Two tandem R2R3 MYB transcription factor genes cooperatively regulate anthocyanin accumulation in potato tuber flesh
Summary
Anthocyanin biosynthesis and accumulation determines the colour of tuber flesh in potato (Solanum tuberosum) and influences nutritional quality. However, the regulatory mechanism behind anthocyanin biosynthesis in potato tuber flesh remains unclear. In this study, we identified the Pigmented tuber flesh (Pf) locus through a genome-wide association study using 135 diploid potato landraces. Genome editing of two tandem R2R3 MYB transcription factor genes, StMYB200 and StMYB210, within the Pf locus demonstrated that both genes are involved in anthocyanin biosynthesis in tuber flesh. Molecular and biochemical assays revealed that StMYB200 promotes StMYB210 transcription by directly binding to a 1.7-kb insertion present in the StMYB210 promoter, while StMYB210 also regulates its own expression. Furthermore, StMYB200 and StMYB210 both activated the expression of the basic helix–loop–helix transcription factor gene StbHLH1 and interacted with StbHLH1 to regulate anthocyanin biosynthesis. An analysis of the StMYB210 promoter in different diploid potato accessions showed that the 1.7-kb insertion is associated with flesh colour in potato. These findings reveal the genetic and molecular mechanism by which the Pf locus regulates anthocyanin accumulation in tuber flesh and provide an important reference for breeding new potato varieties with colourful flesh.
Introduction
Anthocyanins are widely distributed flavonoid secondary metabolites and a primary determinant of colour in plants; they are also antioxidants involved in the response to abiotic and biotic stresses in plants (Allan et al., 2008; Liu et al., 2018). Anthocyanin-rich foods have health-promoting effects in humans (Cappellini et al., 2021). The anthocyanin biosynthesis pathway is highly conserved and involves structural genes encoding the enzymes responsible for anthocyanin biosynthesis, and regulatory genes encoding transcriptional regulators (Cappellini et al., 2021; Tanaka et al., 2008). MYB-type transcription factors (TFs) and basic helix–loop–helix (bHLH) TFs play roles in activating or repressing anthocyanin accumulation in various plant species (Cappellini et al., 2021; Liu et al., 2018; Ma and Constabel, 2019). These MYB TFs and bHLH TFs, together with WD repeat (WDR) proteins, form MYB-bHLH-WDR (MBW) complexes that regulate anthocyanin biosynthesis in a tissue-specific and/or growth-dependent manner, depending on the expression patterns of their encoding genes (Cappellini et al., 2021; Xu et al., 2015).
Potato (Solanum tuberosum L.) is an important tuber crop that originated from Andean and Chilean landraces distributed in the Andes of southern Peru (Comai, 2023; Hardigan et al., 2017; Spooner et al., 2005; Zhang et al., 2022). These landraces exhibit a broad diversity in terms of tuber colours (Lewis et al., 1998; Spooner et al., 2005). The colour of tuber skin and flesh ranges from white to dark purple-blue and results from the accumulation of two main classes of pigments, anthocyanins and carotenoids (Lewis et al., 1998). Three primary loci, developer (D), Red (R) and Purple (P) are involved in anthocyanin accumulation in tuber skin of tetraploid potato; these loci are equivalent to the inhibitor (I), R and P loci in diploid potato, respectively (De Jong, 1991; Dodds and Long, 1955, 1956; Salaman, 1910). R (located on chromosome 2) and P (located on chromosome 11) are required for production of red and purple pigments, respectively, anywhere in the potato plants (Dodds and Long, 1955, 1956), and encode dihydroflavonol 4-reductase (DFR) and flavonoid 3′,5′-hydroxylase (F3′5′H), respectively (De Jong et al., 2003; Jung et al., 2005; van Eck et al., 1993, 1994; Zhang et al., 2009).
The I (or D) locus is required for the tissue-specific expression of anthocyanins in tuber skin (De Jong, 1991; Dodds and Long, 1956). Gene collinearity analysis in the Solanaceae and transgenic verification in potato indicated that the D locus encodes the R2R3-MYB TF Solanum tuberosum anthocyanin 2 (Stan2) (De Jong et al., 2004; Jung et al., 2009). Notably, the Pigmented tuber flesh (Pf) locus regulating anthocyanin accumulation in tuber flesh is tightly linked to the I locus, which maps to chromosome 10 (De Jong, 1987; van Eck et al., 1994). Through a BC1S1 segregating population derived from a cross between potato clones showing red- and yellow-coloured tuber flesh, the locus controlling red flesh colour was mapped to a 377-kb interval from 51.47 to 51.85 Mb (in the reference genome DMv4.03) on chromosome 10 (Xu et al., 2019). Notably, Stan2 is located in this 377-kb interval, further indicating that the I (D) locus is closely linked to the locus regulating tuber flesh colour (Jung et al., 2009). However, the identity of the Pf locus remained to be elucidated.
In this study, we identified the Pf locus on chromosome 10 regulating tuber flesh colour in a diploid potato through a genome-wide association study (GWAS). According to the reference potato genome DMv8.1 (Yang et al., 2023), combined with the RNA-seq data, two tandem R2R3 MYB TFs, DM8C10G21200 (StMYB200) and DM8C10G21210 (StMYB210/Stan2/StAN1), were identified as the candidate genes for the Pf locus. Gene editing established that both genes regulate anthocyanin accumulation in tuber flesh. StbHLH1 functions with StMYB200 and StMYB210 to activate the expression of the structural genes in anthocyanin biosynthesis. In addition, StMYB200 binds to a 1.7-kb insertion in the StMYB210 promoter to modulate anthocyanin accumulation in tuber flesh. These results offer insight into the regulatory mechanisms of tuber flesh colour by the Pf locus, and provide molecular markers for breeding new potato cultivars with pigmented flesh.
Results
A genetic locus on chromosome 10 regulates tuber flesh colour in diploid potato
To identify the loci controlling flesh colour in potato, we surveyed the colour of tuber flesh from 135 diploid landraces (Table S1) (Zhang et al., 2019). The phenotypes fell into two categories: 26 colourful clones with red or purple flesh and 109 colourless clones with white or yellow flesh. Using this binary trait as phenotype, we performed a GWAS for flesh colour. We identified one significant locus at the end of chromosome 10, with nine single-nucleotide polymorphisms above the threshold (P value < 1.0 × 10−8) (Figure 1a,b and Table S2). This genomic region overlapped with the known Pf and I (D) loci (De Jong, 1987; Jung et al., 2009); thus, we designated this interval as the Pf locus.
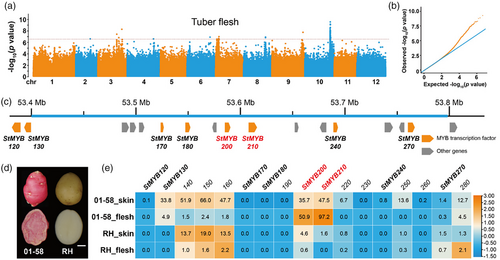
According to the annotation of the reference genome DMv8.1, we determined that the above Pf locus contains eight R2R3 MYB TF genes, designated StMYB120, StMYB130, StMYB170, StMYB180, StMYB200, StMYB210, StMYB240 and StMYB270, and nine other genes (Figure 1c). We had previously mapped a locus controlling red flesh to a 396-kb interval (in DMv8.1) at the end of chromosome 10 using a BC1S1 segregating population (Xu et al., 2019), which overlapped with the above Pf locus. However, StMYB120 and StMYB130 are located upstream and outside of this 396-kb interval. In addition, transcriptome data for tuber skin, and tuber flesh derived from 01 to 58 (a clone with red skin and red flesh) and RH (a clone with white skin and white flesh) indicated that only StMYB200 and StMYB210 are highly expressed in red flesh but rarely expressed in white flesh (Figure 1d,e), supporting the notion that these two tandem MYB TF genes StMYB200 and StMYB210 might be candidate genes underlying the Pf locus.
Two tandem MYB transcription factor genes contribute to anthocyanin accumulation in tuber flesh
To assess the regulatory role of the tandem R2R3 MYB genes StMYB200 and StMYB210 in anthocyanin accumulation in tuber flesh, we designed single guide RNAs targeting each gene and cloned them separately into the modified genome editing vector pCAMBIA2300MGFPuv-sgRNACas (Du et al., 2023). We transformed the red-flesh clone 01–58 and obtained one bi-allelic knockout mutant of StMYB200 (StMYB200ko1) and two bi-allelic knockout mutants of StMYB210 (StMYB210ko1 and StMYB210ko2) (Figure 2a,b). All mutants harboured small insertions or deletions leading to a frameshift and the translation of a truncated protein, suggesting that all mutants were null alleles. Importantly, all three mutants showed an alteration of tuber flesh colour (Figure 2c), indicating that these two MYB TFs were involved in anthocyanin accumulation in tuber.
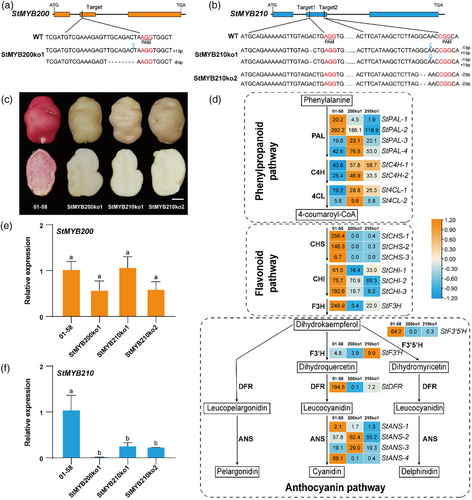
RNA-seq analysis of tuber flesh indicated modest effects on the expression of structural genes in the phenylpropanoid pathway in the StMYB200ko1 and StMYB210ko1 lines (Figure 2d). We observed a downregulation in the expression levels of structural genes, such as Chalcone synthase (StCHS), Chalcone isomerase (StCHI), Flavanone 3-hydroxylase (StF3H), StF3′5′H, StDFR, StANS-1 and StANS-4 involved in flavonoid and anthocyanin pathway, in tuber flesh of StMYB200ko1 and StMYB210ko1 lines. This finding supports the hypothesis that StMYB200 and StMYB210 regulate anthocyanin accumulation by modulating the expression of structural genes in flavonoid and anthocyanin pathways. In addition, RT-qPCR analysis demonstrated that knockout of StMYB200 does not affect the expression of StMYB200 but does decrease the expression of StMYB210 in the tuber flesh (Figure 2e,f). Similarly, knockout of StMYB210 did not alter the expression of StMYB200 but significantly decreased its own expression (Figure 2e,f). The RT-qPCR results were therefore consistent with the expression patterns of StMYB200 and StMYB210 obtained by RNA-seq, suggesting a level of interaction between the two MYB TFs.
StMYB200 directly activates StMYB210 transcription
Based on the above RT-qPCR analysis, we wondered whether StMYB200 might activate StMYB210 expression. Accordingly, we cloned the StMYB210 promoter from the heterozygous clone 01–58. We obtained two haplotypes, one being identical to the reference genome and designated as the Pf genotype, containing a 1757-bp (~1.7-kb) insertion relative to the other haplotype (designated as the pf genotype) (Figure 3a). In the selfed progeny of 01–58, all tubers with the pf/pf genotype had white flesh, whereas all tubers with the Pf/Pf genotype were red (Figure S1a,b). StMYB200 and StMYB210 expression levels in tuber flesh with the Pf/Pf genotype were higher than in those with the pf/pf genotype (Figure S1c and Figure 3b). Additionally, the anthocyanin biosynthesis gene StDFR was expressed at very low levels in lines with pf/pf genotype and at much higher levels in lines with the Pf/Pf genotype (Figure S1d). Based on these findings, along with the StMYB210 expression analysis in the StMYB200ko1 line, we speculated that the 1.7-kb insertion present in the promoter region of StMYB210 might influence its expression in tuber flesh. Furthermore, we hypothesized that StMYB200 promotes StMYB210 expression to regulate anthocyanin biosynthesis through this insertion.
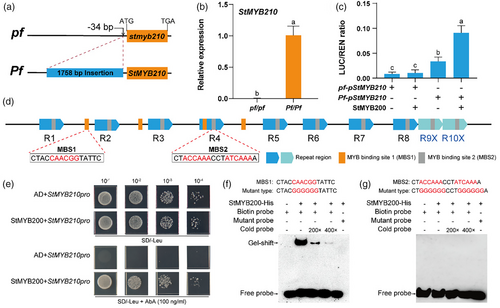
To test this hypothesis, we conducted dual-luciferase (dual-LUC) assays to examine the transcriptional activity from the StMYB210 promoters for the pf and Pf genotypes. To this end, we cloned the StMYB210 promoter fragment from each haplotype to the upstream region of the firefly luciferase (LUC) reporter; we also cloned the full-length StMYB200 coding sequence to the downstream of the cauliflower mosaic virus (CaMV) 35S promoter as an effector construct. We determined that the StMYB210 promoter from the Pf haplotype harbouring the 1.7-kb insertion exhibits higher activity than that from the pf haplotype (Figure 3c), which is enhanced by the presence of StMYB200 (Figure 3c). Importantly, the promoter from the pf haplotype lacking the 1.7-kb insertion failed to be activated by StMYB200 (Figure 3c).
The 1.7-kb insertion in the StMYB210 promoter from the Pf haplotype contains 10 repeated regions, with each repeat being about 100 bp in length and containing one MYB binding site (MBS2, ACCAA/ATCAAA) (Figure 3d and Figure S2). We also identified four additional MYB-binding sites of another type (MBS1 and CAACGG) within the 1.7-kb insertion, either in between or within repeated regions (Figure 3d and Figure S2). Therefore, we suspected that StMYB200 may directly bind to the 1.7-kb insertion in the StMYB210 promoter though one or more MBSs. To test this hypothesis, we performed a yeast-one-hybrid assay using a StMYB210 promoter fragment containing all four MBS1s and three repeat regions (R2, R3 and R4). Indeed, StMYB200 could bind to the StMYB210 promoter in yeast cells (Figure 3e). An electrophoretic mobility shift assay (EMSA) confirmed that recombinant His-tagged StMYB200 specifically binds to a probe containing MBS1 (Figure 3f), but not to one containing MBS2 (Figure 3g). Moreover, the binding ability of StMYB200 declined in the presence of increasing amounts of cold probe, and mutation of the core sequence within MBS1 abolished binding (Figure 3f). In conclusion, these findings provide evidence that StMYB200 directly activates the transcription of StMYB210 by binding to MBS1 in the 1.7-kb insertion present in its promoter region from the Pf haplotype.
StMYB210 possesses self-regulation ability
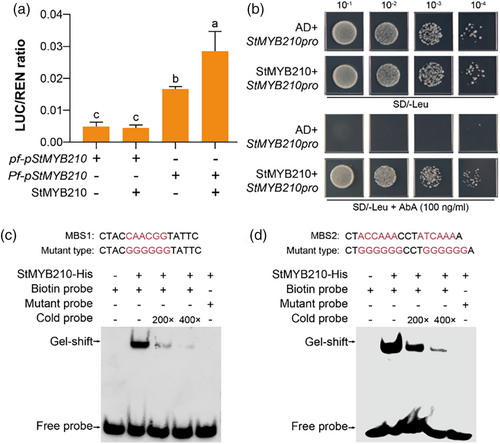
The knockout of StMYB210 also displayed decreased expression of StMYB210 (Figure 2e), suggesting potential self-regulation. We explored this possibility by conducting a dual-LUC assay using StMYB210pro:LUC reporter constructs for the pf and Pf haplotypes, together with construct 35S:StMYB210 as effector. As with StMYB200 above, we established that StMYB210 significantly enhanced transcription from the StMYB210 promoter carrying the 1.7-kb insertion from the Pf haplotype, but not that from the pf haplotype (Figure 4a). A yeast-one-hybrid assay demonstrated that StMYB210 can directly bind to the 1.7-kb insertion in the StMYB210 promoter (Figure 4b). Furthermore, an EMSA confirmed that recombinant purified His-tagged StMYB210 binds to probes containing the MYB-binding sites MBS1 and MBS2 present within the 1.7-kb insertion (Figure 4c,d). Co-incubation with cold probes also decreased the extent of binding ability, and mutation of the core sequence within MBS1 or MBS2 abolished binding. These results demonstrate that StMYB210 can enhance the expression of its encoding gene by binding to MYB-binding sites within the 1.7-kb insertion in its promoter from the Pf haplotype.
StbHLH1 regulates anthocyanin biosynthesis in tuber flesh
Two potato bHLH TFs, StbHLH1 and StJAF13, were earlier identified as regulating anthocyanin biosynthesis by interacting with MYB TFs when tested in the heterologous system of Nicotiana benthamiana leaves (D'Amelia et al., 2014; Liu et al., 2016, 2023a). RNA-seq analysis of tuber flesh showed that StbHLH1 is not expressed in StMYB200ko1 or StMYB210ko1 lines (Figure S3a) and is only barely expressed in the pf/pf genotype lines from the selfed progeny of clone 01–58 (Figure S1e). In addition, dual-LUC assays with a StbHLH1pro:LUC reporter construct demonstrated that transcription from the StbHLH1 promoter is activated by StMYB200 or StMYB210 (Figure S3b). These findings support the hypothesis that both StMYB200 and StMYB210 activate StbHLH1 expression.
To investigate whether StbHLH1 is also involved in the regulation of anthocyanin biosynthesis in tuber flesh, we obtained two StbHLH1 knockout lines for this gene in the red-fleshed clone 01–58. The two bi-allelic mutants, namely StbHLH1ko1 and StbHLH1ko2, harbour small deletions and/or insertions at the target sites that lead to truncated proteins, and thus likely carry null alleles (Figure S3c). Importantly, both mutants have tubers with white flesh (Figure S3d). An RT-qPCR analysis of tuber flesh showed significant downregulation of StbHLH1 expression in the StbHLH1ko1 and StbHLH1ko2 lines compared with the wild type (Figure S3e), along with lower expression levels of the structural gene StDFR in the anthocyanin pathway (Figure S3f). However, knockout of StbHLH1 significantly increased StMYB200 expression without altering that of StMYB210 (Figure S3g,h). These results demonstrate that StbHLH1 not only controls anthocyanin accumulation in potato tuber flesh but also affects the expression of its own gene and StMYB200.
Interactions among StMYB200, StMYB210 and StbHLH1 regulate anthocyanin biosynthesis
MYB and bHLH TFs typically form MBW complexes with WDR proteins to regulate anthocyanin biosynthesis (Xu et al., 2015). The interaction between StMYB210 (also reported as StAN1) and StbHLH1 was previously demonstrated in a yeast two-hybrid (Y2H) assay (D'Amelia et al., 2014). To confirm this physical interaction between StMYB210 and StbHLH1 and investigate their interaction with StMYB200, we conducted a series of assays encompassing Y2H, pull-down, LUC complementation imaging (LCI) and bimolecular fluorescence complementation (BiFC). In the Y2H assay, we established that StMYB210 interacts with StbHLH1 (Figure 5a), consistent with previous studies (D'Amelia et al., 2014). In addition, StMYB210 and StbHLH1 both interacted with StMYB200 in pairwise examinations. In a pull-down assay with glutathione S-transferase (GST)-tagged StbHLH1 incubated with maltose binding protein (MBP)-tagged StMYB200 or MBP-StMYB210, we detected the GST-tagged proteins among the proteins pulled down with amylose resin coated with each MBP-tagged protein, but not with MBP alone (Figure 5b,c). Furthermore, GST-tagged StMYB200 interacted with MBP-tagged StMYB210 but not with MBP alone in another MBP pull-down assay (Figure 5d). BiFC and LCI assays detected yellow fluorescent protein (YFP) signal or LUC activity in N. benthamiana leaves infiltrated with the corresponding StbHLH1 constructs, along with separate constructs for StMYB200 or StMYB210, respectively (Figure 5e–h). In another set of BiFC and LCI assays, we observed the interaction of StMYB200 with StMYB210 (Figure 5g,h). These in vitro and in vivo protein–protein interaction assays demonstrate that StbHLH1 interacts with StMYB200 and StMYB210 separately, whereas StMYB200 also interacts with StMYB210.
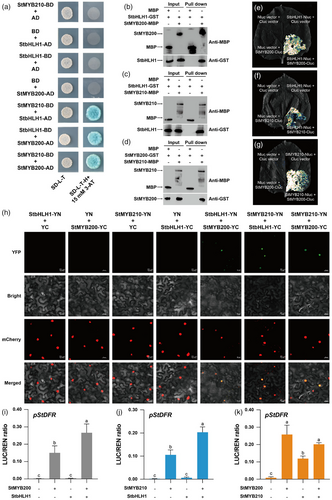
To assess whether these interactions among StMYB210, StMYB200 and StbHLH1 enhance the expression of anthocyanin pathway genes, we cloned the StDFR promoter from the potato accession 01–58 with red skin and flesh to the upstream region of the LUC reporter gene. In dual-LUC assays in N. benthamiana leaves, co-infiltrating the DFRpro:LUC reporter and the effector construct 35S:StMYB200 or 35S:StMYB210 significantly increased relative LUC activity derived from the StDFR promoter, but not when using 35S:StbHLH1 alone as effector construct (Figure 5i–k). Relative LUC activity driven by the StDFR promoter was stronger when StbHLH1 and StMYB200 or StMYB210 were co-expressed compared with StMYB200 or StMYB210 alone (Figure 5i,j). However, relative LUC activity from the StDFRpro:LUC reporter was not significantly enhanced when StMYB200 and StMYB210 were co-expressed compared with expressing StMYB200 alone (Figure 5k). In addition, further dual-LUC assays indicate that the interactions among StMYB200, StMYB210 and StbHLH1 activate the expression of anthocyanin pathway gene StF3H in a similar manner (Figure S4). Together with the above findings, we conclude that StbHLH1 interacts with StMYB200 and StMYB210 to regulate the transcription of structural genes within the anthocyanin pathway.
The 1.7-kb insertion in the StMYB210 promoter is associated with flesh colour in potato
To investigate the correlation between the promoter haplotype and expression levels of StMYB210 as well as tuber flesh colour, we chose 24 accessions further for analysis, representing wild species (Solanum candolleanum), and examples of the landraces (StGp tuberosum, StGp Stenotomum, StGp Phureja and StGp Goniocalyx) (Table S3). The skin and flesh from tubers of four wild accessions were white, whereas those of diploid landraces varied (Figure 6a). We then amplified the StMYB210 promoter, by PCR for genotyping, which revealed that the 1.7-kb insertion was absent in the promoters of all four wild clones. Of the 11 landraces with colourless flesh, five contained one copy of the 1.7-kb insertion, and six carried the pf haplotype lacking the insertion (Figure 6b). All nine landraces with colourful flesh had the 1.7-kb insertion, with two of these being homozygous for the insertion (Figure 6b). End point RT-PCR analysis for the expression of StMYB210 was consistent with the presence or absence of the 1.7-kb insertion. Indeed, we detected StMYB210 expression in all landraces with at least one copy of the insertion (Figure 6c), indicating that the 1.7-kb insertion in the StMYB210 promoter is associated with StMYB210 expression and anthocyanin accumulation in tuber flesh. However, the expression of this TF gene alone may not result in anthocyanin accumulation in tuber flesh, which also requires the expression of structural genes.
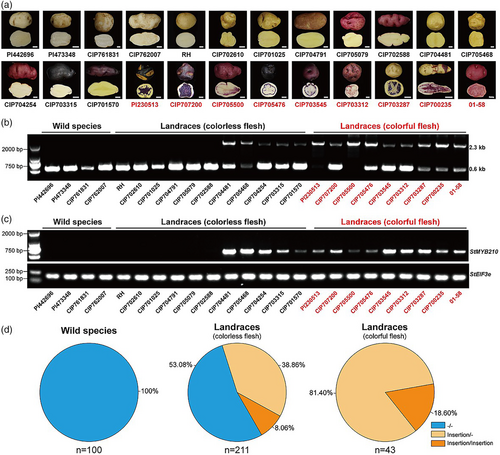
To investigate the frequency of the 1.7-kb insertion in a population of diploid potatoes, we amplified the StMYB210 promoter from 354 accessions belonging to 100 wild species, 211 landraces with colourless flesh and 43 landraces with colourful flesh (Table S3). Notably, the 1.7-kb insertion was not detected in the StMYB210 promoter in any of the 100 wild species tested (Figure 6d and Tables S3 and S4). Nearly half of all landraces with colourless flesh contained the 1.7-kb insertion in the StMYB210 promoter, and all 43 landraces with colourful flesh had the 1.7-kb insertion (Figure 6d and Tables S3 and S4). These results suggest that the allele frequency of the 1.7-kb insertion in the StMYB210 promoter has significantly increased in diploid landraces, especially those with colourful flesh, further demonstrating that the presence of the 1.7-kb insertion is required for anthocyanin accumulation in tuber flesh.
Discussion
Potato cultivars with pigmented tuber flesh provide an opportunity to enhance certain aspects of the food value of potato and processed foods made from potato (Brown et al., 2003). The Pf locus controlling tuber flesh colour is located on the end of chromosome 10 (De Jong, 1987, 1991; Xu et al., 2019), as confirmed by GWAS in this study using a population of diploid potato accessions (Figure 1a,b). We here demonstrated that the tandem MYB TF genes StMYB200 and StMYB210 located within the Pf locus regulate anthocyanin accumulation in tuber flesh and skin by activating the expression of anthocyanin structural genes. Notably, StMYB200, also reported as StMYBA1 or Stan3, was also shown to promote anthocyanin accumulation in potato tuber skin and in tobacco leaves under light conditions (Jung et al., 2009; Liu et al., 2016, 2017; Zhao et al., 2023). Stan2, an allele of StMYB210, was earlier identified as the candidate gene of the I locus, which is required for anthocyanin accumulation in tuber skin and is also tightly linked to the Pf locus (De Jong, 1987; Jung et al., 2009). A construct consisting of Stan2 expressed from a double 35S promoter and transformed into the light red-skinned cultivar Désirée, could enhance anthocyanin accumulation in tuber skin and flesh (Jung et al., 2009). Together, these findings indicate that StMYB200 and StMYB210 in the I-Pf linked group are required for anthocyanin accumulation in the skin and flesh of potato tubers.
Multiple MYB TFs cooperatively regulate anthocyanin biosynthesis (Luan et al., 2024; Sun et al., 2020; Yan et al., 2020); however, few genes encoding these MYB TFs have been reported to be located within the same genomic region. The two neighbouring MYB TF genes FveMYB10 and FveMYB10L function independently in the fruit and petiole, respectively, of wild strawberry (Fragaria vesca) to regulate anthocyanin biosynthesis (Luo et al., 2023). In this study, we determined that two tandem MYB TF genes, StMYB200 and StMYB210, cooperatively promote anthocyanin biosynthesis in the flesh and skin of potato tubers. We also demonstrated that StMYB200 activates the transcription of the adjacent gene StMYB210 by binding to a 1.7-kb insertion present within the StMYB210 promoter in one haplotype, and that StMYB210 also regulates the expression of its encoding gene. StMYB200 and StMYB210 activate StbHLH1 transcription and interact with the encoded TF to regulate anthocyanin biosynthesis. We propose a working model based on these findings that illustrates the regulatory mechanism of StMYB200 and StMYB210 for anthocyanin accumulation in potato tuber flesh (Figure 7).
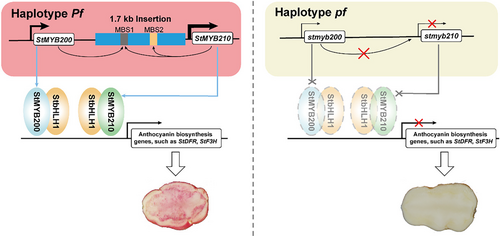
Tuber colour is among several typical domestication traits, as the tubers from wild species are colourless, whereas the tubers of landraces and cultivars have diverse colours (Hardigan et al., 2017; Spooner et al., 2005). We confirmed that the presence of the 1.7-kb insertion in the StMYB210 promoter is associated with high StMYB210 expression and anthocyanin accumulation in tuber flesh. None of the 100 wild species tested in this study contained the 1.7-kb insertion in their StMYB210 promoter, whereas we detected the 1.7-kb insertion in more than half of all landraces, especially those landraces with colourful flesh (Figure 6b,d), suggesting that the 1.7-kb insertion in the StMYB210 promoter may have been selected, along with the tuber colour trait during the domestication and improvement of potato varieties.
Given that some clones harbouring the 1.7-kb insertion exhibited expression of StMYB210 in tuber flesh, but their flesh was colourless (Figure 6a–c), this suggested that other regulators and structural genes may also be involved in anthocyanin accumulation in tuber flesh. Multiple quantitative trait loci (QTLs) influencing the extent of flesh pigmentation have previously been reported to map on chromosomes 5, 8 and 9 of potato (Zhang et al., 2009). Moreover, the structural genes DFR and F3′5′H required for anthocyanin accumulation exhibit differences among potato cultivars (Jung et al., 2005; Zhang et al., 2009). Some other TFs may also be involved in the regulation of anthocyanin biosynthesis in tuber flesh (Liu et al., 2023a, 2023b; Zhang et al., 2022, 2023). In addition, some landraces without the 1.7-kb insertion in the StMYB210 promoter also have colourful skin (Figure 6a,b), indicating that 1.7-kb insertion is not required for anthocyanin accumulation in tuber skin and that StMYB200 and StMYB210 may regulate the colour of tuber skin in different ways. These clones with either colourful or colourless skin, lacking the 1.7-kb insertion, offer an ideal system in which to explore the other regulatory mechanisms underlying tuber skin colour.
The Pf and I loci are also linked to the B locus (required for anthocyanin accumulation in seed spots, the nodal band, the floral abscission layer and tuber eyebrows) and the F locus (required for anthocyanin accumulation in flowers) (De Jong, 1991; Dodds and Long, 1956; Hermsen and Verdenius, 1973). According to the reference genome DM8.1 (Yang et al., 2023), the Pf-I-B-F linkage group contains eight MYB TF genes (Figure 1c). Of these, StMYB130/StFlAN2/StMYB113 was identified as the candidate gene for the F locus regulating flower colour, in agreement, transgenic introduction of StFlAN2 in white-flowered homozygous doubled-monoploid (DM) plants resulted in a recovery of purple flowers (Laimbeer et al., 2020). Overexpression of the StMYB130/StMYB113/StFlAN2 gene also promoted anthocyanin accumulation in the skin of potato tubers (Zhang et al., 2023). In addition, the flowers of the four transgenic lines harbouring the StMYB210/Stan2 gene under the control of a doubled CaMV 35S promoter in the Désirée accession exhibited a dark red pigmentation, in contrast to the light pink colour of the untransformed control (Jung et al., 2009). Based on these collective findings, we hypothesize that the eight MYB TF genes within the Pf-I-B-F linkage group might regulate various aspects of anthocyanin accumulation in potatoes, with distinct alleles likely promoting their biosynthesis in tuber flesh or skin, seed spots, cotyledon nodes, leaf axils and flowers. Additional genetic and biochemical studies are needed to clarify the regulatory mechanism of each MYB TF gene in this group related to anthocyanin biosynthesis. Importantly, these findings reveal the genetic and molecular mechanism by which the Pf locus regulates anthocyanin accumulation in potato tuber flesh and serve as a valuable reference for molecular design breeding of pigmented potatoes.
Materials and methods
Plant materials
The 135 diploid landraces used for GWAS in this study were kindly provided by the International Potato Center, Peru, and were reported in our previous study (Zhang et al., 2019) (Table S1). These diploid landraces, along with another 100 wild species, 102 landraces with colourless flesh and 17 landraces with colourful flesh, were used to identify the 1.7-kb insertion in the StMYB210 promoter (Table S3). Flesh colour for all clones was observed after harvest. The diploid clone 01–58, an accession with red skin and red flesh and harbouring the S-locus inhibitor (Sli) gene, was used for functional analysis of candidate genes and for constructing the selfed F2 population to analyse the segregation pattern of the I locus. Clone RH89-039-16 (RH), kindly provided by the Department of Plant Breeding at Wageningen University, has white flesh and white skin.
Genome-wide association study (GWAS)
Whole-genome resequencing data for the 135 diploid landraces were obtained from our previous study (Zhang et al., 2019). These genome sequences were aligned to the reference potato genome (DMv8.1) (Yang et al., 2023). A total of 5 852 788 bi-allelic SNPs with a missing rate below 20% and a minor allele frequency (MAF) of at least 5% were identified. Phenotypic data were defined as binary traits, with 1 representing red or purple colour and 0 representing yellow or white colour in tuber flesh. GWAS was performed using the EMMAX algorithm with kinship and population structure added as covariates into the mixed linear model as described previously (Kang et al., 2010). The threshold value was set to 3.85e-07 using Bonferroni correction as shown in the Manhattan plot for identifying the peak association signals.
RNA extraction, reverse transcription quantitative PCR (RT-qPCR) and end point RT-PCR
Tuber flesh and tuber skin from all accessions and the knockout lines were collected after harvest and ground in liquid nitrogen. Total RNA was extracted from each sample using the TRIzol Reagent (Invitrogen, Carlsbad, California, USA). First-strand cDNA was synthesized from 1 μg total RNA per each sample using a PrimeScript™ FAST RT reagent Kit with gDNA Eraser (TaKaRa, Kusatsu, Shiga Prefecture, Japan) and diluted five times with distilled water. Quantitative PCR (qPCR) for each sample was performed in a total reaction volume of 20 μL containing 2 μL diluted cDNA and 1.6 μL 10 μM primers (forward and reverse) using TB Green® Premix Ex Taq™ II (Tli RNaseH Plus) with ROX plus (TaKaRa, Kusatsu, Shiga Prefecture, Japan) on an ABI Step One System thermocycler (Applied Biosystems, Foster City, California, USA). Amplification followed the program: 95 °C for 30 s, then 40 cycles of 5 s at 95 °C, 15 s at 58 °C and 25 s at 72 °C. The StEIF3e (DM8C10G27650) gene was used as an internal control gene (Zhang et al., 2019), and three biological replicates were performed. Relative expression levels were calculated using the method (Livak and Schmittgen, 2001).
StMYB210 expression in the tuber flesh of 24 accessions from different species was examined by end point RT-PCR with the StEIF3e as a reference. cDNAs were amplified by PCR as follows: 1 μL of the cDNA produced as above was added to PrimeSTAR Max Premix (2X) (TaKaRa, Kusatsu, Shiga Prefecture, Japan) and 0.2 μM of each primer in a final volume of 25 μL. An initial step lasting 5 min at 94 °C was followed by 20–29 cycles of 10 s at 98 °C, 15 s at 58 °C and 20 s at 72 °C, with a final 5-min extension at 72 °C. The appropriate number of PCR cycles was determined for each primer pair in turn when the brightness of the PCR products by gel electrophoresis rose at a rate proportional to the addition of cDNA (Marone et al., 2001). The primers used for RT-qPCR and end point RT-PCR are listed in Table S5.
RNA-seq and data analysis
Samples from tuber skin and tuber flesh of clone 01–58 and knockout lines were collected for RNA-seq in three biological replicates. Sequencing libraries were generated using a NEBNext Ultra RNA Library Prep Kit for Illumina (NEB, Ipswich, Massachusetts, USA) and sequenced on an Illumina NovaSeq platform (paired-end 150 bp reads) to generate 4 Gb data of Raw reads by Annoroad Gene Technology (Beijing, China). RNA-seq data for clone RH89-039-16 were obtained from a previous study (Potato Genome Sequencing Consortium, 2011), with data from tuber peel used for tuber skin; data from tuber cortex and tuber pith were mixed and considered as tuber flesh. The clean reads were mapped to the reference potato genome (DMv8.1) (Yang et al., 2023) using hisat2 (Kim et al., 2019), and the FPKM values were calculated by StringTie (Pertea et al., 2015). The heat map was created using log2 (FPKM) values by TBtools (Chen et al., 2020, 2023).
CRISPR/Cas9 vector construction and plant transformation
CRISPR/Cas9 vector construction was performed as described previously (Xing et al., 2014). The single guide RNAs targeting each gene of interest were designed using CRISPRdirect tools (https://crispr.dbcls.jp/) and cloned into the modified genome editing vector pCAMBIA2300MGFPuv-sgRNACas (Du et al., 2023). The resultant CRISPR/Cas9 vectors were transformed into Agrobacterium (Agrobacterium tumefaciens) strain GV3101. The diploid red-flesh clone 01–58 was used as recipient for transformation as described previously (Ye et al., 2018). Transgenic plants were obtained by selection for kanamycin resistance and fluorescence observation (Du et al., 2023). Genomic DNA was extracted from each transgenic plant for PCR and Hi-TOM sequencing analysis of the putative target sites (Liu et al., 2019). Bi-allelic mutants were grown to harvest tubers. The primers used in vector construction and Hi-TOM sequencing are listed in Table S5.
Dual-LUC assays
StMYB210 promoter fragments from genotypes pf/pf and Pf/Pf of the diploid clone 01–58, the StbHLH1 promoter, the StDFR promoter and the StF3H promoter were individually amplified by genomic PCR and cloned into the pGreenII0800-LUC vector to generate reporter constructs. The resulting pGreenII0800-LUC constructs were transformed into Agrobacterium GV3101 carrying the helper plasmid pSoup19. The full-length coding sequences of StMYB200, StMYB210 and StbHLH1 were individually amplified from tuber flesh cDNA of diploid clone 01–58 and cloned into the pHB vector (Mao et al., 2005) and transformed into Agrobacterium strain GV3101 to generate effector constructs. Agrobacterium cultures carrying the reporter or the effector construct were mixed in the appropriate pairs and at a 1:2 (v/v) ratio before infiltration into N. benthamiana leaves. After 48 h, the infiltrated leaves were harvested for a Dual-LUC assay using a dual luciferase reporter assay kit (Vazyme, Nanjing, Jiangsu, China). Four biological replicates were measured for each combination. The primers used in vector construction are listed in Table S5.
Yeast one-hybrid (Y1H) assays
The full-length coding sequences of StMYB200 and StMYB210 were cloned individually into the pGADT7 vector. The StMYB210 promoter fragment containing the MBS1 (CAACGG) and MBS2 (AC/TCAAA) motifs was cloned into the pAbAi vector. The Y1H assays were performed according to the protocol of the Matchmaker Gold Yeast One-Hybrid Library Screening System (Clontech, San Francisco, CA). The primers used in vector construction are listed in Table S5.
Electrophoretic mobility shift assays (EMSA)
The full-length coding sequences of StMYB200 and StMYB210 were cloned individually into the pET-30 a (+) vector to generate the StMYB200-His and StMYB210-His expression vectors. The resulting plasmids were transformed into Escherichia coli strain BL21 (DE3). After the bacteria were cultured at 16 °C overnight, 0.6 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) was added to induce the production of recombinant StMYB200-His and StMYB210-His proteins. The recombinant proteins were purified according to a protocol described previously (Zhou et al., 2023). Probes representing the StMYB210 promoter containing the MBS1 (CAACGG) and MBS2 (AC/TCAAA) motifs were synthesized and labelled with biotin by Shanghai Sangon Biotechnology (Shanghai, China). Unlabelled DNA with the same sequence was used as a competitor. The EMSA was performed according to the recommendation of the Light Shift Chemiluminescent EMSA Kit (Beyotime, China). The primers and the oligonucleotide probes used in this study are listed in Table S5.
Yeast two-hybrid (Y2H) assays
The Y2H assays were performed following the manufacturer's instructions (Clontech). The full-length coding sequences of StMYB210 and StbHLH1 were cloned individually into the bait vector pGBKT7 (BD), and the full-length coding sequence of StbHLH1 was cloned into the prey vector pGADT7 (AD). Combinations of prey and bait vectors were co-transformed into cells of yeast strain AH109, which were then grown on synthetic defined (SD) medium lacking leucine and tryptophan (SD/−Leu/−Trp) at 28 °C. Positive transformants were tested on SD medium lacking leucine, tryptophan, histidine and adenine (SD/−Leu/−Trp/−His/−Ade) and containing 40 mg L − 1 X-α-gal and 15 mM 3-AT to detect the interaction. Transformants carrying empty vectors (BD or AD) were used as negative controls. The primers used in vector construction are listed in Table S5.
Pull-down assays
The full-length coding sequences of StMYB200, StMYB210 and StbHLH1 were cloned into the pGEX-6P-1 or pMAL-c2x vectors. The resulting constructs were introduced into DE3, and protein production was induced by the addition of IPTG. The recombinant proteins StMYB200-GST, StbHLH1-GST, StMYB200-MBP and StMYB210-MBP were purified according to a previously described protocol (Zhou et al., 2023). These pull-down assays were performed according to the protocol of the Pierce™ GST Protein Interaction Pull-Down Kit (ThermoFisher Scientific, Waltham, Massachusetts, USA). The pulled-down proteins were detected by immunoblotting using anti-MBP antibodies (Abmart, Code M30111, dilution 1:1000) and anti-GST antibodies (Abmart, Code M20007, dilution 1:1000). The primers used in vector construction are listed in Table S5.
Luciferase complementation imaging (LCI) assays
LCI assays were carried out as described previously (Zhou et al., 2018). The full-length coding sequences of StMYB210 and StbHLH1 were cloned individually into the pCAMBIA1300-Nluc vector (Nluc), whereas the full-length coding sequences of StMYB200 and StMYB210 were cloned into the pCAMBIA1300-Cluc vector (Cluc). All constructs were transformed into Agrobacterium strain GV3101, and then the transformants carrying the Nluc or Cluc vectors were mixed in a 1:1 (v/v) ratio before infiltration into N. benthamiana leaves. After 48 h, the infiltrated leaves were sprayed with D-Luciferin (1 mM; Vazyme, Nanjing, Jiangsu, China) and then kept in the dark for 10 min. Images representing luciferase activity were captured using a chemiluminescence imaging system (Tanon, Shanghai, China). N. benthamiana leaves infiltrated with Agrobacterium strains carrying empty vectors (Nluc or Cluc) were used as negative controls. The primers used in vector construction are listed in Table S5.
Bimolecular fluorescence complementation (BiFC) assays
For BiFC assays, the full-length coding sequences of StMYB210 and StbHLH1 without the stop codon were cloned into the pSPYNE vector, and the full-length coding sequences of StMYB200 and StbHLH1 were cloned into the pSPYCE vector. All constructs were individually transformed into Agrobacterium strain GV3101, respectively. The Agrobacterium strains containing one nYFP construct or one cYFP construct were mixed with an Agrobacterium strain carrying a plasmid with a nuclear localization control (pCAMBIA2300-35S-H2B-mCherry) in a 1:1:1 (v/v/v) ratio and infiltrated into N. benthamiana leaves. YFP signal was observed 48 h later with a confocal laser-scanning microscope. The primers used in vector construction are listed in Table S5.
DNA extraction and identification of the 1.7-kb insertion in the StMYB210 promoter
Genomic DNA was extracted from diploid potato accessions. Conserved genomic regions in the promoter and gene body of StMYB210 were identified from the resequencing data of some accessions to design primers annealing on either side of the 1.7-kb insertion. To identify the 1.7-kb insertion, PCR amplification was performed using PrimeSTAR GXL polymerase (Takara, Kusatsu, Shiga Prefecture, Japan). The primers used in identifying the insertion are listed in Table S5.
Statistical analyses
Data are means ± SD (n ≥ 3). In this study, one-way ANOVA and Student's t-test were used to analyse the statistical data by GraphPad Prism v.8.2.1 for Windows. Different letters indicate significant differences (P < 0.05), and ns indicates no significant difference.
Accessions numbers
Sequence data in this article can be found in the web site (http://www.bioinformaticslab.cn/pubs/dm8/) under the following accessions number: StMYB200 (DM8C10G21200), StMYB210 (DM8C10G21210), StMYB120 (DM8C10G21120), StMYB130 (DM8C10G21130), StMYB170 (DM8C10G21170), StMYB180 (DM8C10G21180), StMYB240 (DM8C10G21240), and StMYB270 (DM8C10G21270), 140 (DM8C10G21140), 150 (DM8C10G21150), 160 (DM8C10G21160), 190 (DM8C10G21190), 220 (DM8C10G21220), 230 (DM8C10G21230), 250 (DM8C10G21250), 260 (DM8C10G21260), 280 (DM8C10G21280), and 290 (DM8C10G21290); StPAL-1 (DM8C05G27310), StPAL-2 (DM8C09G05090), StPAL-3 (DM8C09G05100), StPAL-4 (DM8C10G21350), StC4H-1 (DM8C06G32330), StC4H-2 (DM8C06G32340), St4CL-1 (DM8C03G32680), St4CL-2 (DM8C06G24210), StCHS-1 (DM8C05G24190), StCHS-2 (DM8C09G29030), StCHS-3 (DM8C09G29040), StCHI-1 (DM8C05G02010), StCHI-2 (DM8C05G02020), StCHI-3 (DM8C05G22750), StF3H (DM8C02G23930), StF3′H (DM8C03G29990), StF3′5′H (DM8C11G21030), StDFR (DM8C02G24950), StANS-1 (DM8C01G28350), StANS-2 (DM8C01G42270), StANS-3 (DM8C07G19220) and ANS-4 (DM8C08G26790); StbHLH1 (DM8C09G19810) and StEIF3e (DM8C10G27650).
Acknowledgements
We thank Prof. William John Lucas, University of California Davis, USA, for the revisions to this manuscript. We also thank Dr. Zhen Wang from School of Life Sciences, Anhui Agricultural University, Dr. Jinjing Sun from Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences and Dr. Huimin Zhang from College of Horticulture, Qingdao Agricultural University, for their critical discussions and comments. We are thankful to Dr. Jieping Li from International Potato Center (CIP) China Center for Asia Pacific (CCCAP), for his help during the experiment. We are grateful to all teachers from Yunnan Key Laboratory of Potato Biology, the AGISCAAS-YNNU Joint Academy of Potato Sciences, Yunnan Normal University, for their help during these studies.
Conflict of interest
The authors declare no competing interests.
Author contributions
H.D., G.Z. and C.Z. planned and designed the research. H.D., ZF.Z. and J.P. performed experiments and analysed data. P.W. and Y.Z. performed the materials planting and phenotypic survey. Z.Z. performed a GWAS. J.L., R.W. and L.H. performed plant transformation. D.L. and K.C. provided technical support. H.D., ZF.Z. and C.Z. wrote the manuscript. C.Z., G.Z., H.D., ZF.Z. and J.P. revised the manuscript. All authors read and approved the final manuscript.
Funding
The work was supported by China National Key Research and Development Program (2023YFF1000400), National Natural Science Foundation of China (32372695, 32102375, U2002204 and 32488302), Guangdong Major Project of Basic and Applied Basic Research (2021B0301030004) and Agricultural Science and Technology Innovation Program (CAAS-ZDRW202404).
Open Research
Data availability statement
The datasets generated and/or analysed during the current study are provided with this paper. The RNA-seq data in this article are available at NCBI with BioProject accession number PRJNA1160339 (https://www.ncbi.nlm.nih.gov/sra/PRJNA1160339).




