The MdBBX22–miR858–MdMYB9/11/12 module regulates proanthocyanidin biosynthesis in apple peel
Summary
Proanthocyanidins (PAs) have antioxidant properties and are beneficial to human health. The fruit of apple (Malus × domestica Borkh.), especially the peel, is rich in various flavonoids, such as PAs, and thus is an important source of dietary antioxidants. Previous research on the regulation of PAs in apple has mainly focussed on the transcription level, whereas studies conducted at the post-transcriptional level are relatively rare. In this study, we investigated the function of mdm-miR858, a miRNA with multiple functions in plant development, in the peel of apple fruit. We showed that mdm-miR858 negatively regulated PA accumulation by targeting MdMYB9/11/12 in the peel. During fruit development, mdm-miR858 expression was negatively correlated with MdMYB9/11/12 expression and PA accumulation. A 5′-RACE experiment, GUS staining assays and transient luminescent assays indicated that mdm-miR858 cleaved and inhibited the expression of MdMYB9/11/12. Overexpression of mdm-miR858 in apple calli, tobacco and Arabidopsis reduced the accumulation of PAs induced by overexpression of MdMYB9/11/12. Furthermore, we found that MdBBX22 bound to the mdm-miR858 promoter and induced its expression. Overexpression of MdBBX22 induced the expression of mdm-miR858 to inhibit the accumulation of PAs in apple calli overexpressing MdMYB9/11/12. Under light stress, MdBBX22 induced mdm-miR858 expression to inhibit PA accumulation and thereby indirectly enhanced anthocyanin synthesis in the peel. The present results revealed that the MdBBX22–miR858–MdMYB9/11/12 module regulates PA accumulation in apple. The findings provide a reference for further studies of the regulatory mechanism of PA accumulation and the relationship between PAs and anthocyanins.
Introduction
Proanthocyanidins (PAs), also termed condensed tannins, are important polyphenolic compounds synthesized in plants. These compounds are present in the fruit, bark, seeds and leaves of plants (Dauer et al., 2003; Gabetta et al., 2000; Prior et al., 2001; Savitri Kumar et al., 2009). The structural characteristic of PAs in containing multiple phenolic hydroxyl groups confers the compounds with a stronger capability to scavenge oxygen free radicals than that of vitamins C and E (Bagchi et al., 1997). As natural antioxidants, PAs can reduce melanin production and inhibit the formation of age spots (Furumura et al., 2012; Kim and Yokozawa, 2009). Proanthocyanidins also function in protecting the cardiovascular system, preventing hypertension and inducing tumour cell apoptosis (Bagchi et al., 2003; Kawakami et al., 2011; Roy et al., 2005). In addition, PAs impart flavour and astringency to beverages, such as fruit juice, wine and tea and may function as feeding deterrents to protect plants from herbivore damage (Forkner et al., 2004). The peel and pulp of apple fruit contain PAs, which are more abundant in the peel (Henry-Kirk et al., 2012).
Proanthocyanidins, flavonols and anthocyanins are products of the flavonoid pathway. Their synthesis is controlled by genes encoding enzymes involved in the formation of the biochemical structure of the compound and regulatory genes that control the expression of these synthetic enzymes (Hichri et al., 2011; Koes et al., 2005). Leucoanthocyanidin reductase (LAR) and anthocyanidin reductase (ANR) are specific catalytic enzymes of the PA branch that convert leucocyanidin and cyanidin into catechins and epicatechins, respectively (Tanner et al., 2003; Xie et al., 2003). These monomers are further condensed to form PA polymers (Dixon et al., 2005). The transcription factors that regulate the expression of these two enzymes have been identified in multiple species. For example, Arabidopsis TRANSPARENT TESTA 2 (TT2) promotes PA accumulation in the seed by inducing the expression of BANYULS (which encodes ANR) (Nesi et al., 2001). In grape, VvMYB5b and VvMYBPA2 promote PA accumulation in the peel of youngberries by inducing the expression of VvLAR and VvANR (Deluc et al., 2008; Terrier et al., 2009). In persimmon, DkMYB4 binds to the DkANR promoter, and decrease in DkMYB4 expression leads to a decline in PA biosynthesis and the resulting nonastringent-type traits (Akagi et al., 2009). FaMYB9 and FaMYB11 promote PA accumulation in strawberry and activate the expression of Arabidopsis ANR (Schaart et al., 2013). In apple, MdMYB9/11 can bind to the promoters of the PA branch genes MdLAR and MdANR, but not the promoter of the anthocyanidin branch-specific gene MdUFGT. Co-transformation of MdbHLH3 with MdMYB9/11 significantly enhances the transcriptional activity of the MdANR promoter (An et al., 2015). MdMYB12 is able to interact with MdbHLH33 and MdbHLH3, and co-transformation of MdbHLH33 and MdMYB12 significantly enhances the transcriptional activity of the MdLAR promoter (Wang et al., 2017b). Further, overexpression of MdMYB9/11/12 in apple calli promotes PA accumulation (An et al., 2015; Wang et al., 2017b).
The B-box (BBX) protein is a zinc finger protein containing one or two B-box motifs (Reymond et al., 2001). This transcription factor regulates flavonoid accumulation in plants (Song et al., 2020). In Arabidopsis, AtBBX21 and AtBBX22 interact with AtHY5 and enhance its biochemical activity, leading to increased expression of flavonoid biosynthetic genes and accumulation of anthocyanins. In addition, AtBBX21 and AtBBX22 directly activate AtCHI transcription to promote anthocyanin accumulation (Datta et al., 2007, 2008). MdBBX22, MdBBX20, PpBBX16 and PpBBX18 also interact with HY5 to induce the expression of MdMYB1 or PpMYB10 and thus promote anthocyanin accumulation in apple and pear peel (Bai et al., 2014, 2019a,b; Fang et al., 2019b). In our previous study, we observed that MdBBX21 can bind to the MdHY5, MdBBX20 and MdBBX22 promoters, and induces their expression and ultimately the accumulation of anthocyanidins in apple peel (Zhang et al., 2021). However, whether BBX proteins regulate miRNA expression and PA accumulation in apple has not been reported.
In addition to transcriptional regulators, microRNA (miRNA), a type of noncoding endogenous small RNA composed of 18–24 nucleic acids (nt), can negatively regulate its target gene at the post-transcriptional level through two silencing mechanisms: cleavage or translational inhibition of mRNA expression (Zhang et al., 2006). Increasing evidence has shown that miRNA is involved in the regulation of plant growth and development, and plays an important regulatory role in the response to various stresses (Nonogaki, 2010; Sun et al., 2019; Vashisht and Nodine, 2014; Zhang, 2015). In plants, miR858 is a conserved miRNA that has been reported to have multiple functions (Kozomara and Griffiths-Jones, 2011). In cotton, miR858 targets GhMYB2 to regulate fibre development (Guan et al., 2014). In the grape, miR858 promotes anthocyanin accumulation by regulating VvMYB114 (Tirumalai et al., 2019). Overexpression of miR858 interferes with Heterodera schachtii parasitism in Arabidopsis and results in reduced susceptibility (Piya et al., 2017). In addition, Arabidopsis miR858 can target the flavonol regulators AtMYB11, AtMYB12 and AtMYB111 to regulate flavonol accumulation (Sharma et al., 2016). At the same time, miR858 also targets the anthocyanin inhibitor AtMYBL2 to promote anthocyanin accumulation in Arabidopsis, but miR858 is incapable of targeting AtTT2 (Wang et al., 2016).
In apple, there are currently few reports on the regulation of PA at the post-transcriptional level. In addition, the relationship between PAs and anthocyanins in apple is unclear. miRNA mainly participates in plant growth, development and stress response by regulating gene expression at the post-transcriptional level (Nonogaki, 2010; Sun et al., 2019). In this study, we characterized the function of mdm-miR858. mdm-miR858 could target MdMYB9/11/12 to regulate PA accumulation. Under light stress, MdBBX22 induced the expression of mdm-miR858 to inhibit PA accumulation and altered metabolism to enhance anthocyanin synthesis to promote rapid colouration of the peel. The present research lays a foundation for further investigation of the regulatory mechanism of PA accumulation and the relationship between PAs and anthocyanins.
Results
Expression of mdm-miR858 is negatively correlated with PA accumulation during apple peel development
Contents of catechin, epicatechin, procyanidin B1, procyanidin B2 and total PAs decreased rapidly from 30 to 60 days after full bloom (DAFB), then increased slightly from 60 to 90 DAFB and thereafter decreased during peel development in apple ‘Starkrimson Delicious’ fruit. The contents of quercetin glycosides showed distinct trends. Among these compounds, quercetin-3-rutinoside, quercetin-3-galactoside and quercetin-3-arabinoside contents decreased rapidly from 30 to 60 DAFB and gradually increased from 60 to 140 DAFB. Quercetin-3-glucoside and quercetin-3-xyloside contents remained unchanged from 60 to 140 DAFB. Chlorogenic acid content declined throughout the fruit growth and development period. Cyanidin-3-galactoside content decreased from 30 to 60 DAFB, was almost undetectable from 60 to 90 DAFB and rapidly accumulated after 90 DAFB (Figure 1a,b; Figure S1).
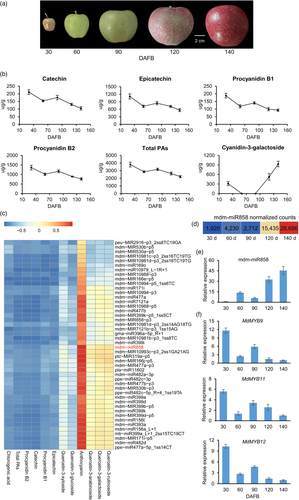
To identify miRNAs involved in the regulation of PA accumulation, apple peel samples were collected at 30, 60, 90, 120 and 140 DAFB and used for sRNA sequencing. In total, 5106 unique mature miRNAs were identified. All miRNAs were classified into three groups: known miRNAs (617), conservative miRNAs (60) and novel miRNAs (4429) (Table S1). A correlation analysis of the expression of these miRNAs with total PA content was performed. The expression of 188 miRNAs (39 known miRNAs, 7 conservative miRNAs and 142 novel miRNAs) was negatively correlated with PA content (r ≤ −0.8) (Table S2, Figure 1c). Prediction of their target genes revealed that only the predicted target gene of mdm-miR858, MdMYB9/11/12, is involved in flavonoid biosynthesis (Table S3). The expression pattern of mdm-miR858 based on sRNA sequencing data was validated by the results of qRT-PCR analysis (Figure 1d,e). These results suggested that mdm-miR858 may be involved in the regulation of PA accumulation.
MdMYB9/11/12 are the target genes of mdm-miR858
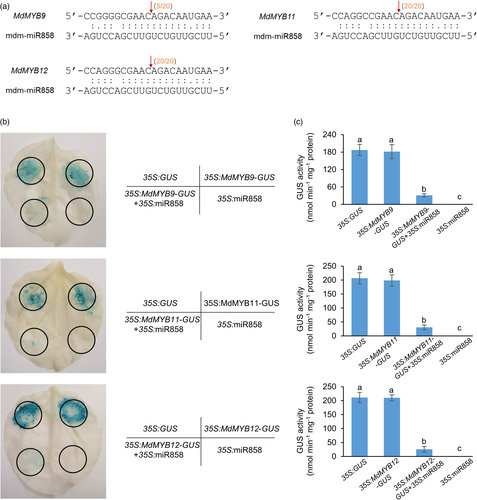
To demonstrate that mdm-miR858 regulates PA accumulation by targeting MdMYB9/11/12, we determined the relative expression of MdMYB9/11/12 genes (Figure 1f). The expression patterns of MdMYB9/11/12 were in contrast to mdm-miR858 and consistent with the changes in total PA content. MdLAR1/2 and MdANR1/2 showed the same expression pattern as MdMdMYB9/11/12 (Figure S2). Further, a 5′ rapid amplification of cDNA ends (5′-RACE) experiment showed that mdm-miR858 cleaved the target genes MdMYB9/11/12 at the 10th and 11th bases of the complementary base-pairing region (Figure 2a). To confirm the regulation of mdm-miR858 on MdMYB9/11/12, we applied co-transformation technology to tobacco (Nicotiana benthamiana) in accordance with a previously described method (Wang et al., 2017a) (Figure 2b). The recombinant vector containing the GUS reporter gene was introduced into tobacco leaves through an Agrobacterium-mediated transformation system. Histochemical staining revealed that the leaves infiltrated with 35S:MdMYB9/11/12-GUS and the 35S:GUS control leaves showed a similar GUS phenotype. The GUS phenotype was not observed in the leaves infiltrated with 35S:mdm-miR858, in which GUS sequence was replaced by the precursor of mdm-miR858. The intensity of GUS staining was notably reduced when 35S:mdm-miR858 and 35S:MdMYB9/11/12-GUS were co-transformed into tobacco leaves. To verify these histochemical observations, a fluorospectrophotometer was used to determine the GUS content in the injection area of each leaf (Figure 2c). The measurements were consistent with the phenotypic observations. These results provided additional evidence that mdm-miR858 targets MdMYB9/11/12.
mdm-miR858 targets MdMYB9/11/12 to regulate the expression of downstream structural genes
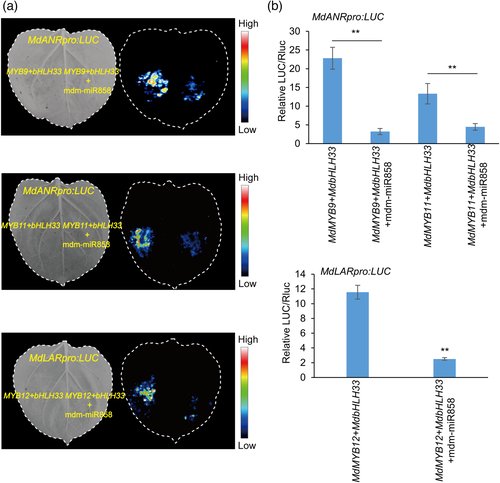
MdMYB9, MdMYB11 and MdMYB12 in apple can combine with MdbHLH33 and MdbHLH3 to promote the expression of the structural genes MdANR and MdLAR (An et al., 2015; Wang et al., 2017b). In this experiment, tobacco leaves co-transformed with 35S:MdMYB9+35S:MdbHLH33 +MdANRpro:LUC showed strong fluorescence, but if 35S:mdm-miR858 was co-infiltrated with 35S:MdMYB9+35S:MdbHLH33+MdANRpro:LUC, the fluorescence intensity was significantly reduced (Figure 3a). Similarly, when we co-transformed 35S:mdm-miR858 with 35S:MdMYB11+ 35S:MdbHLH33+MdANRpro:LUC or 35S:mdm-miR858 with 35S:MdMYB12+35S:MdbHLH33+ MdLARpro:LUC, the fluorescence intensity was significantly reduced compared with that in the absence of mdm-miR858 (Figure 3a). The results of luciferase (LUC) activity determination were similar to the phenotypic observations (Figure 3b). These results indicated that mdm-miR858 targets MdMYB9/11/12 to regulate the expression of downstream structural genes.
mdm-miR858 targets MdMYB9/11/12 to inhibit PA accumulation in apple calli
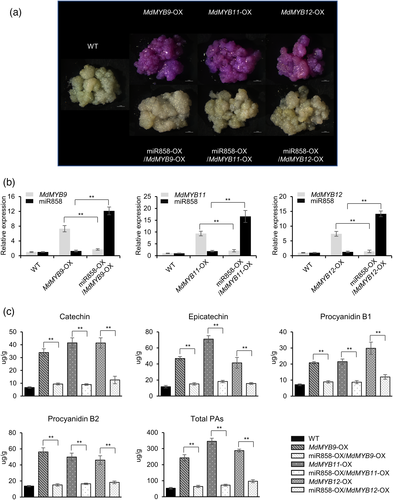
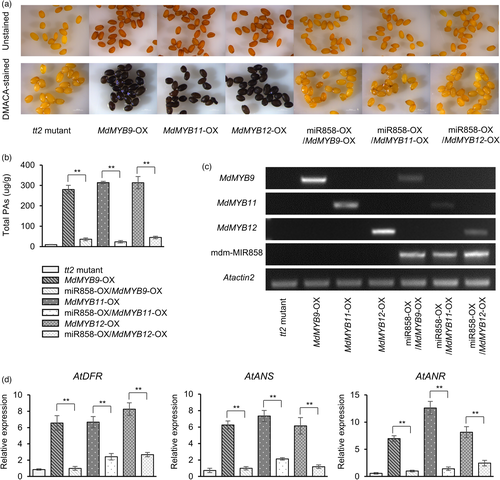
Phylogenetic analysis indicates that MdMYB9/11/12 belongs to a putative PA-specific clade in other plant species (Figure S3), which suggests that these proteins may be functionally redundant. To test this hypothesis, we silenced MdMYB9, MdMYB11 and MdMYB12 in apple peel and apple calli (Figure S4). Silencing of MdMYB9, MdMYB11 and MdMYB12 individually reduced the total PA content in both apple peel and apple calli; however, when MdMYB9, MdMYB11 and MdMYB12 were silenced simultaneously, the total PA content decreased more significantly (Figure S4). These results suggested that MdMYB9, MdMYB11 and MdMYB12 were functionally redundant in regulating PAs. To verify that mdm-miR858 inhibits PA accumulation, MdMYB9-OX, MdMYB11-OX and MdMYB12-OX calli were generated. After staining with 4-dimethylaminocinnamaldehyde (DMACA), MdMYB9-OX, MdMYB11-OX and MdMYB12-OX calli were stained purple (Figure 4a). Quantitative reverse transcription PCR (RT-qPCR) analysis showed that the expression of MdMYB9, MdMYB11 and MdMYB12 in MdMYB9-OX, MdMYB11-OX and MdMYB12-OX calli was significantly increased compared with that in wild-type calli (Figure 4b). HPLC analysis showed that the contents of catechin, epicatechin, procyanidin B1, procyanidin B2 and total PAs in MdMYB9-OX, MdMYB11-OX and MdMYB12-OX calli were higher than those detected in wild-type calli (Figure 4c). When mdm-miR858 was overexpressed in MdMYB9-OX, MdMYB11-OX and MdMYB12-OX calli, the expression levels of MdMYB9, MdMYB11 and MdMYB12 were significantly reduced in mdm-miR858-OX/MdMYB9-OX, mdm-miR858-OX/MdMYB11-OX and mdm-miR858-OX/MdMYB12-OX calli (Figure 4b). After staining with DMACA, the purple colouration was absent in mdm-miR858-OX/MdMYB9-OX, mdm-miR858-OX/MdMYB11-OX and mdm-miR858-OX/MdMYB12-OX calli (Figure 4a). Catechin, epicatechin, procyanidin B1, procyanidin B2 and total PA contents were significantly reduced compared with those of MdMYB9-OX, MdMYB11-OX and MdMYB12-OX calli (Figure 4c). The expression levels of the structural genes MdLAR1, MdLAR2, MdANR1 and MdANR2 were reduced compared with those of MdMYB9-OX, MdMYB11-OX and MdMYB12-OX calli (Figure S5).
mdm-miR858 targets MdMYB9/11/12 to inhibit PA accumulation in tobacco
Tobacco flowers contain flavonoids, such as anthocyanins, PAs and flavonols (Han et al., 2012). To verify that mdm-miR858 targets MdMYB9/11/12 to inhibit PA accumulation, we first overexpressed MdMYB9/11/12 in tobacco (Figure S6a). It was interesting that MdMYB9-OX, MdMYB11-OX and MdMYB12-OX tobacco produced flowers with either a pale pink or pure white corolla (Figure S6a). Further testing revealed that the anthocyanin (Cyanidin-3-O-rutinoside) content in flowers of MdMYB9-OX, MdMYB11-OX and MdMYB12-OX tobacco was significantly reduced. The contents of catechins, epicatechins and total PAs increased significantly, but that of flavonols did not change notably (Figure S6c). When mdm-miR858 was transferred to MdMYB9-OX, MdMYB11-OX and MdMYB12-OX tobacco, mdm-MIR858 (pre-mdm-miR858) and mdm-miR858 were expressed in large quantities, whereas the expression of MdMYB9, MdMYB11 and MdMYB12 was significantly reduced (Figures S6b and Figure S7). The corolla colour of the tobacco flowers was comparable to that of the wild type (Figure S6a). HPLC analysis showed that the anthocyanin content in flowers of mdm-miR858-OX/MdMYB9-OX, mdm-miR858-OX/MdMYB11-OX and mdm-miR858-OX/MdMYB12-OX tobacco increased. However, the contents of catechins, epicatechins and total PAs decreased, whereas the contents of other flavonols remained unchanged (Figure S6c). In addition, we detected the relative expression of genes associated with the flavonoid metabolism pathway. The expression of NtDFR, NtANS, NtLAR, NtANR1 and NtANR2 increased in MdMYB9-OX, MdMYB11-OX and MdMYB12-OX tobacco flowers, whereas the expression levels of these genes were significantly reduced in flowers of mdm-miR858-OX/MdMYB9-OX, mdm-miR858-OX/MdMYB11-OX and mdm-miR858-OX/MdMYB12-OX tobacco. The expression of other structural genes remained unchanged (Figure S8).
mdm-miR858 targets MdMYB9/11/12 to inhibit PA accumulation in Arabidopsis
The Arabidopsis TT2 gene encodes an R2R3-MYB domain protein that regulates PA accumulation in the seed. The seed coat of the tt2 mutant is a buff colour (Nesi et al., 2001). When we transferred MdMYB9/11/12 into the tt2 mutant, the colour of the seed coat changed to brown and DMACA-stained the seed coat blue-black (Figure 5a). The PA contents and the relative expression levels of MdMYB9, MdMYB11 and MdMYB12 in the seeds of MdMYB9-OX/tt2, MdMYB11-OX/tt2 and MdMYB12-OX/tt2 Arabidopsis were significantly increased compared with those of the tt2 mutant (Figure 5b,c). When mdm-miR858 was transformed into MdMYB9-OX/tt2, MdMYB11-OX/tt2 and MdMYB12-OX/tt2 Arabidopsis, mdm-MIR858 (pre-mdm-miR858) and mdm-miR858 were expressed in large quantities (Figure 5c; Figure S9), The seed coat was a buff colour and was not stained by DMACA (Figure 5a). The content of total PAs and the expression of MdMYB9/11/12 in the seed were significantly reduced (Figure 5b,c; Figure S9). In addition, the expression of AtDFR, AtANS and AtANR was significantly reduced compared with those of MdMYB9-OX/tt2, MdMYB11-OX/tt2 and MdMYB12-OX/tt2 Arabidopsis (Figure 5d). The expression of other structural genes remained unchanged (Figure S10).
MdBBX22 binds to the mdm-miR858 promoter and induces mdm-miR858 expression
The promoter fragment of mdm-miR858 (Figure S11a) was used for yeast one-hybrid library screening to identify mdm-miR858-associated proteins. The results revealed that MdBBX22 was sequenced (Table S4, Figure S12). To study whether MdBBX22 can activate the transcription of mdm-miR858, the promoter of mdm-miR858 was fused with the LUC reporter gene. Co-transformation of 35S:MdBBX22 and mdm-miR858pro:LUC significantly increased the luminescence intensity (Figure 6a,b). This result indicated that MdBBX22 positively regulated the expression of mdm-miR858.
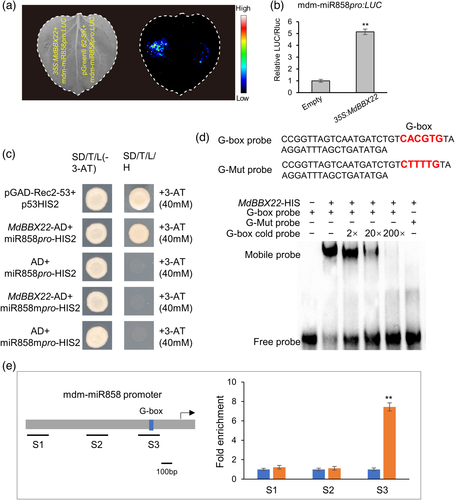
Previous studies have shown that the BBX protein regulates transcription by binding to the G-box motif (Datta et al., 2008; Xiong et al., 2019; Xu et al., 2016). The G-box motifs (5′-CACGTG-3′) in the mdm-miR858 promoter were mutated to 5′-CTTTTG-3′ and then cloned into the pHIS2 reporter vector to obtain mdm-miR858mpro-HIS2 (Figure S11b). Yeast cells co-transformed with pGADT7-MdBBX22 and mdm-miR858pro-HIS2 grew on SD/−His/−Leu−Trp screening medium supplemented with 3-AT. However, yeast cells co-transformed with pGADT7-MdBBX22 and mdm-miR858mpro-HIS2 did not grow under the same conditions (Figure 6c). This result indicated that MdBBX22 was able to bind to the G-box motif in the mdm-miR858 promoter. The results of electrophoretic mobility shift assays (EMSAs) further supported this result (Figure 6d).
To confirm that MdBBX22 is bound to the promoter of mdm-miR858 in vivo, we conducted chromatin immunoprecipitation (ChIP)-PCR assay. The coding sequence (CDS) of MdBBX22 fused to the GFP-tagged sequence was overexpressed in apple calli. The presence of MdBBX22 substantially enhanced the PCR-based detection of the mdm-miR858 promoter fragment S3 containing the G-motif (Figure 6e), indicating that MdBBX22 bound to the mdm-miR858 promoter in vivo.
MdBBX22 inhibits MdMYB9/11/12-induced PA accumulation by promoting the expression of mdm-miR858 in apple calli
To further verify that MdBBX22 induced the expression of mdm-miR858 to inhibit PA synthesis, MdBBX22 was overexpressed in MdMYB9-OX, MdMYB11-OX and MdMYB12-OX apple calli. The MdBBX22-OX/MdMYB9-OX, MdBBX22-OX/MdMYB11-OX and MdBBX22-OX/MdMYB12-OX calli were stained pale pink by DMACA (Figure 7a). RT-qPCR analysis showed that MdBBX22 and mdm-miR858 were highly expressed in MdBBX22-OX/MdMYB9-OX, MdBBX22-OX/MdMYB11-OX and MdBBX22-OX/MdMYB12-OX calli, whereas the expression levels of MdMYB9, MdMYB11 and MdMYB12 were significantly reduced compared with those of MdMYB9-OX, MdMYB11-OX and MdMYB12-OX calli (Figure 7b). HPLC analysis showed that the contents of catechin, epicatechin, procyanidin B1, procyanidin B2 and total PAs in MdBBX22-OX/MdMYB9-OX, MdBBX22-OX/MdMYB11-OX and MdBBX22-OX/MdMYB12-OX calli were significantly reduced compared with those of MdMYB9-OX, MdMYB11-OX and MdMYB12-OX calli (Figure 7c). The relative expression of the structural genes MdLAR1, MdLAR2, MdANR1 and MdANR2 also decreased (Figure S13).
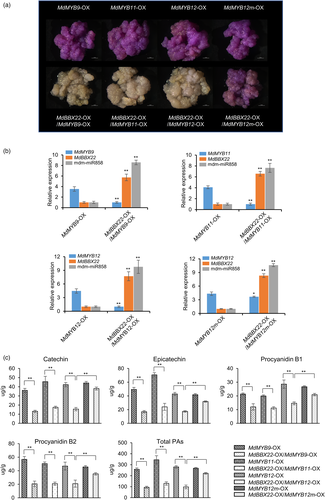
To further demonstrate that MdBBX22 inhibition of MdMYB9/11/12 function is mediated by mdm-miR858, we generated constructs harbouring 35S:MYB12m, a miR858-resistant form of MdMYB12 with 5 bp of synonymous mutations in the miR858 target site under the control of the Cauliflower mosaic virus 35S (CaMV35S) promoter (Figure S14a) and generated MdMYB12m-OX apple calli (Figure 7a). The contents of catechin, epicatechin, procyanidin B1, procyanidin B2 and total PAs in MdMYB12m-OX apple calli were similar to those in MdMYB12-OX apple calli. However, the contents of catechin, epicatechin, procyanidin B1, procyanidin B2 and total PAs in MdMYB12m-OX calli were reduced by 14%–24% under MdBBX22 overexpression. In contrast, these contents were reduced by 49%–65% in MdMYB12-OX calli when MdBBX22 was overexpressed (Figure 7c). These results revealed that mutation at the mdm-miR858 target site is effective in attenuating the inhibitory effect of MdBBX22 on MdMYB12-induced PA. The increased expression of mdm-miR858 was essentially the same in MdMYB12-OX and MdMYB12m-OX calli under MdBBX22 overexpression (Figure 7b). Taken together, these findings suggested that MdBBX22 inhibited MdMYB9/11/12-induced PA accumulation by promoting the expression of mdm-miR858 in apple calli.
MdBBX22 induces the expression of mdm-miR858 to inhibit PA accumulation and alters metabolism to enhance anthocyanin synthesis in apple peel under light stress
MdBBX22 is a light-responsive transcription factor in apple (An et al., 2019; Bai et al., 2014). This led us to speculate that MdBBX22 might regulate the expression of mdm-miR858 and thus affect PA accumulation under light stress. First, apple fruit were bagged at 45 DAFB, and at 120 DFAB, the fruit were unpacked and placed in a light incubator for exposure to continuous light. The content of total PAs decreased under light stress, whereas the anthocyanin content increased (Figure 8a). RT-qPCR analysis showed that the expression of MdBBX22 and mdm-miR858 increased under light stress, whereas the expression of MdMYB11 and MdMYB12 decreased under light stress (Figure 8b), and MdMYB9 always showed a low expression level. These results indicated that MdBBX22 may be involved in the regulation of PA by up-regulating mdm-miR858 expression under light stress.
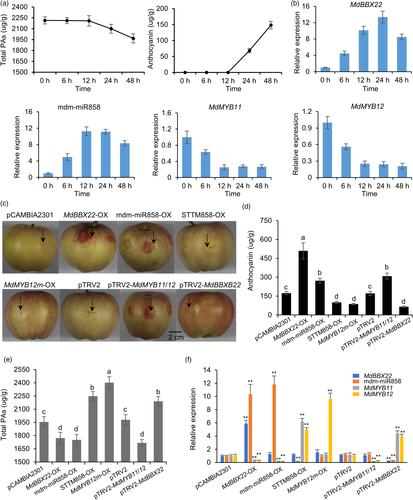
To verify that MdBBX22 can induce the expression of mdm-miR858 to inhibit PA accumulation in apple peel under light stress, we overexpressed MdBBX22 in apple peel (Figure 8c). The expression levels of MdBBX22 and mdm-miR858 and the anthocyanin content in MdBBX22-OX apple peel were increased (Figure 8d,f), whereas the expression of MdMYB11 and MdMYB12 and the content of total PAs were decreased (Figure 8e,f). In contrast, the expression of MdBBX22 and mdm-miR858 and the anthocyanin content were decreased in pTRV2-MdBBX22 apple peel (Figure 8d,f), whereas the expression of MdMYB11 and MdMYB12 and the content of total PAs increased (Figure 8e,f). These results indicated that MdBBX22 induces the expression of mdm-miR858 to inhibit PA accumulation in apple peel under light stress.
The opposite patterns of change in anthocyanin and total PA contents in the aforementioned experiment led us to speculate that inhibition of the PA pathway under light stress may contribute to the rapid synthesis of anthocyanins. To test this hypothesis, mdm-miR858 was overexpressed and MdMYB11/12 was silenced in apple peel (Figure 8c). The expression of MdMYB11/12 was reduced in mdm-miR858-OX apple peel and pTRV2-MdMYB11/12 apple peel accompanied by a decrease in total PA content, whereas the anthocyanin content increased (Figure 8d–f). Previous studies have shown that short tandem target mimics (STTMs) can inhibit miRNA function (Yan et al., 2012). Therefore, we constructed the 35S:STTM858 vector (Figure S14b) under the control of the CaMV35S promoter. In STTM858-OX and MdMYB12m-OX apple peel, the total PA content increased, whereas the anthocyanin content decreased (Figure 8d,e). These results revealed that inhibition of the PA pathway under light stress contributed to the rapid synthesis of anthocyanins. Taken together, these findings suggested that under light stress, MdBBX22 inhibited the expression of MdMYB11 and MdMYB12 by inducing the expression of mdm-miR858 and ultimately led to the decrease in PA accumulation in the apple peel, and that inhibition of the PA branch promoted the rapid synthesis of anthocyanins.
Discussion
Expression of mdm-miR858 negatively correlates with PA synthesis in apple peel
Proanthocyanidins have many functions that are beneficial to human health, such as antioxidation, anti-inflammation, antitumour activity and beauty care (Bagchi et al., 2000; Cos et al., 2004; Katiyar, 2016; Vaid et al., 2016). Apple fruit is rich in a variety of polyphenols, including PAs, and a variety of vitamins (Radenkovs et al., 2020; Tsao et al., 2003). Hence the saying, ‘An apple a day keeps the doctor away’. Fruit development is accompanied by changes in total PA content (Li et al., 2002). In grape, PA synthesis mainly occurs in the exocarp of youngberries and the PA content of the peel decreases during the ripening process (Bogs et al., 2005; Tirumalai et al., 2019). The content of PAs in young fruit of strawberry and peach is higher than that of mature fruit (Schaart et al., 2013; Zhou et al., 2015). In the present study, PAs accumulate in large amounts in apple peel during the young fruit stage and then gradually decrease at advanced stages (Figure 1b). This pattern is similar to the change in PA contents in other fruit, such as grape, strawberry and peach.
The biosynthesis of flavonols, PAs and anthocyanins share common steps in the flavonoid pathway (Holton and Cornish, 1995; Shirley et al., 1992; Winkel-Shirley, 2001). The activity of specific enzymes associated with flavonols, PAs and anthocyanins only leads to the synthesis of corresponding compounds (Boss et al., 1996; Devic et al., 1999; Holton et al., 1993; Tanner et al., 2003). These specific enzymes are regulated by different transcription factors. For example, AtMYB11/12/111, VvMYBF1 and MdMYB22 can regulate the expression of the flavonol-specific enzyme flavonol synthase (Czemmel et al., 2009; Mehrtens et al., 2005; Stracke et al., 2007; Wang et al., 2017b). AtPAP1/2, VvMYBA1 and MdMYB1 can activate the expression of the anthocyanin-specific enzyme UDP-glucose:flavonoid 3-O-glucosyltransferase (UFGT) (Borevitz et al., 2000; Kobayashi et al., 2002, 2004; Takos et al., 2006; Tohge et al., 2005). AtTT2, VvMYBPA1/2 and MdMYB9/11/12 can regulate the expression of the PA-specific enzymes ANR or LAR (An et al., 2015; Bogs et al., 2007; Nesi et al., 2001; Terrier et al., 2009; Wang et al., 2017b). In the current study, the expression of MdMYB9/11/12 was positively correlated with PA synthesis in apple peel, indicating that MdMYB9/11/12 regulates the accumulation of PAs during apple peel development. The expression patterns of MdMYB22 and MdMYB1 indicated that they were unrelated to PA accumulation (Figure S2). Sequence alignment showed that mdm-miR858 could only complement MdMYB9/11/12 and not MdMYB22 and MdMYB1 (Figure 2a). However, ath-miR858 could target the flavonol regulatory genes AtMYB11/12/111 (homologous to MdMYB22) and the anthocyanin-suppressor gene AtMYBL2 but not the PA regulatory gene AtTT2 (homologous to MdMYB9/11/12) in Arabidopsis (Sharma et al., 2016; Wang et al., 2016). Furthermore, grape miR858 positively regulates anthocyanin accumulation by targeting the anthocyanin repressor VvMYB114 (Tirumalai et al., 2019). However, it is not clear whether miR858 targets regulate PA accumulation. In the present study, expression of the homolog of VvMYB114, MD09G1183800, was consistently extremely low during apple development. These results indicated that miR858 may function differentially in apple, Arabidopsis, and grape. Correlation analysis showed that mdm-miR858 expression was significantly and negatively correlated with total PA content during apple development (Figure 1c). These results supported the conclusion that mdm-miR858 was involved in regulating PA accumulation during peel development.
mdm-miR858 targets MdMYB9/11/12 to inhibit PA synthesis
In plants, miRNAs induce cleavage between the 10th and 11th nucleotides of their target mRNA in almost perfectly complementary sites (Rhoades et al., 2002). In the present study, 5′-RACE confirmed that mdm-miR858 cleaves MdMYB9/11/12 between the 10th and 11th nucleotides (Figure 2a). The results of GUS staining assays and transient luminescent assays showed that mdm-miR858 can regulate the expression of MdMYB9/11/12 at the post-transcriptional level, thereby affecting the expression of downstream genes (Figures 2b,c and 3). Previous studies have reported that overexpression of MdMYB9/11/12 can induce PA accumulation in apple calli (An et al., 2015; Wang et al., 2017b), with which the present results were consistent (Figure 4a,c). In addition, we observed that MdMYB9/11/12 was functionally redundant in regulating PAs (Figure S4). Overexpression of VvMYBPA1, MtBAN and MdANR in tobacco leads to anthocyanin loss and PA accumulation (Han et al., 2012; Passeri et al., 2017; Xie et al., 2003). In the present study, the expression levels of NtANR and NtLAR increased in flowers of tobacco overexpressing MdMYB9/11/12, whereas the expression level of NtUFGT was not affected (Figure S8). In addition, the total PA content increased significantly, whereas the anthocyanin content decreased significantly (Figure S6c). These results indicated that MdMYB9/11/12 strongly induced NtANR and NtLAR expression, thus increasing the metabolic substrate flow to PAs and resulting in the reduction of anthocyanin accumulation. Arabidopsis seeds contain a large amount of PAs, which imparts the brown colour to the seed coat. Mutation of TT2 causes AtANR not to be expressed in the seed and PAs are not synthesized, and as a result, the seed coat colour is buff (Nesi et al., 2001). With overexpression of MdMYB9/11/12 in the tt2 mutant, the seed coat colour is brown (Figure 5a), indicating that heterologous overexpression of MdMYB9/11/12 complements the function of TT2 to promote PA accumulation in Arabidopsis seeds. However, with overexpression of mdm-miR858 in apple calli, tobacco and Arabidopsis overexpressing MdMYB9/11/12, the relative expression of MdMYB9/11/12 was significantly reduced and accompanied by a decrease in PA content (Figures 4 and 5; Figure S6). These results indicated that mdm-miR858 could target MdMYB9/11/12 to inhibit PA accumulation.
MdBBX22 induces mdm-miR858 expression to inhibit PA accumulation and alters metabolism to promote anthocyanin synthesis in apple peel under light stress
Light signals mediate many physiological and developmental processes in plants, such as photomorphogenesis and pigment accumulation (Gangappa and Botto, 2014). Emerging evidence suggests that BBX proteins play a central role in these light-mediated physiological processes (Song et al., 2020). Among these proteins, AtBBX22 is a positive regulator of the light signalling pathway and its expression can be induced in Arabidopsis seedlings during the transition from dark to light exposure. Knockdown or overexpression of AtBBX22 can reduce or increase anthocyanin content in Arabidopsis seedlings under light (Chang et al., 2008). In addition to AtBBX22, AtBBX21 and AtBBX23 positively regulate anthocyanin synthesis under light (Datta et al., 2007; Zhang et al., 2017), whereas AtBBX24 and AtBBX25 inhibit light-induced anthocyanin biosynthesis (Gangappa et al., 2013; Job et al., 2018). In apple, the light-induced MdBBX22–MdHY5 heterodimer promotes the expression of MdMYB1 and thus positively regulates anthocyanin accumulation (An et al., 2019; Bai et al., 2014). In addition, MdBBX20, MdBBX21 and MdBBX37 are induced by light and ultimately promote anthocyanin accumulation (An et al., 2020; Fang et al., 2019b; Zhang et al., 2021), whereas MdCOL4 (AtBBX24 homolog), whose expression is inhibited by Ultraviolet-B, interacts with MdHY5 to synergistically inhibit the expression of MdMYB1 (Fang et al., 2019a). In pear, PpBBX18 and PpBBX21 physically interact with PpHY5 and antagonistically regulate anthocyanin biosynthesis in Arabidopsis and pear under light (Bai et al., 2019b). Previous studies have focussed more on the regulation of anthocyanins by BBX proteins, but whether BBX regulates PA synthesis has not been reported previously.
In the present study, a number of molecular and biochemical assays showed that MdBBX22 can bind to the mdm-miR858 promoter and promote the expression of mdm-miR858 (Figure 6). With overexpression of MdBBX22 in MdMYB9-OX, MdMYB11-OX and MdMYB12-OX apple calli, the expression of mdm-miR858 increased significantly, whereas the expression of MdMYB9/11/12 and the total PA content decreased significantly. In addition, the inhibitory effect of MdBBX22 on MdMYB12-induced PA was significantly reduced following mutation of the mdm-miR858 target site in MdMYB12 (Figure 7). These results indicated that MdBBX22 could induce the expression of mdm-miR858 to inhibit MdMYB9/11/12 expression and ultimately led to a reduction in PA accumulation. Under light stress, the expression of MdBBX22 and mdm-miR858 increased, whereas the expression of MdMYB11 and MdMYB12 and the total PA content decreased (Figure 8a,b). Overexpression of MdBBX22 in the peel led to increased expression of mdm-miR858, but the decreased expression of MdMYB11 and MdMYB12 and decreased total PA content. In contrast, with the silencing of MdBBX22, the expression of mdm-miR858 decreased, whereas the expression of MdMYB11 and MdMYB12 increased accompanied by an increase in the total PA content (Figure 8c–f). These results further indicated that MdBBX22 could induce the expression of mdm-miR858 to inhibit MdMYB11 and MdMYB12 expression, which ultimately led to a reduction in PA accumulation in the peel of apple fruit under light stress.
The relationship between PAs and anthocyanins has been investigated in several plant species. Overexpression of PtrMYB6 in poplar leads to an increase in anthocyanin and PA accumulation, and overexpression of VvMYB5a in tobacco promotes the accumulation of anthocyanins and PAs (Deluc et al., 2006; Wang et al., 2019). Heterologous overexpression of the maize (Zea mays) Leaf colour (Lc) regulatory gene in apple also promotes the increase in PA and anthocyanin contents (Li et al., 2007). However, overexpression of VvMYBPA1 and ANS from Medicago truncatula and apple in tobacco resulted in a decrease in anthocyanin content and an increase in total PA content (Han et al., 2012; Passeri et al., 2017; Xie et al., 2003). Deluc et al. (2006) consider that PAs and anthocyanins may be allowed to increase simultaneously when the substrate availability is sufficient, and there may be competition between PAs and anthocyanins when the substrate is insufficient. In the present experiment, the total PA and anthocyanin contents under light stress showed opposite trends (Figure 8a). In addition, overexpression of MdBBX22 and mdm-miR858 or silencing of MdMYB11/12 led to a decrease in total PA content and an increase in anthocyanin content. In contrast, inhibition of MdBBX22 or overexpression of STTM858 or MdMYB12m resulted in an increase in total PA content and a decrease in anthocyanin content (Figure 8c–f). These results indicated that PAs and anthocyanins exhibit a competitive relationship under light stress. The inhibition of PA accumulation aids the rapid synthesis of anthocyanins.
In conclusion, the present research shows that mdm-miR858 can target MdMYB9/11/12 to inhibit the accumulation of PAs. Under light stress, MdBBX22 binds to the mdm-miR858 promoter to induce mdm-miR858 expression to inhibit PA accumulation by targeting MdMYB9/11/12, and inhibition of the PA branch promotes the rapid synthesis of anthocyanins in the peel of apple fruit. Our findings reveal a novel regulatory mechanism for PA synthesis at the post-transcriptional level. In addition, the present results clarify the relationship between PAs and anthocyanins in apple peel under light stress.
Experimental procedures
Plant materials
Six-year-old trees of apple ‘Starkrimson Delicious’ planted at the Baishui Apple Experimental Farm of Northwest A&F University, Shaanxi Province, China were used as experimental materials. Fruit were harvested at 30, 60, 90, 120 and 140 DAFB. For each sample, 18 fruit were harvested, of which six fruit were considered as one biological repeat. Some fruit were bagged with a paper bag (Hongtai, Shanxi, China) at 45 DAFB, then harvested (still enclosed in the bags) at 120 DAFB for transient injection and light stress treatment. The peel was excised with a fruit peeler, immediately frozen with liquid nitrogen and stored at −80 °C until use.
For the light stress treatments, the bagged fruit were harvested at 120 DAFB and placed in the dark at 23 °C for 24 h to ensure the temperatures inside and outside of the paper bag were consistent. The fruit were unpacked and placed in a light incubator at 23 °C under continuous white light (500 μmol/m2/s). The peel was excised at different time points for experiments.
DMACA staining of PAs and determination of phenolic compounds
Proanthocyanidins in plant tissues were detected using DMACA solution [0.2% (w/v) DMACA in a mixture of methanol and 6 m HCI (1:1, v/v)] following a previously described method (Li et al., 1996). Calli were stained for 24 h and dried seeds were stained for 48 h. The total soluble PA content was determined using the method described by Li et al. (1996) and An et al. (2015). The determination of polyphenols followed the method described by Wang et al. (2013).
RNA extraction, gene expression analysis and phylogenetic analyses
Total RNA was extracted using the TRIzol RNA Plant Plus Reagent (Tiangen, Beijing, China). Genomic DNA was removed using DNase I (TaKaRa, Dalian, China). First-strand cDNA was synthesized using the PrimeScript™ RT Reagent Kit (TaKaRa). The reverse transcription of mdm-miR858 used specific stem-loop primers. Semi-quantitative RT-PCR was performed using the PCR Master Mix (Tiangen). RT-qPCR analysis was conducted using SYBR® Premix Ex Taq™ II (TaKaRa) on an ABI StepOnePlus™ Real-Time PCR System (Applied Biosystems, Waltham, MA). MdU6 and MdActin were used as internal controls for miRNA and coding genes, respectively. The method was used to calculate the relative expression. The primer sequences used are listed in Table S5.
MiRNA library construction, sequencing and analysis
Total RNA was extracted from the peel at 30, 60, 90, 120 and 140 DAFB with three biological replicates. One microgram of total RNA from each sample was used to construct miRNA libraries using the TruSeq Small RNA Sample Preparation Kit (Illumina, San Diego, CA) in accordance with the manufacturer’s instructions. These libraries were used for single-end sequencing with an Illumina HiSeq2500 platform by LC-BIO (Hangzhou, China). MiRNA sequences were identified by ACGT101-miR (LC Sciences, Houston, TX) as described by Zhou et al. (2016). Pearson correlation analysis of the expression of these miRNAs, based on normalized deep-sequencing counts, with total PA content was performed. Target genes of miRNAs were predicted using the psROBOT sRNA analysis toolbox (Wu et al., 2012).
Yeast one-hybrid assay
The mdm-miR858 promoter fragment (Figure S11) amplified from genomic DNA extracted from ‘Starkrimson Delicious’ fruit peel was inserted into the reporter vector pHIS2 to form the recombinant vector mdm-miR858pro-HIS2. The mdm-miR858m promoter fragment (Figure S11) was synthesized by GENERAL BIOL (Anhui, China), The mdm-miR858m promoter fragment was inserted into the pHIS2 vector to generate the recombinant vector miR858mpro-HIS2. The CDS of MdBBX22 was inserted into the pGADT7 vector to generate the recombinant vector pGADT7-MdBBX22. The primer sequences used are listed in Table S5. Yeast strain Y187 cells carrying the miR858pro-HIS2/miR858mpro-HIS2 vector were cultured in a screening medium (SD/−His/−Trp) supplemented with different concentrations of 3-amino-1,2,4-triazole (3-AT). The miR858pro-HIS2/miR858mpro-HIS2 and pGADT7-MdBBX22 constructs were co-transformed into yeast strain Y187 cells, then screened on selective medium (SD/−Leu/−Trp/−His) containing the optimal concentration of 3-AT.
In vivo GUS protein degradation assay
The relationship between mdm-miR858 and target genes was verified using a GUS protein degradation assay in tobacco (N. benthamiana) leaves. First, the CDS sequences of MdMYB9, MdMYB11 and MdMYB12 were inserted separately into the pBI121 vector to generate the 35S:MdMYB9-GUS, 35S:MdMYB11-GUS and 35S:MdMYB12-GUS vectors. The GUS sequence in the pBI121 vector was replaced with the Md-pri-miR858 sequence (with an additional ~100 bp at both ends based on the Md-pre-miR858 sequence) to generate the vector pBI121-35S:mdm-miR858. The pBI121-GFP vector was generated by replacing the GUS sequence in the pBI121 vector with the GFP sequence to serve as a negative control. The primer sequences used are listed in Table S5. Agrobacterium tumefaciens strain EHA105 carrying the recombinant vector was cultured to OD600 ~0.8 and finally resuspended to OD600 0.4. Four different areas of the leaf were injected in accordance with the method described by Wang et al. (2017a). The leaves were sampled to measure GUS activity 72 h after the infiltration as described by Jefferson et al. (1987).
Transient dual-luciferase assay
The Md-pri-miR858 sequence and the CDS sequences of MdMYB9, MdMYB11, MdMYB12, MdbHLH33 and MdBBX22 were inserted separately into pGreenII 62-SK effector vectors. The empty vector pGreenII 62-SK was used as a negative control. The promoter fragments from MdANR, MdLAR and mdm-miR858 were inserted into the reporter vector pGreenII 0800-LUC. The primer sequences used are listed in Table S5. The Agrobacterium-mediated transient transformation was performed on leaves from 4-week-old tobacco (N. benthamiana) plants using a method described previously (Zhang et al., 2020).
RNA ligase-mediated 5′-RACE
To verify the cleavage of MdMYB9, MdMYB11 and MdMYB12 by mdm-miR858, RNA ligase-mediated RACE experiments were conducted using the SMARTer RACE 5′/3′ Kit (TaKaRa). First, total RNA was extracted from the peel of apple fruit at 30, 60, 90, 120 and 140 DAFB and equal amounts were mixed, then reverse-transcribed using SMARTScribe™ Reverse Transcriptase and connected to the 5′ adaptor. Next, PCR was performed using 5′ universal primers supplied with the kit, 3′ gene-specific primers and the reverse-transcribed cDNA. The PCR product was recovered by gel purification and ligated into the pMD19-T vector (TaKaRa) for sequencing. The primers used in the experiment are listed in Table S5.
Generation of transgenic plant materials
To obtain MdMYB9-OX, MdMYB11-OX and MdMYB12-OX apple calli, the CDSs of MdMYB9, MdMYB11 and MdMYB12 were inserted in the pCAMBIA2301 vector under the control of the CaMV35S promoter to generate the constructs 35S:MdMYB9-2301, 35S:MdMYB11-2301 and 35S:MdMYB12-2301. The sequence of MdMYB12m was synthesized by GENERAL BIOL (Anhui, China) and inserted into the pCAMBIA2301 vector to generate 35S:MdMYB12m-2301. The primer sequences used are listed in Table S5. Calli of apple ‘Orin’ was used for transformation using a previously reported method with some modifications (Xie et al., 2012; Zhang et al., 2021). Three-week-old calli were infected with Agrobacterium tumefaciens strain LBA4404 carrying 35S:MdMYB9-2301, 35S:MdMYB11-2301, 35S:MdMYB12-2301 and 35S:MdMYB12m-2301. To overexpress mdm-miR858 in MdMYB9-OX, MdMYB11-OX and MdMYB12-OX calli, the kanamycin sequence in the pCAMBIA2301 vector was replaced with the hygromycin sequence to generate pCAMBIA2301(H) by digesting the two XhoI sites. The Md-pri-miR858 sequence and MdBBX22 CDS were inserted into pCAMBIA2301(H) to generate 35S:mdm-miR858-2301(H) and 35S:MdBBX22-2301(H). The 35S:mdm-miR858-2301(H) and 35S:MdBBX22-2301(H) constructs were transformed into MdMYB9-OX, MdMYB11-OX, MdMYB12-OX and MdMYB12m-OX calli. Kanamycin in the screening medium was replaced with 20 mg/L hygromycin.
The constructed vectors 35S:MdMYB9-2301, 35S:MdMYB11-2301, 35S:MdMYB12-2301 and 35S:mdm-miR858-2301(H) were transformed into Agrobacterium strain GV3101. Tobacco (Nicotiana tabacum ‘Petite Havana SR1’) was transformed following the method described by Horsch et al. (1985). The same method was used to transfer hygromycin-resistant 35S:mdm-miR858-2301(H) into T3 MdMYB9-OX, MdMYB11-OX and MdMYB12-OX tobacco, and T2 hygromycin-resistant transgenic tobacco was used for the analysis of gene expression and flavonoid compounds.
Agrobacterium strain GV3101 carrying 35S:MdMYB9-2301, 35S:MdMYB11-2301 and 35S:MdMYB12-2301 was used to infect the Arabidopsis tt2 mutant (SALK_208156) using the floral-dip method (Clough and Bent, 1998). The 35S:mdm-miR858-2301(H) construct was transformed into T3 MdMYB9-OX, MdMYB11-OX and MdMYB12-OX Arabidopsis, and T3 transgenic Arabidopsis was used to analyze PA compounds.
Electrophoretic mobility shift assay
The MdBBX22 CDS was inserted into the pET28a vector and transformed into Escherichia coli strain BL21 (DE3). Expression of the MdBBX22-HIS fusion protein was induced at 16 °C and purified using the His-Tagged Protein Purification Kit (Sangon Biotech, Shanghai, China). The EMSAs were implemented using biotin-labelled probes, MdBBX22-HIS proteins and the LightShift Chemiluminescent EMSA Kit (Thermo Scientific, Waltham, MA). All operations were strictly implemented in accordance with the kit manufacturer’s instructions. The reaction mixture was incubated at 25 °C for 30 min. The reaction products were separated in 6.5% native polyacrylamide gel and then transferred to a nylon membrane. After crosslinking, the biotin-labelled DNA on the membrane was detected by chemiluminescence using a ChemiDoc XRS system (Bio-Rad, Hercules, CA).
Chromatin immunoprecipitation-PCR analysis
ChIP-PCR assays were performed following the method described by Bowler et al. (2004). Apple calli were crosslinked for 10 min in 1% formaldehyde. Chromatin was isolated and sonicated to shear DNA into 0.2–0.5 kb fragments. After centrifugation at 4 °C for 5 min at 13 000 g, chromatin was retained in the upper aqueous phase. ChIP-grade protein A/G agarose beads (Thermo Fisher) were used to preclear the chromatin supernatant. An anti-GFP antibody (Abcam, Cambridge, UK) was added to the supernatant and incubated overnight at 4 °C with gentle agitation. Immune complexes were collected with protein A/G agarose beads after incubation for 1 h at 4 °C with gentle agitation. The beads were centrifuged at 1000 g and then washed five times. Immune complexes were eluted and resuspended. Crosslinking was reversed by overnight incubation at 65 °C in 5 m NaCl. Each ChIP assay was repeated three times and the enriched DNA fragments in each ChIP sample were used as one biological replicate for qPCR. Primers used are listed in Table S5.
Transient transformation assays in apple fruit
The sequence of STTM858 was synthesized by GENERAL BIOL and inserted into the pCAMBIA2301 vector to generate 35S:STTM858-2301. The specific 300 bp fragment in the CDS of MdMYB11 and MdMYB12 was selected and inserted into the pTRV2 vector in tandem to form the recombinant vector pTRV2-MdMYB11/12. The specific 300 bp fragment in MdBBX22 was also inserted into the pTRV2 vector to generate pTRV2-MdBBX22. The primer sequences used are listed in Table S5. Apple fruit were injected using the method described previously (Zhang et al., 2020).
Acknowledgements
This work was supported by the Earmarked Fund for Modern Agro-industry Technology Research System, China (CARS-27), the National Natural Science Foundation of China (32072555)
Conflict of interest
The authors declare that they have no competing interests.
Author contributions
B.Z., H.Y. and Z.Y.Z. conceived the original screening and research plans. B.Z., Y.Y. and Z.Z.Z. supervised the experiments. B.Z., H.Y. and D.Q. analyzed the data. B.Z. wrote the article. H.Y. revised the manuscript.




