can better reflect the spatiotemporal dynamics of eATP at subcellular scale without requirement of reagents. However, compared with luciferase-based in vivo imaging method, FRET sensor has a narrower detection range and lower sensitivity to eATP (Kim et al., 2023b; Myers et al., 2022; Shi et al., 2023) (Figure 5 and Table 4).
| Plant species | Organ/Tissue | Treatment | Detection method | Detection range | Concentration |
|---|---|---|---|---|---|
| Arabidopsis thaliana | Leaf | Wounding | Extraction of extracellular fluid | Depending on the commercial ATP detection kit, the detection range is usually between 1 nM and 10 μM | 40 ± 22 μM (Song et al., 2006) |
| Nicotiana benthamiana | Leaf | Xylanase induced Systemic acquired resistance | Collection of guttation fluid | 100 ~ 200 nM (Chivasa et al., 2010) | |
| Arabidopsis thaliana | Suspension cells | Salt and osmotic stresses | Collection of suspension cell medium | 0 ~ 30 nM (Ramachandran et al., 2019) | |
| Arabidopsis thaliana | Leaf | Wounding | Luciferase-based in vivo imaging | Accurate quantitative relationship between 0 and 140 nM | ~ 35 nM (Myers et al., 2022) |
| Arabidopsis thaliana | Leaf | Salt stress | Luciferase-based in vivo imaging | ~ 8 nM (Kim et al., 2023b) | |
| Arabidopsis thaliana | Whole plant | Salt and wounding | FRET sensor | 0.1–2.5 mM of exogenous applied ATP | Not determined at physiological situations (Shi et al., 2023) |
Perspectives of future research
Dissecting eATP signalling in crop plants
Currently, most research in this field has focused on the model dicot plant Arabidopsis, whereas eATP signalling in crops, such as rice, maize, wheat and tomato, remains largely unexplored. In Arabidopsis, eATP regulates various biological processes relevant to crop research (Clark et al., 2024; Sabharwal et al., 2022; Veerappa et al., 2019; Yang et al., 2015). Thus, investigating the role of eATP and its signalling components in crops is a promising strategy for genetic improvement. The primary task is to clarify the biological effects of eATP on crops, and analyse the similarity and differences of eATP signalling network between crops and Arabidopsis.
Degradation mechanisms of eATP at ECM
An incomplete eATP degradation pathway, lacking the initial step, has been identified. Extracellular ATP and ADP are hydrolysed by an unknown enzyme to produce AMP. AMP is then converted to eAdo by 5′NT (Zrenner et al., 2006). eAdo is either transported into the cytoplasm by ENT3 (Traub et al., 2007) or catalysed by NSH3 to produce adenine (Ade) (Jung et al., 2011), which is subsequently transported into the cell by PUP (Möhlmann et al., 2014).
Unlike mammals, none of the Arabidopsis apyrases target the PM. They function within the endomembrane system, including the Golgi apparatus, endoplasmic reticulum and vesicles. These apyrases hydrolyse ATP intracellularly, limiting ATP release and indirectly reducing eATP concentration. However, this method is inefficient for responding rapidly to external changes. For instance, the eATP concentration peaks 1 h after osmotic stress treatment, whereas APY1 and APY2 expression remained uninduced. By 3 h post-treatment, the eATP concentration declined by 50%, coinciding with the peak expression of APY1 and APY2 (Kim et al., 2009). This temporal lag between eATP reduction and APY1/APY2 induction suggests that APY1 and APY2 are not the primary factors responsible for eATP degradation.
To address this issue, the functional roles of PAPs in eATP degradation should be inspected. Besides, a systemic survey of apyrase should be conducted in plant kingdom to find out if there are real ecto-apyrases in plants.
Molecular mechanisms for attenuating eATP responses
Balancing growth and defence is crucial for plant survival, as the constitutive activation of defence signalling often impairs growth. For instance, the constitutive activation of JA signalling in the higher order jaz mutant jazD enhances herbivore and fungal resistance, but results in stunted vegetative growth and reproductive deficiencies (Guo et al., 2018). Similarly, excessive eATP accumulation leads to the growth retardation and defects in pollen germination (Steinebrunner et al., 2003; Wolf et al., 2007; Wu et al., 2007). To prevent the overactivation of defence pathways, PM-localized receptors are regulated by negative feedback mechanisms. For instance, flagellin-sensing 2 (FLS2) undergoes polyubiquitination by PUB12 and PUB13 to promote its turnover and prevent excessive PTI response (Lu et al., 2011). Additionally, the S-acyltransferases PAT5 and PAT9 can inhibit the kinase activity of P2K1 through S-acylation (Chen et al., 2021). Furthermore, CPK28 serves as a substrate for P2K1 and can suppress eATP responses via an unknown mechanism (Sowders et al., 2024a). These findings highlight the negative regulatory mechanisms of eATP signalling, demonstrating the need for further research to expand our understanding of this area. Specifically, CPK28 is a known suppressor of PTI response (Monaghan et al., 2014). The mechanism by which CPK28 inhibits eATP response is still unknown, and whether CPK28 mediates eATP and PTI signalling crosstalk needs to be investigated. Moreover, the degradation mechanisms of key components of eATP signalling such as P2K1 remains to be explored.
Transcriptome reprograming mechanisms in response of eATP stimuli
Transcriptome analysis has revealed that most, though not all, eATP-responsive genes can also be regulated by JA signalling (Jewell et al., 2019). However, no clear intersection between the eATP and JA signalling pathways has been identified to date, suggesting that eATP likely regulates gene expression independently of the JA signalling pathway. The possibility that a transcription factor (TF) regulated by the upstream eATP receptor is responsible for modulating the expression of eATP-responsive genes remains an open question. In addition to specific TF, epigenetic regulation, kinase activity changes also mediate transcriptome reprogramming. Therefore, a systemic analysis needs to be conducted to dissect the mechanisms of plant response to eATP stimuli at transcriptional, chromatin and protein levels.
Mechanisms behind plants' biphasic response to ATP treatment
Many studies have shown that plants exhibit distinct responses to low and high ATP concentrations, indicating a dose-dependent response to eATP. In Arabidopsis, low ATP concentrations can promote stomatal opening, whereas high concentrations can induce stomatal closure (Clark et al., 2011). In cotton (Gossypium hirsutum), low concentrations (30 μM) of ATPγS and ADPβS increased average fibre length, whereas 150 μM of these compounds suppressed fibre growth (Clark et al., 2010). Similarly, we observed that low ATP concentrations promoted root growth in rice, whereas high concentrations inhibited it. Interestingly, eATP regulates multiple aspects of plant growth and development, but p2k1 mutants showed normal growth phenotypes which cannot be distinguished from wild-type plants, suggesting the existence of other eATP sensing system (Roux, 2014; Zhu et al., 2017). In summary, these observations suggest the possibility of two distinct eATP-sensing systems in plants, a hypothesis that warrants further investigation. In order to identify new eATP receptors, taking advantage of root inhibition assay is a promising strategy, because eATP treatment disrupts Auxin distribution to inhibit root growth in Arabidopsis. It can be useful supplement to AEQ report system.
Mechanism of coordinating intracellular energy status and eATP level
As mentioned earlier, the eATP comes from intracellular ATP (iATP) through diverse routes, and the level of iATP reflects intracellular energy status (Xiao et al., 2024). Therefore, we wondered if energy status within cell affects eATP concentration. Previous work reported that eATP release linked to cell metabolism (Shan et al., 2022). Shi et al. demonstrated that treatment of antimycin A, an inhibitor of mitochondrial electron transport complex III, decreased the eATP concentration slightly (Shi et al., 2023). In turns, eATP signalling shapes cell metabolism landscape (Cho et al., 2022; Jewell et al., 2022) and depletion of eATP elicits the accumulation of intracellular ATP synthases (Chivasa et al., 2011). These data indicated that eATP and iATP are mutually regulated. The destination of iATP after synthesis, most of it is used intracellularly to maintain the energy homeostasis, and the rest may be released to ECM as signalling molecule. Therefore, the mechanism of coordinating intracellular energy status and eATP level is an interesting topic. We believe that investigating the molecular mechanism of ATP synthase accumulation induced by eATP depletion is good start point for answering this question.
Acknowledgements
This work is supported by the National Science Foundation of China (32302379, 32372482) to DPY and YHX. This work is also funded by the China Postdoctoral Science Foundation (2024M751528) to DPY.
Conflicts of interest
The authors declare no conflict of interest.
Author contributions
DPY and YHX conceived the article; DPY and DK wrote the manuscript; DPY prepared the artwork. All authors read and approved of the content of this article.
Open Research
Data availability statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.




