Manipulation of the brown glume and internode 1 gene leads to alterations in the colouration of lignified tissues, lignin content and pathogen resistance in wheat
Summary
Lignin is a crucial component of the cell wall, providing mechanical support and protection against biotic and abiotic stresses. However, little is known about wheat lignin-related mutants and their roles in pathogen defence. Here, we identified an ethyl methanesulfonate (EMS)-derived Aegilops tauschii mutant named brown glume and internode 1 (bgi1), which exhibits reddish-brown pigmentation in various tissues, including internodes, spikes and glumes. Using map-based cloning and single nucleotide polymorphism (SNP) analysis, we identified AET6Gv20438400 (BGI1) as the leading candidate gene, encoding the TaCAD1 protein. The mutation occurred in the splice acceptor site of the first intron, resulting in a premature stop codon in BGI1. We validated the function of BGI1 using loss-of-function EMS and gene editing knockout mutants, both of which displayed reddish-brown pigmentation in lignified tissues. BGI1 knockout mutants exhibited reduced lignin content and shearing force relative to wild type, while BGI1 overexpression transgenic plants showed increased lignin content and enhanced disease resistance against common root rot and Fusarium crown rot. We confirmed that BGI1 exhibits CAD activity both in vitro and in vivo, playing an important role in lignin biosynthesis. BGI1 was highly expressed in the stem and spike, with its localisation observed in the cytoplasm. Transcriptome analysis revealed the regulatory networks associated with BGI1. Finally, we demonstrated that BGI1 interacts with TaPYL-1D, potentially involved in the abscisic acid signalling pathway. The identification and functional characterisation of BGI1 significantly advance our understanding of CAD proteins in lignin biosynthesis and plant defence against pathogen infection in wheat.
Introduction
Bread wheat (Triticum aestivum L.) is a crucial staple crop worldwide, contributing about 20% of caloric and protein intake for the human population. However, wheat production is threatened by various factors, including diseases and lodging. Common root rot (CRR) caused by Bipolaris sorokiniana and Fusarium crown rot (FCR) caused by multiple Fusarium species are important diseases in many arid and semi-arid cropping regions worldwide, such as Australia, the United States, Canada, China and South Africa (Bozoğlu et al., 2022; Kazan and Gardiner, 2018). These two diseases are major concerns in regions with extensive wheat-maize rotation and straw returning practices, as seen in China (Su et al., 2021). In addition to diseases, lodging is also a significant limiting factor for wheat production, reducing grain yield and causing several knock-on effects, including decreased grain quality and increased drying costs (Berry and Spink, 2012). Enhancing lignin content to improve lodging resistance is a crucial breeding objective (Zhang et al., 2016).
Lignin is a complex and heterogeneous aromatic polymer that forms the secondary cell wall together with cellulose and hemicellulose. Lignin plays an important role in mechanical support, water conductance and protection against biotic and abiotic stresses during plant growth and development (Barros et al., 2015; Gallego-Giraldo et al., 2020). Lignin is gaining increasing attention due to its crucial role in enhancing both abiotic stress tolerance and pathogen resistance (Lee et al., 2019; Rong et al., 2016; Xu et al., 2020). The synthesis of lignin involves the polymerisation of monolignols, which are produced through the phenylpropanoid pathway and subsequently undergo hydroxylation and methylation processes (Boerjan et al., 2003; Ralph et al., 2004). Polymerisation of the three main monolignols (p-coumaryl, coniferyl and sinapyl alcohols) resulted in the formation of various monomers, namely p-hydroxyphenyl (H), guaiacyl (G) and syringyl (S), respectively (Vanholme et al., 2010). The monomers undergo oxidation by peroxidases and/or laccases, resulting in the formation of monolignol radicals that combinatorially couple with each other and with the developing lignin polymer.
Cinnamyl alcohol dehydrogenase (CAD) plays a crucial role in the lignin biosynthesis pathway. It is responsible for catalysing the nicotinamide adenine dinucleotide phosphate (NADPH)-dependent reduction of cinnamaldehydes to cinnamyl alcohol, the final step in monolignol biosynthesis before polymerisation in the cell wall (Goffner et al., 1992; Vanholme et al., 2010). Disruption of CAD activity significantly increases the incorporation of cinnamaldehyde into the lignin polymer (Liu et al., 2021c). Plants with impaired CAD function may exhibit delayed flowering, reduced plant height and a characteristic brownish-red to tan pigmentation in the leaf midrib, particularly in C4 grasses (Chen et al., 2012; Liu et al., 2021c; Trabucco et al., 2013). Previous studies indicated that a high level of coniferyl aldehyde leads to the reddish-brown colouration (Tsai et al., 1998). Complete genome sequencing and annotation have enabled the determination of the number of CAD genes in various species, which has led to their classification into three classes (Barakat et al., 2009). Class I CADs exhibit a highly conserved primary sequence structure across most species and are involved in lignin deposition in the secondary cell walls during plant growth and development (Park et al., 2018). Class II CADs represent a more extensive and diverse group of CAD isoforms, known to be associated with stress resistance (Rong et al., 2016). Class III CAD members may show redundancy with Class I CADs, yet their precise functions remain unclear (Peracchi et al., 2024; Xu et al., 2011).
In Arabidopsis, although nine CAD genes have been identified in the published reference genome (Sibout et al., 2005), only three (AtCAD1, AtCAD4 and AtCAD5) have been validated as the enzymes involved in monolignol biosynthesis (Rong et al., 2016). Similarly, among the 12 CAD genes identified in the rice genome, OsCAD2 has been directly linked to lignin biosynthesis (Zhang et al., 2006), while OsCAD7 plays a role in culm mechanical strength (Li et al., 2009). In maize, ZmCAD2 (GRMZM5G844562) is a member of the CAD family that plays a crucial role in lignification. Mutants exhibiting brown leaf midribs were first identified in ZmCAD2, leading to their designation as brown midrib1 (bm1) (Chen et al., 2012). In sorghum, the bmr6 phenotype resulted from a mutation in the CAD gene, which is orthologous to the maize BM1 gene (Saballos et al., 2009). The brown midrib trait is a valuable genetic characteristic that enhances the digestibility and quality of maize and sorghum, especially in livestock feed and bioenergy production. Recently, in silico analysis of the hexaploid wheat genome has revealed 47 high-confidence TaCAD gene copies (Peracchi et al., 2024). Among these TaCAD genes, TaCAD1 was speculated to be involved in lodging resistance (Chen et al., 2021; Ma, 2010), and TaCAD12 was found to contribute to host resistance against sharp eyespot (Rong et al., 2016). However, the functionality of these genes has not been experimentally verified in wheat.
The challenges posed by the size and complexity of the bread wheat genome have historically impeded the identification and characterisation of TaCAD genes. Currently, there is a lack of data on lignin-related CAD mutants or overexpression in wheat. This study addresses this gap by using a lignin-deficient mutant called brown glume and internode 1 (bgi1) identified from an EMS-mutagenised library of the diploid Aegilops tauschii accession PI 511383. The bgi1 mutant plants exhibit a reddish-brown pigmentation in various tissues, including internodes, spikes and glumes. Through map-based cloning and single nucleotide polymorphism (SNP) analysis, we isolated the BGI1 gene, which encodes the TaCAD1 protein. We validated the function of the cloned candidate gene using loss-of-function EMS and gene editing knockout mutants. Furthermore, we investigated the regulatory mechanisms determining the role of BGI1 in lignin biosynthesis and demonstrated that BGI1 overexpression increases lignin content and enhances resistance against both CRR and FCR.
Results
Characterisation of the bgi1 mutant
We identified a M2 mutant line, M326 (bgi1), in an EMS-mutagenised population of the Ae. tauschii accession PI 511383. bgi1 mutants exhibited normal development under greenhouse conditions (Figure 1a). However, at the flowering stage (Z65, Zadoks growth scale), various parts of the bgi1 mutant plants, including the internode, spike, spike rachilla, glume and lemma, had a reddish-brown pigmentation (Figure 1b–g). The reddish-brown pigmentation became evident at the jointing stage and reached its peak at the flowering stage. The spikes showed a reddish-brown colouration (Figure 1c), and the node of the spike rachilla facing the spikelet displayed the most vivid reddish-brown hue (Figure 1d). Interestingly, the interior of the glume displayed a deeper hue compared to the exterior (Figure 1e,f).
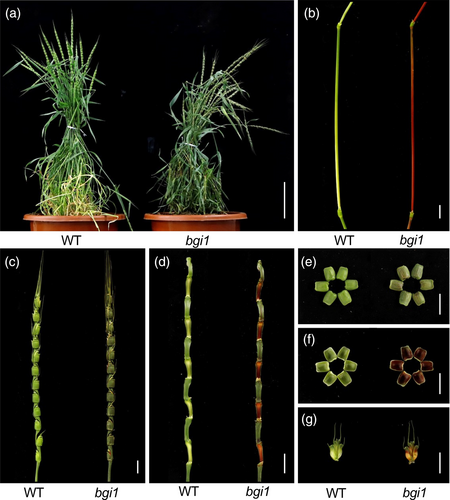
Crossing the bgi1 mutant with the WT (PI 511383) produced F1 plants that uniformly exhibited the WT phenotype. Within a subset of 94 F2 plants derived from this cross, 68 plants displayed normal colouration while 26 plants exhibited reddish-brown glumes and internodes, which fits the expected 3:1 segregation ratio for a single recessive gene (χ2 = 0.35, P = 0.55).
Map-based cloning of bgi1
To map the causal gene, two F2 mapping populations were generated: one comprising 182 F2 plants derived from the M326 × AL8/78 cross and the other comprising 1872 F2 plants from the M326 × PI 511383 cross. Through RNA sequencing and SNP calling, 225 high-confidence EMS-induced SNPs were identified between M326 and PI 511383. Bulked segregant RNA sequencing (BSR-Seq) analysis revealed 18 SNPs on chromosome 6D, ranging from 40.2 to 324.1 Mb (AL8/78 Aet v4.0; Table S1), which are significantly associated with the phenotype in the M326 × PI 511383 population. This finding indicates that the bgi1 gene is located on chromosome 6D.
To confirm the map location, seven SNPs within the candidate region on chromosome 6D (Table S1) were selected for developing D-genome-specific cleaved amplified polymorphic sequence (CAPS) or kompetitive allele-specific PCR (KASP) markers (Table S2). Genotyping of 94 F2 plants from the M326 × PI 511383 cross with these markers pinpointed the bgi1 gene within a 2.2-cM interval flanked by CAPS markers pkw1225 and pkw2754 (Figure 2b). In the mapping population derived from the M326 × AL8/78 cross, ten PCR markers were developed (Table S2), mapping bgi1 within a 1.65-cM interval flanked by markers ucw139 and ucw239 (Figure S1). Notably, both populations exhibited recombination suppression in regions approximately from 157 to 239 Mb of chromosome 6D.
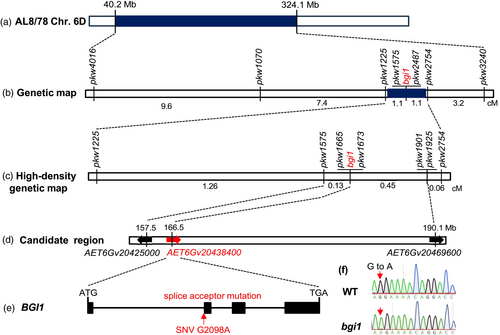
To further narrow down the chromosome location of bgi1, an additional 1778 F2 plants from the M326 × PI 511383 cross were screened for recombinants between markers pkw1225 and pkw2754. Using these recombinants and four newly developed markers (Table S2), the bgi1 gene was further mapped to a 0.58-cM region flanked by markers pkw1575 and pkw1901, and was completely linked to markers pkw1665 and pkw1673 (Figure 2c). No recombination was detected within the bgi1 candidate region, likely due to its proximity to the centromere.
The 0.58-cM candidate region encompasses a 32.6-Mb region (157.5–190.1 Mb) in the Ae. tauschii reference genome (AL8/78 Aet v4.0), containing 213 annotated high-confidence genes (Table S3). RNA sequencing results identified only two high-confidence SNPs (G166511490A and G167343806A) between M326 and PI 511383 within the candidate region (Table S1). The SNP, G167343806A, is located within the 5′ untranslated region (UTR) or the intron of AET6Gv20439300 (depending on the alternative splice forms), which encodes a glycerophosphodiester phosphodiesterase protein. Analysis of published RNA-seq data from the wheat expVIP database (https://www.wheat-expression.com/) revealed very low expression of this gene in the stem and spike (Figure S2a), suggesting it is unlikely to be the bgi1 gene.
The other SNP, G166511490A, located within the candidate gene AET6Gv20438400, is a splice acceptor mutation (Figure 2e). Expression profile analysis revealed that AET6Gv20438400 is highly expressed in various wheat tissues, particularly in the stem and spike (Figure S2b). Sanger sequencing confirmed the presence of a mutation (G > A) at the splicing acceptor site of the first intron of the candidate gene AET6Gv20438400, leading to premature termination of protein (Figure 2f).
AET6Gv20438400 (BGI1) spans 4233 bp from the start to the stop codon, comprising four exons and three introns (Figure 2e). The complete coding sequence of 1083 bp encodes a predicted protein of 360 amino acids. BGI1 is orthologous to the maize gene BM1 (Halpin et al., 1998), the rice gene GH2 (Zhang et al., 2006), the sorghum gene BMR6 (Li et al., 2015) and the Brachypodium distachyon gene BdCAD1 (Bouvier d'Yvoire et al., 2013). Phylogenetic analysis showed that BGI1 was grouped in a clade with these known CAD proteins (Figure S3). Mutations in these CAD genes are associated with pigmentation changes in lignified tissues (Bouvier d'Yvoire et al., 2013; Halpin et al., 1998; Li et al., 2015; Zhang et al., 2006). These results make BGI1 a highly promising candidate.
Loss-of-function EMS mutations in BGI1 homeologs showed reddish-brown phenotypes
Within the tetraploid and hexaploid wheat genomes, the homeologs of BGI1 exhibit a protein similarity exceeding 98.9% (Figure S4), suggesting potential functional redundancy. To confirm the casual relationship between BGI1 and observed phenotypic alterations, two mutant lines, K3288 and K3088, were selected from a sequenced mutant population of the tetraploid wheat variety Kronos (Krasileva et al., 2017). The mutant line K3288 harbours a single nucleotide mutation in the fourth exon of the A-genome copy (C3623T), resulting in a premature stop codon (Q312*; Figure 3a). Similarly, the mutant line K3088 carries a G42A mutation in the first exon the B-genome copy, leading to a premature stop codon (W14*; Figure 3a). Using genome-specific markers K3288-A1 and K3088-B1 (Table S2), we identified mutant plants homozygous for either the bgi-A1 or bgi-B1 allele (Figure 3b). In greenhouse experiments, neither bgi-A1 nor bgi-B1 homozygous plants exhibited reddish-brown phenotypes. Subsequently, we crossed the K3288 and K3088 mutants, and selected F2 plants homozygous for all four possible combinations of the BGI1 homeologs-WT, bgi-A1, bgi-B1 and bgi1 double mutants for further evaluation. In this experiment, only the bgi1 double mutant plants displayed reddish-brown pigmentation at the flowering stage (Z61) while neither the bgi-A1 nor the bgi-B1 mutants exhibited phenotypic alterations (Figure 3c–i). These results demonstrate that BGI-A1 and BGI-B1 genes exhibit functional redundancy in tetraploid wheat.
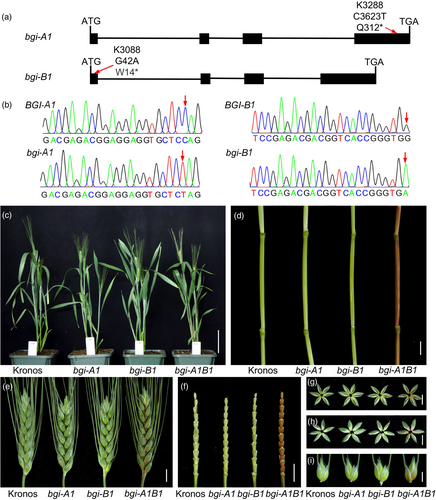
In the sequenced EMS-mutagenised population of hexaploid wheat variety Cadenza (Krasileva et al., 2017), we selected truncation mutations for the A, B and D genome homeologs of BGI1 (Figure S5a). To generate loss-of-function EMS mutants in hexaploid wheat background, we combined the splice site mutations in the A- and B-genome homeologs (bgi-A1 and bgi-B1) with a premature stop codon mutation (Q322*) in the D-genome homeolog (bgi-D1) by crossing and selection in the F2 generation. The procedure for generating single-gene mutants, double mutants and the bgi1 triple mutant is detailed in Figure S6. Phenotypic analysis revealed that only the bgi1 triple mutant plants exhibited a reddish-brown phenotype at the flowering stage (Z61), and none of the single or double mutants showed phenotypic alterations (Figure S5b–h). These results confirm the redundant roles of these three homeologs in determining the colouration of glumes and internodes.
Validation of BGI1 using barley stripe mosaic virus (BSMV)-sgRNA-based gene editing
To validate the phenotypic observations associated with the EMS-induced bgi1 mutant, we employed a BSMV-sgRNA-based gene editing approach (Li et al., 2021) to generate independent edited wheat plants. We designed an sgRNA that specifically targeted a conserved region in the first exon, enabling the simultaneous knockout of all three BGI1 homeologs (Figure 4a). The wheat transgenic line expressing the Cas9 gene in the Bobwhite genetic background (Cas9-transgenic Bobwhite) was infected with BSMV vectors. Of the eleven infected M0 plants in Cas9-transgenic Bobwhite, ten were successfully edited, as confirmed by genotyping with the CAPS marker HL553 from flag leaf DNAs (digested with PshAI; Figure S7a,b).
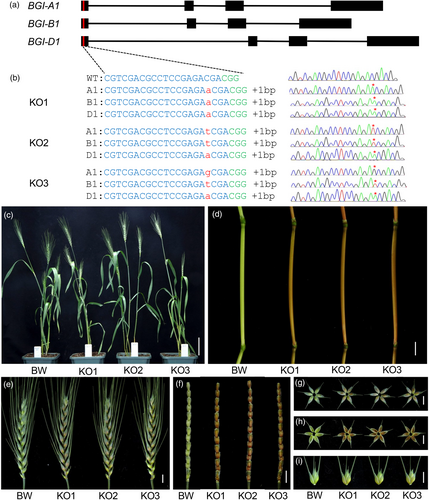
Subsequently, we genotyped 99 M1 plants derived from the selected M0 edited plants using the genome-specific markers HL554, HL555 and HL556 (Table S2). Sanger sequencing revealed mutations in 87 of the 99 M1 plants tested, yielding a mutation frequency of 87.9% (Table S4). Among these 87 edited M1 plants, 41 harboured mutations in all three BGI1 homeologs, with three being homozygous knockout (KO) mutants wherein six alleles were edited concurrently (referred to as KO1, KO2 and KO3; Table S4).
These three independent homozygous KO mutant lines carry insertions of an ‘A’, ‘T’ or ‘G’ at position 25 from the ATG (3 bp upstream of the CGG PAM site) in all three BGI1 homeologs (Figure 4b). These frameshift insertions alter more than 97.2% of the protein sequences, resulting in loss-of-function in all three homeologs. Phenotypic investigations showed that single and double mutant plants exhibit no phenotypic changes (Figure S8). In contrast, the homozygous KO mutant lines KO1, KO2 and KO3 exhibited reddish-brown pigmentation in the internode, spike, spike rachilla, glume and lemma at the flowering stage (Figure 4c–i).
Taken together, the genetic mapping, SNP analysis, the loss-of-function EMS mutations and BSMV-sgRNA-based gene editing demonstrated that BGI1 is the causal gene responsible for the bgi1 mutant phenotype.
Disrupting or overexpressing BGI1 results in significant changes in lignin content and composition
To investigate the cellular basis of the reddish-brown colouration observed in the mutant line KO1, we performed histological examinations of transverse sections from internodes at the flowering stage (Z61). Reddish-brown pigmentation was predominantly localised to lignified tissues, mainly in the mechanical tissue (MT) and vascular bundle (VB) regions (Figure 5a,d). Subsequent staining with Wiesner reagent revealed pronounced differences between WT and KO1 mutant samples (Figure 5b,e). The cross-sections of WT displayed a purple hue from the epidermal layer to the MT and VB regions (Figure 5b), whereas the KO1 mutant exhibited significantly reduced staining (Figure 5e), indicating a lower level of lignin accumulation in the mutant internodes. Mäule staining (Meyer et al., 1998), which differentiates G residues as yellow and S residues as red, was used to assess lignin composition. The strong red colouration of lignified tissues observed in WT was significantly reduced in the KO1 mutant samples (Figure 5c,f), suggesting that KO1 impedes the formation of S residues in lignified stem tissues. Apart from the differences mentioned, no other significant differences were observed between WT and KO1 plants across the seven agronomic traits examined under greenhouse conditions (Figure S9).
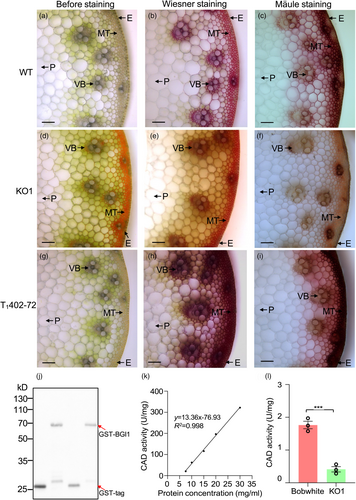
To determine the impact of BGI1 overexpression on lignin content, we generated nine independent T0 transgenic plants overexpressing BGI1 driven by the maize ubiquitin (UBI) promoter. Transcript levels of BGI1 were significantly higher (P < 0.001) in all transgenic plants compared to the Fielder control (Figure S10). When stained with Wiesner reagent, transverse sections of plants from transgenic family T1402-72 displayed significantly increased staining relative to WT (Figure 5h), indicating higher lignin content. When subjected to Mäule staining, the MT and VB regions exhibited intense red staining in the T1402-72 plants (Figure 5i), suggesting that BGI1 overexpression enhances the formation of S units in the stems.
To quantitatively evaluate lignin content, Klason lignin analyses were carried out on dried internodes to determine the lignin content in WT, KO1 and OE transgenic plants. The total lignin content and Klason lignin levels were significantly reduced in KO1 compared to WT while both were significantly increased in T1402-72 and T1402-73 transgenic plants (P < 0.05; Figure S11). At the flowering stage, the penultimate internodes from WT, KO1 and OE transgenic plants were cut into equal-length segments (~10 cm) for shearing force measurement. KO1 mutant plants exhibited a significant reduction in shearing force compared to WT plants, whereas OE transgenic plants showed an increased shearing force relative to WT (Figure S12).
Overexpression of BGI1 enhances resistance to both CRR and FCR
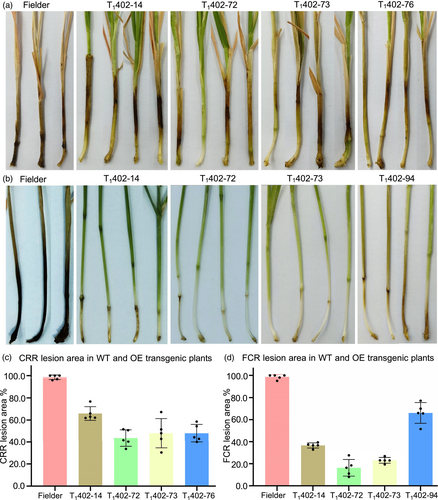
Lignin accumulation in cell walls can establish mechanical barriers to pathogen invasion and has been associated with plant defence against pathogen infection (Rong et al., 2016; Tronchet et al., 2010). Based on the observed high accumulation of lignin in the stems of BGI1-OE transgenic lines, we hypothesised that the plant resistance to stem base rot diseases such as CRR and FCR will be enhanced by overexpression of BGI1. To test this hypothesis, we challenged independent OE transgenic lines and the Fielder control with CRR and FCR in growth chambers. All T1 transgenic plants containing the transgene exhibited increased resistance against both CRR and FCR compared to Fielder (Figures 6 and S13). The average lesion area in OE transgenic lines was significantly smaller (P < 0.001) than in the Fielder control (Figure 6c,d). A negative correlation was observed between BGI1 overexpression levels and average lesion area (R = −0.76). These findings demonstrate that BGI1 overexpression enhances resistance to both CRR and FCR. Both KO1 mutant plants and the Bobwhite control exhibited high susceptibility when challenged with CRR or FCR, with no significant difference observed between them (Figure S14).
To further investigate the underlying mechanism of enhanced resistance, we analysed the transcript levels of three pathogenesis-related (PR) genes in the stems of OE transgenic plants and Fielder at the flowering stage. qRT-PCR experiments revealed that the transcript levels PR1, PR4 and PR5 showed no significant difference between OE transgenic plants and the Fielder control (Figure S15). These findings suggest that the enhanced resistance to pathogen infection in OE transgenic plants may be attributed to increased lignin content and the associated mechanical barrier rather than induced systemic resistance.
BGI1 has CAD activity
To characterise the enzymatic activity of BGI1, a fusion protein of glutathione S-transferase (GST)-BGI1 was expressed and purified in Escherichia coli expression system. Western blot analysis confirmed the expression of the recombinant GST-BGI1 protein with an expected molecular weight of 66 kD (Figure 5j). The enzymatic activity of the purified recombinant GST-BGI1 protein was evaluated using cinnamaldehydes and NADPH as substrates, with the activity quantified by the rate of NADPH consumption (Chabannes et al., 2001). A correlation was observed between the enzyme activity of BGI1 and the concentration of the recombinant protein provided (Figure 5k), demonstrating the in vitro CAD activity of the recombinant BGI1 protein.
To investigate the catalytic activity of native BGI1 in vivo, the KO mutant plants were further examined. A comparison of CAD activity in the internodes between WT Bobwhite and the KO1 mutant plants at the flowering stage revealed a significant reduction in enzyme activity in KO mutant plants compared to WT (P < 0.001; Figure 5I). Conversely, the CAD activities of OE transgenic lines (T1402-72 and T1402-73) were significantly increased compared to Fielder (Figure S16). These findings demonstrate that BGI1 exhibits CAD activity both in vitro and in vivo.
Subcellular localisation and expression pattern of BGI1
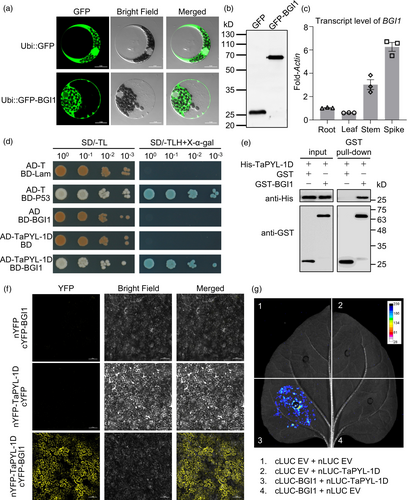
To determine the subcellular localisation of BGI1, we performed transient expression assays in wheat protoplasts using green fluorescent protein (GFP) and GFP-BGI1 fusion protein constructs derived by the UBI promoter. In contrast to the ubiquitous distribution of free GFP, the fluorescence signal of GFP-BGI1 was exclusively confined to the cytoplasm (Figure 7a). Anti-GFP immunoblots confirmed that both GFP and GFP-BGI1 proteins were expressed at their expected sizes (Figure 7b). Furthermore, quantitative reverse transcription PCR (qRT-PCR) analysis revealed that BGI1 was expressed in all examined tissues, including the root, leaf, stem and spike, with notably higher expression levels observed in the stem and spike (Figure 7c).
The regulatory networks and interaction protein of BGI1
To understand the regulatory networks associated with BGI1, we performed RNA-seq analysis to compare the transcriptomes of WT and KO mutants at the flowering stage. This analysis identified 6551 differentially expressed genes (DEGs) between WT and KO1 mutant plants (FDR <0.01, |log2 fold change| > 1, P value <0.05; Figure S17a, Table S5). Among these, 2345 DEGs were upregulated while 4206 were downregulated in the KO1 mutant plants compared to WT (Figure S17a, Table S5). Gene Ontology (GO) annotations of these DEGs revealed significant enrichment in biological processes such as lignin biosynthetic process, pigment biosynthetic process and abscisic acid (ABA) metabolic process (Figure S17b). The RNA-seq data indicated that lignin biosynthesis and ABA-related genes were downregulated in KO1 plants compared to WT (Figure S18). qRT-PCR analysis confirmed the downregulation of two lignin biosynthesis related genes TaCOMT1 (TraesCS3A02G534900) and TaLAC4 (TraesCS3B02G392700) in KO1 plants (Figure S19). Conversely, these genes were upregulated in the OE transgenic lines compared to WT (Figure S20). Furthermore, we validated the expression levels of ABA-related genes TaABA1 (TraesCS2A02G317000, TraesCS2B02G335400 and TraesCS2D02G314900), TaPP2C (TraesCS1B02G242300) and TaSnRK (TraesCS1B02G347100 and TraesCS3B02G225800), and found that they were downregulated in KO1 (Figure S21), supporting the RNA-seq results.
To identify potential protein interactions with BGI1, a yeast two-hybrid (Y2H) screen was conducted using the coding sequence of BGI1 as bait against a wheat cDNA library. The Y2H analysis successfully identified an interaction between BGI1 and TaPYL-1D (TraesCS1D02G126900), a protein with a PYR/PYL/RCAR-like domain known for its role in the ABA signalling pathway (Liu et al., 2021a). This interaction was further verified by GST pull-down, bimolecular florescence complementation (BiFC) and luciferase complementation imaging (LCI) assays (Figure 7d–g). These findings suggest a potential regulatory mechanism involving BGI1 in lignification and the ABA signalling pathway.
Discussion
The bgi1 mutant is defective in lignin biosynthesis
CAD mutants have been previously characterised in several plant species, including bm1 in maize (Halpin et al., 1998), bmr6 in sorghum (Li et al., 2015; Scully et al., 2016), gh2 in rice (Zhang et al., 2006) and Bdcad1 in B. distachyon (Bouvier d'Yvoire et al., 2013). While these studies identified CAD mutants, to our knowledge, no corresponding CAD mutants has been identified and characterised in wheat. Using map-based cloning and SNP analysis, we identified the BGI1 gene encoding a CAD protein and found that bgi1 is a lignin-deficient mutant, leading to a reddish-brown colouration phenotype in lignified tissues.
In maize and sorghum, the bm1 and bmr6 mutant plants exhibit reddish-brown pigments in the stalk pith and leaf midrib (Halpin et al., 1998; Saballos et al., 2009). In rice, the gh2 mutant plants display pigments in the internode, hull and basal leaf sheath (Zhang et al., 2006). Consistent with previous studies, our research identified that the bgi1 mutant plants show a reddish-brown colouration in the internode, spike, spike rachilla, glume and lemma (Figure 1). All the above CAD mutants exhibit reddish-brown colouration in lignified tissues, suggesting that these CADs play a conserved role in lignin biosynthesis across maize, sorghum, rice and wheat. However, phenotype differences were observed, such as the reddish-brown colouration of the inner glumes in the bgi1 mutant, a trait not seen in other mutants. The difference in phenotypes could be attributed to two key factors. First, these CAD orthologous genes may have some degree of functional differentiation among different plant species. The maize BM1 (Halpin et al., 1998) and sorghum BMR6 (Li et al., 2015) genes were expressed strongly in leaves while rice GH2 (Zhang et al., 2006), B. distachyon BdCAD1 (Bouvier d'Yvoire et al., 2013) and wheat BGI1 genes were expressed slightly in the same tissue. Second, the type of mutation (missense or splice acceptor) may lead to different levels of reduction in CAD enzymatic activity. The gh2 mutant has an amino acid (G185D) change in the causal gene (Zhang et al., 2006). In Brachypodium, the Bd4179 and Bd7591 mutant lines carry one (G192D) or two (G99V and S286F) amino acid changes in BdCAD1 (Bouvier d'Yvoire et al., 2013). In contrast, the bgi1 mutant plants carry a mutation (G > A) in the splicing acceptor site of the first intron of BGI1, leading to premature termination (Figure 2f). These different types of mutations may contribute to the phenotypic differences observed across various plant species.
The KO mutant plants of BGI1 exhibited reduced CAD activity and lower lignin content (Figures 5 and S11), which is consistent with observations in some monocotyledonous plants, such as maize (Halpin et al., 1998; Xiong et al., 2020), sorghum (Scully et al., 2016), rice (Zhang et al., 2006) and B. distachyon (Bouvier d'Yvoire et al., 2013). However, the estimates of lignin concentration can vary greatly depending on the extraction method used. Some studies have observed reduced CAD activity without a corresponding decrease in lignin content (Baucher et al., 1996, 1999; Christiane Marque et al., 1998).
Overexpressing BGI1 results in increased lignin content and shearing force
Numerous studies have documented CAD mutants, yet research on the phenotypic changes resulting from CAD overexpression in cereal crops remains relatively scarce. In Artemisia annua, overexpression of the AaCAD gene led to significantly higher lignin content in transgenics compared with WT plants (Ma et al., 2018). Transgenic Arabidopsis plants overexpressing IbCAD1 exhibited increased lignin content in stems and roots, with a higher proportion of S lignin compared to G lignin (Kim and Huh, 2019). PpCAD2 overexpression transgenic tomato plants had a higher lignin content and CAD enzymatic activity in the leaf, stem and fruit pericarp tissues (Li et al., 2019). Although none of these studies were conducted in cereal crops, these results support our observation that overexpression of BGI1 leads to increased lignin content (Figure S11).
In addition to increasing lignin content, BGI1 overexpression significantly enhances stem strength, as evidenced by increased shearing force of stems (Figure S12). Recent correlation analyses have identified a significant positive relationship between TaCAD1 gene expression and stem strength (Chen et al., 2021). Lodging is a common issue in wheat production, causing yield losses ranging from 10% to 80% (Easson et al., 1993; Peng et al., 2014). Lignin accumulation has been positively and significantly correlated with internode breaking strength and culm lodging resistance (Peng et al., 2014). Thus, overexpression of BGI1 could be a potential strategy for improving lodging resistance in wheat.
Interestingly, overexpression of PpCAD2 in tomato resulted in increased plant height, longer roots and larger stem diameter (Li et al., 2019). Overexpression of IbCAD1 in Arabidopsis enhanced seed germination rate and increased tolerance to reactive oxygen species (Kim and Huh, 2019). Although the BGI1 overexpression transgenic plants did not exhibit any significant phenotypic changes (Figure S22), comprehensive agronomic and quality evaluations are necessary.
Overexpression of BGI1 enhances pathogen resistance
Yield losses caused by B. sorokiniana are often severe, ranging from 15% to 20%. Under favourable conditions of heat and drought, this disease can reduce wheat production by up to 70% and cause significantly seed quality deterioration (Sharma and Duveiller, 2007). FCR infection is estimated to cause a 35% yield loss in winter wheat in the Pacific Northwest region of the United States, with wheat seeds likely to become contaminated with fungal toxins (Smiley et al., 2005; Su et al., 2021). Additionally, there are only a limited number of genetic loci conferring resistance to CRR or FCR (Su et al., 2021). In our study, transgenic plants overexpressing BGI1 exhibited increased resistance against both CRR and FCR infections (Figure 6a,b). This enhanced resistance to pathogen infections in OE transgenic plants is linked to the increased lignin content.
Lignin serves as a mechanical defence barrier and is known to be involved in plant defence against pathogen infections (Miedes et al., 2014; Vance et al., 1981). Lignification can form protective barriers against pathogen invasion, modify cell walls to resist pathogen-released degrading enzymes, enhance the resistance of cell walls to toxins diffusion from pathogens to hosts, generate free radicals and toxic precursors and lignify to entrap pathogens (Bhuiyan et al., 2009; Rong et al., 2016). In Arabidopsis, genetic and functional analyses indicate that CAD-C and CAD-D not only serve as key enzymes in lignin biosynthesis but also play vital roles in plant defence against Pseudomonas syringae pv. tomato (Tronchet et al., 2010). In wheat, increased lignin accumulation has been associated with increased resistance against FCR (Yang et al., 2021). Likewise, wheat lines overexpressing TaCAD12 demonstrated significantly enhanced resistance to the fungus Rhizoctonia cerealis throughout all growth stages (Rong et al., 2016). These results support our findings that overexpression of BGI1 leads to increased pathogen resistance.
In Arabidopsis, cad-C and cad-D mutations reduced the expression of PR1 and PR5 genes after inoculation with Pseudomonas syringae pv. Tomato (Tronchet et al., 2010). In wheat, transcriptional levels of PR10 and PR17c were significantly elevated in the stems of TaCAD12-overexpression wheat plants, contributing to increased resistance against R. cerealis (Rong et al., 2016). However, our study did not observe changes in transcript levels of PR genes in BGI1-overexpressing plants upon pathogen inoculation (Figure S15), suggesting that CAD overexpression may involve different mechanisms in plant defence. The precise molecular mechanisms underlying the pathogen resistance caused by BGI1 overexpression require further investigation.
The interaction between BGI1 and TaPYL-1D may contribute to the process of lignification
Previous studies have reported that the hormone ABA plays a crucial role in regulating plant secondary cell-wall deposition and lignification (Brookbank et al., 2021; Liu et al., 2021a). ABA signalling is mediated by intracellular PYL receptors, which bind to and inhibit PP2Cs, thereby releasing protein kinases SnRK2s from inhibition (Chen et al., 2020; Park et al., 2009). In muskmelon, exogenous ABA enhanced CAD activity and promoted the production of lignin monomers, lignin content and phenolic acids in fruit wounds (Wang et al., 2024b). In Kenaf (Hibiscus cannabinus L.), ABA treatment induced a biphasic expression pattern of HcCAD2, with significant induction at 6, 24 and 48 h (Choi et al., 2016). Additional studies revealed that ABA increased the expressions of lignin biosynthesis genes, such as CAD, 4-coumarate-CoA ligase and cinnamate 4-hydroxylase, as well as the activities of lignifying enzymes, thereby promoting lignin accumulation (Cheng et al., 2013; Liu et al., 2021b; Xu et al., 2020). These studies suggest a potential regulatory mechanism involving ABA, PYL receptors and CAD proteins in lignin biosynthesis. Our study identified a physical interaction between BGI1 and TaPYL-1D using Y2H, LCI, BiFC and GST pull-down assays (Figure 7d–g). This finding aligns with GO annotations indicating enrichment in the ABA metabolic process (Figure S17b), suggesting a regulatory role for BGI1 in ABA signalling. Our results provide a foundation for further investigation into the functional mechanisms of BGI1 and TaPYL-1D in regulating lignin biosynthesis in wheat.
In summary, we successfully isolated the bgi1 gene in wheat, which encodes the TaCAD1 protein. Functional validation through loss-of-function EMS and CRISPR mutations in BGI1 results in a reddish-brown colouration of lignified tissues. Disruption of BGI1 significantly reduces lignin content without affecting plant development, potentially enhancing the use of wheat residues for biofuel production and other bio-based products. Conversely, overexpression of BGI1 elevates lignin content and boosts pathogen resistance, which could lead to the development of wheat varieties with improved disease tolerance and lodging resistance. Specifically, increased disease resistance for CRR and FCR could reduce reliance on chemical control methods, contributing to more sustainable agricultural practices.
Materials and methods
Plant materials and mapping populations
Mutant lines were generated by treating approximately 14 800 seeds from the Ae. tauschii accession PI 511383 with 0.4% EMS (Sigma-Aldrich Co., MO, USA). Seeds from 2156 independent M1 mutant plants were harvested. We identified a M2 mutant line (M326) segregating plants with reddish-brown pigmentation in various tissues. The M2 plants exhibiting this pigmentation were transplanted and self-pollinated to produce a homozygous M3 family. As the M2 seeds of M326 were exhausted, the M3 plants were crossed with the WT PI 511383, generating an F2 population of 1872 individuals. To accelerate the development of molecular markers, we generated an additional mapping population from a cross between M326 and AL8/78 (Luo et al., 2017), a genotype that is highly polymorphic compared to PI 511383.
BSR-Seq analysis
Based on the phenotypic data, 20 F2 plants displaying the WT phenotype and 20 F2 plants exhibiting the mutant phenotype from the M326 × PI 511383 cross were selected to generate the wild bulk (W-bulk) and mutant bulk (M-bulk) for BSR-Seq analysis. Total RNA was isolated from both parents and W/M-bulks using the NucleoZol reagent (Macherey-Nagel, Düren, Germany) and purified with the Direct-zol RNA MiniPrep Plus Kit (Zymo Research, CA, USA). RNA sequencing was carried out at Novogene Bioinformatics Technology Co., Ltd. (Beijing, China). The raw sequencing data are available in the National Genomics Data Center (NGDC) database under the accession number PRJCA028685. Procedures for sequencing reads analysis and variant calling were adopted from previously reported methods (Bai et al., 2024; Jiang et al., 2023). SNP-index and Δ|SNP-index| were calculated across the genome to identify potential genomic regions of interest (Takagi et al., 2013).
Development of CAPS and KASP markers
SNPs identified within the candidate region on chromosome 6D were used to develop both CAPS (Konieczny and Ausubel, 1993) and KASP markers (Semagn et al., 2014). For CAPS markers, primers were designed using Primer3 (https://bioinfo.ut.ee/primer3-0.4.0/primer3/). The resulting PCR products were digested with appropriate restriction endonucleases (New England BioLabs Inc., Hitchin, UK). For KAPS markers, forward primers were designed with standard FAM-compatible tails (5′-GAAGGTGACCAAGTTCATGCT-3′) or HEX tails (5′-GAAGGTCGGAGTCAACGGATT-3′), incorporating the polymorphism between the parents at the 3′ end. Common reverse primers were specifically designed for chromosome 6D.
Phylogenetic analysis
Using the sequence of BGI1 as a query, homoeologous sequences from various plant species were obtained from the publicly available plant genome database (http://plants.ensembl.org/). Protein sequences were aligned using Muscle, as implemented in the software MEGA v7 (Kumar et al., 2016). Phylogenetic trees were constructed using the Neighbor-Joining method. The resulting phylogenetic tree was visualised using the Interactive Tree Of Life (iTOL) v6 (https://itol.embl.de/).
EMS mutants in tetraploid and hexaploid wheat
The sequenced EMS mutagenised populations of the tetraploid wheat variety Kronos and the hexaploid cultivar Cadenza (Krasileva et al., 2017) are available online at https://www.wheat-tilling.com/. Using BLASTN with the sequence of BGI1 as a query, we identified two Kronos mutant lines (K3288 and K3088) that harbour premature stop codons in BGI-A1 and BGI-B1, respectively. We crossed the K3288 mutant with the K3088 mutant, self-pollinated the F1 plants and selected F2 plants that were homozygous for BGI1 homeologs-WT, bgi-A1, bgi-B1 and the bgi1 double mutant.
Similarity, we identified three Cadenza mutant lines (Cadenza0376, Cadenza0749, and Cadenza0183) carrying premature stop codons in BGI-A1, BGI-B1 and BGI-D1, respectively. Through crossing and selection in the F2 generation, we generated single-gene mutants (bgi-A1, bgi-B1 and bgi-D1), double mutants (bgi-A1B1, bgi-A1D1 and bgi-B1D1) and the bgi1 triple mutant (bgi-A1B1D1).
BSMV-mediated gene editing and OE transgenic validation
The previously reported plasmids used for BSMV-mediated gene editing in wheat were employed (Li et al., 2021). The resulting BSMV-sgRNA constructs were transformed into A. tumefaciens strain EHA105 and subsequently co-infiltrated into N. benthamiana leaves. The inoculated leaves were then homogenised and rub-inoculated onto the leaves of Bobwhite Cas9-transgenic plants. Sanger sequencing was used to identify plants homozygous for single-gene, double and triple mutants in the M2 population.
The coding sequence of BGI1 was introduced into the modified binary vector pCAMBIA1300 and then transformed into the hexaploid wheat variety Fielder via A. tumefaciens-mediated transformation at the Peking University Institute of Advanced Agricultural Sciences transformation facility.
Subcellular localisation assay
The coding sequence of BGI1 was cloned into the pJIT163-Ubi-GFP vector (Luo et al., 2022). Both the empty vector (EV) and the resulting ubi::GFP-BGI1 construct were transformed into wheat protoplasts (variety Fielder) using the PEG-mediated method (Luo et al., 2022). Protoplast images were captured using a confocal microscope (A1 HD25 Nikon, Tokyo, Japan).
qRT-PCR and transcriptome analysis
Stem, leaf, and spike samples from Ae. tauschii accession PI 511383 were collected at the flowering stage while root samples were collected at the three-leaf stage. qRT-PCR was conducted on an Applied Biosystems ABI QuantStudio 5 Real-Time PCR System (Applied Biosystems, CA, USA). RNA expression levels were normalised using the endogenous control TaActin (Zhang et al., 2017), and BGI1 transcript levels were calculated using the 2−ΔCT method as previously described (Chen et al., 2018). The primers for PR genes were previously described (Zhang et al., 2017).
Differentially expressed genes (DEGs) were identified using edgeR software (Robinson et al., 2010). Genes showing a false discovery rate (FDR) < 0.01, |log2 fold change| > 1 and P-value <0.05 were classified as DEGs. GO enrichment analysis of DEGs was conducted using agriGO v2.0 (http://systemsbiology.cau.edu.cn/agriGOv2/).
BGI1 protein expression and purification in E. coli
The coding sequence of BGI1 was inserted into the pGEX-4T-1 vector using the In-Fusion HD cloning kit (Takara Biotech, Shiga, Japan). Subsequently, the pGEX-4T-1 and GST-BGI1 constructs were transformed into the E. coli expression strain BL21. Protein expression was achieved by adding 0.3 mM isopropyl-β-D-thiogalactopyranoside (IPTG) for 16 h at 16 °C. Purification of the GST and recombinant GST-BGI1 proteins was carried out using Glutathione Magnetic Agarose Beads (Thermo Fisher, Waltham, MA, USA). The purified proteins were quantified using a BCA protein assay kit (Cwbio, Jiangsu, China).
Western blotting analysis
Purified proteins were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (GenScript, Nanjing, China). The separated proteins were then electrotransferred onto a polyvinylidene fluoride (PVDF) membrane (Roche, Basel, Switzerland). Immunoblotting was conducted using a commercial HRP-conjugated Mouse anti GST-Tag antibody (Abclonal, Wuhan, China). A primary anti-GFP antibody (Abcam, Cambridge, UK) and a secondary Goat anti-Rabbit IgG-HRP antibody (Abmart, Shanghai, China) were used in subcellular localisation assays.
BGI1 enzyme activity assay
Enzyme activity assays were conducted on second internode tissues (~100 mg) following the methods described previously (Chabannes et al., 2001). Both in vivo and in vitro CAD activities were measured using the CAD Activity Assay Kit (Sangon Biotech, Shanghai, China).
Histochemical staining
Wiesner staining (Blaschek et al., 2020) and Mäule staining (Sibout et al., 2005) were used to localise lignin accumulation. For Wiesner staining, fresh hand-cut cross-sections of wheat second internodes at the flowering stage were initially incubated in a phloroglucinol solution for 10 min, followed by a 5-min treatment with 18% HCl. For Mäule staining, fresh cross-sections were sequentially treated: 1% KMnO4 for 15 min, rinsed with water, 10% HCl for 1 min, rinsed again and then mounted in 5% NaHCO3 for 10 min (Sibout et al., 2005). After treatment, the cross-sections were immediately photographed using a Leica microscope (Leica, Hamburg, Germany).
Lignin content and composition analysis
Internodes were collected at the flowering stage, oven-dried and then ground into powder. The power was sieved through a 40-mesh screen for lignin content and composition analysis. Klason lignin was determined using the previously reported method (Zhang et al., 2021).
Y2H assays
A yeast two-hybrid assay was conducted following the Clontech Yeast Two-Hybrid System protocol (Clontech, Mountain View, CA, USA). BGI1 was used as bait for screening a cDNA library constructed from Fielder (Li et al., 2023). To validate the interaction between BGI1 and TaPYL-1D, the BGI1 coding sequence was ligated into the pGBKT7 vector while the TaPYL-1D coding sequence was cloned into the pGADT7 vector. The constructs were co-transformed into the yeast strain Y2H Gold and cultured on SD/-Trp/-Leu medium. Co-transformants were then plated on selective medium (SD/-Trp/-Leu/-His +40 μg/mL X-α-gal).
In vitro GST pull-down assays
The BGI1 coding sequence was inserted into the pGEX-4 T-1 vector to generate a GST-tagged BGI1 protein. Similarly, the TaPYL-1D coding sequence was cloned into the pET-28a vector to produce His-tagged TaPYL-1D protein. Both GST and His fusion proteins were purified using glutathione magnetic agarose beads and HisPur Ni-NTA magnetic beads (Pierce, Waltham, MA, USA), respectively. Pull-down assays were performed following the manufacturer's protocol (Pierce, Waltham, MA, USA). The bait-prey mixtures were denatured at 95 °C for 10 min, separated by 10% SDS-PAGE and immunoblotted with anti-His (Abclonal, Wuhan, China) and anti-GST antibodies (Abclonal, Wuhan, China).
In vivo BiFC assays
The coding sequence of BGI1 was inserted into pCAMBIA1300-35S-cYFP while TaPYL-1D was fused with pCAMBIA1300-35S-nYFP. These constructs were transformed into Agrobacterium strain EHA105 and then infiltrated into 4-week-old N. benthamiana leaves. YFP fluorescence signals were detected two days post-infiltration using a confocal microscope (A1 HD25 Nikon, Tokyo, Japan).
LCI assays
The coding sequences of BGI1 and TaPYL-1D were inserted into the pCAMBIA1300-cLuc and pCAMBIA1300-nLuc vectors, respectively (Zhou et al., 2018). The constructs, cLuc-BGI1 and nLuc-TaPYL-1D, were transformed into Agrobacterium strain EHA105 and co-infiltrated into 4-week-old N. benthamiana leaves. Luciferase activities were captured 48 h post-infiltration using the ChemiDoc MP Imaging System (Bio-Rad, Hercules, USA). Fluorescence signals in the injected leaves were quantified using ImageJ software (Schneider et al., 2012).
Evaluation of pathogen resistance
Inoculation experiments with B. sorokiniana and Fusarium pseudograminearum were performed at Hebei Agricultural University, Baoding, Hebei, China. The plants were grown in growth chambers under controlled conditions (25 °C and 16 h photoperiod). The dominant strains of B. sorokiniana and F. pseudograminearum (Wz2-8A) prevalent in Hebei Province, China, were isolated from infected wheat plants and cultured on potato dextrose agar (PDA) medium. Procedures for inoculation and statistical analyses of infection types have been previously reported (Wang et al., 2024a; Yuan et al., 2024).
Author contributions
LH, RS and XH performed most of the experimental tasks; JZ conducted the BGI1 enzymatic activity assays; YL contributed to subcellular localisation; JL contributed to mapping; HL contributed to expression analysis; GW designed the BGI1 sgRNA; SR contributed to the writing process; JW and DF provided and analysed EMS mutants; XW and XR designed and performed the pathogen infection experiments; CZ developed and screened the PI 511383 EMS mutant population. LH and SC analysed the data and wrote the first version of the manuscript. SC, CZ, XW and YD proposed and supervised the project. SC generated the final version of the paper. All authors revised the manuscript and provided suggestions.
Acknowledgements
The authors thank Dr. Jorge Dubcovsky (University of California, Davis, USA) for providing the Kronos and Cadenza mutant lines. Work at SC laboratory was supported by the Key R&D Program of Shandong Province (ZR202211070163, 2024LZGC034, 2023LZGC022), the Young Yuandu Scholars Program of Weifang City, the National Natural Science Foundation of China (32472159), and the Young Taishan Scholars Program of Shandong Province. Work at YD laboratory was supported by the Shandong Provincial Natural Science Foundation (ZR2020MC029). Work at XW laboratory was supported by the National Key Research and Development Program of China (2023YFD1201002), Provincial Natural Science Foundation of Hebei (C2022204010) and S&T Program of Hebei (23567601H).
Conflicts of interest
The authors declare that they have no conflict of interests.
Open Research
Data availability statement
Data supporting the findings of this work are available within the paper and its supplementary information files. All the raw sequencing data generated for this project are archived at the China National Genomics Data Center (NGDC) under BioProject accession number PRJCA028685.




