Ketofol simulations for dosing in pediatric anesthesia
Summary
Background
Propofol mixed with racemic ketamine (or ‘ketofol’) is popular for short procedural sedation and analgesia. Use is creeping into anesthesia, yet neither the optimal combination nor infusion rate is known. The EC50 of propofol's antiemetic effect is reported to be 0.343 mg·l−1, while ketamine analgesia is thought to persist with concentrations above 0.2 mg·l−1. We aimed to determine a ketofol dosing regimen for anesthesia 30-min and 1.5-h duration in a healthy child that did not unduly compromise recovery.
Methods
Pharmacokinetic–pharmacodynamic parameters were used to simulate drug concentration and effect profiles over time for different ratios of propofol to ketamine ratios (1 : 1 to 10 : 1) and rates. The target effect was the 95% probability of loss of response to a 5-s transcutaneous tetanus (P05). Combined effects were additive, with a propofol EC50 of 3.1 mg·l−1, ketamine EC50 of 0.64 mg·l−1, and slope of 5.4. The time to predicted 50% probability of return of this response after ceasing infusion (P50) was determined for a 5-year-old 20-kg healthy child.
Results
The addition of ketamine to propofol infused using a manual infusion regimen (loading dose 3 mg·kg−1, then 15 mg·kg−1·h−1 for 15 min, 13 mg·kg−1·h−1 for 15 min, 11 mg·kg−1·h−1 for 30 min, and 10 mg·kg−1·h−1 for 1–2 h) caused prolonged postoperative sedation. The P50 after a 1.5-h infusion using a 1 : 1 mixture was 4.5 h, 2 : 1 mixture was 3.25 h, 5 : 1 mixture was 1.6 h, and 10 : 1 mixture was 40 min. These P50 estimates could be reduced by slowing administration infusion rates to 20%, 33%, 50%, 67%, 80%, and 90% for mixtures 1 : 1, 2 : 1, 3 : 1, 5 : 1, 6.7 : 1, and 10 : 1, respectively. These rates achieve a P50 of approximately 20 min for 30-min duration anesthesia and 60 min for 1.5-h duration anesthesia.
Conclusions
The addition of ketamine to propofol infusion will prolong recovery unless infusion rates are decreased. We suggest an optimal ratio of racemic ketamine to propofol of 1 : 5 for 30-min anesthesia and 1 : 6.7 for 90-min anesthesia. Delivery of these ratios achieves propofol concentrations above an antiemetic threshold for longer than the ketamine concentration above the analgesic threshold during, potentially reducing postoperative nausea incidence.
Introduction
Ketofol is a neologism coined to refer to the combination of ketamine and propofol mixed together in one syringe. These two drugs are pharmacologically compatible 1, 2, and this combination has been used since the early 1990s for surgical anesthesia. There has been increasing interest in this mixture for anesthesia in both children 3-8 and adults 9-11, subsequent to reports of fewer adverse events, less vomiting and earlier discharge times after procedural sedation and analgesia. The ideal propofol to ketamine ratio for anesthesia remains unknown although an optimal ratio of racemic propofol to ketamine of 1 : 3 for short procedures (10–20 min) has been suggested 12. The duration of effect for ketamine is context sensitive 13. Consequently, a ratio of 1 : 3 may not be optimal for anesthesia of duration longer than 20 min. There is increasing enthusiasm for total intravenous anesthesia in children 14, 15, and ketamine may find a niche in this role because it maintains respiration and lacks opioid-associated adverse effects (respiratory depression, postoperative nausea and vomiting, pruritus, chest muscle wall rigidity). Ketamine augmented with other drugs such as propofol, dexmedetomidine, or midazolam has been suggested as an alternative to inhalation agents in children with neuromuscular disorders because of concerns about rhabdomyolysis 16.
Debate continues as to whether the interaction between propofol and ketamine is synergistic, additive or infra-additive for sedation and anesthesia 17, 18. We have assumed additivity of effect between propofol and ketamine, based on information from the study by Hui et al. 17. We used pharmacokinetic–pharmacodynamic (PK–PD) models reported in the literature to simulate ketofol effects when used for surgical anesthesia in a 5-year-old 20-kg child. We aimed to determine the optimum mixture ratio, and the best dosing regimen.
Methods
This was a simulation study in which drug concentrations (plasma and effect site) and anesthesia probability profiles over time were estimated for racemic ketamine with propofol. We used reported pharmacodynamic parameter estimates for ketamine and propofol in adults to predict probability of anesthesia defined as loss of response to verbal command and loss of response to a 5-s transcutaneous tetanus 17. We simulated response for endpoints of probability of consciousness (defined by response to tetanic stimuli) after anesthesia and recovery times using different dose combination ratios in a 5-year-old 20-kg child.
Simulations
Simulations were performed in Excel 2010 using PK–PD Tools for Excel© 2009, version 1.31 (www.pkpdtools.com). Plasma and effect-site concentrations were estimated using pharmacokinetic parameter sets available in the literature. We assumed the pharmacokinetic parameter set described by Kataria et al. 19 adequately described propofol pharmacokinetics in children aged 3–11 years. Ketamine pharmacokinetics were described for children using the parameter set reported by Herd et al. 20. Allometric scaling 21 was used to correct for size.
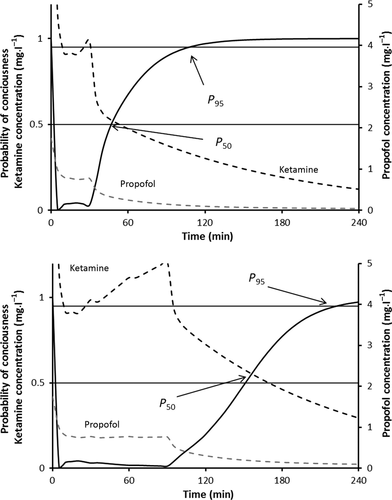
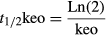 (1)
(1)A keo of 0.89 min−1 for propofol effects on the bispectral index (BIS) has been reported 23. This was used to estimate propofol effect-site concentrations in the child.
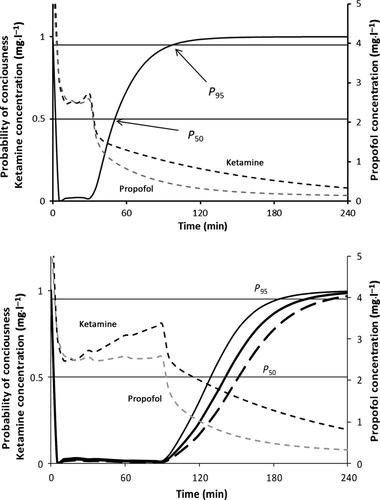
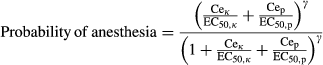 (2)
(2)Simulations were then performed with the PK–PD model to investigate the best dosing regimens for both boluses and infusions.
Dose regimen selection
We aimed to identify the optimum mixture ratio and dosing that provides effective anesthesia [i.e., 95% probability of loss of response (P05)] with the shortest time to emergence, longest antiemetic effect of propofol, and the easiest practical drug mixing and dosing volumes [based on the formulations commonly available in Australasia: racemic ketamine (10%, 2 ml vial) and propofol (1%, 20 ml vial)]. Propofol to ketamine ratios from 1 : 1 to 1 : 10 were investigated as infusion for both 30 min and 90 min. We used the base infusion rate for propofol described by McFarlan et al. 29. A loading dose of 2.5 mg·kg−1 followed by an infusion rate of 15 mg·kg−1·h−1 for the first 15 min, 13 mg·kg−1·h−1 from 15 to 30 min, 11 mg·kg−1·h−1 from 30 to 60 min, 10 mg·kg−1·h−1 from 1 to 2 h.
Results
Dosing regimens
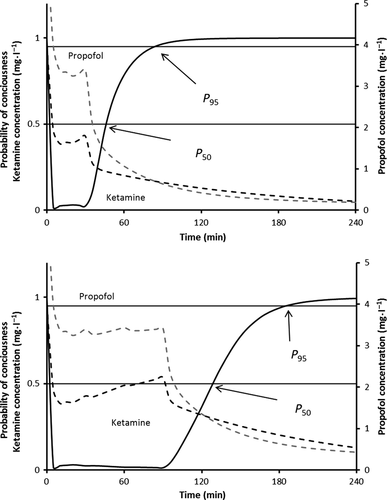
Infusion regimens that maintained a probability of response <0.05 are shown in Table 1. The addition of ketamine to the base propofol regimen (suggested by McFarlan) resulted in extremely delayed awakening (P50) of over 4 h when the propofol:ketamine 1 : 1 mixture was used. This decreased to a delay of 50 min when a 10 : 1 ratio was used. However, reduction in infusion rate to maintain a probability of anesthesia >95% resulted in times to P50 of 16–24 min after 30-min exposure and 40–60 min after 90-min exposure in all scenarios (Table 2). Figures 1-3 show the probability of consciousness, propofol concentrations, and ketamine concentrations after infusion regimens using propofol:ketamine mixes of 1 : 1, 5 : 1, and 10 : 1 with infusion reduction schedules (Table 1). Figure 2 shows the impact of age on return to consciousness after a 90-min infusion; the P50 was 40 min in a 2-year-old and 65 min for a 10-year-old child. The propofol:ketamine ratio of 5 : 1 achieved a balance where propofol concentrations were above 0.343 μg·ml−1 and ketamine above 0.2 μg·ml−1 for 83 min after a 30-min infusion (Table 3). A propofol:ketamine ratio of 6.7 : 1 revealed a longer duration of propofol concentrations (135 min) over ketamine (150 min). Clearance, expressed as l·h−1·kg−1, increased with decreasing age 21. Consequently, recovery is slower as age increases (Figure 2). Infusion rates and recovery rates shown in the tables are for a 5-year-old child.
| Rate as a percentage of McFarlan regime | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20% | 33% | 50% | 67% | 80% | 90% | |||||||||
| Ratio P:K | 1 : 1 | 2 : 1 | 3 : 1 | 5 : 1 | 6.7 : 1 | 10 : 1 | ||||||||
| Propofol alone (McFarlan) | Ketamine alone | P | K | P | K | P | K | P | K | P | K | P | K | |
| Initial loading doses (mcg·kg−1) | ||||||||||||||
| 3000 | 1000 | 800 | 800 | 1500 | 750 | 2000 | 666 | 2500 | 500 | 2750 | 410 | 3000 | 300 | |
| Subsequent infusion rates (mcg·kg−1·min−1) | ||||||||||||||
| 0–15 min | 250 | 70 | 50 | 50 | 82.5 | 41.25 | 125 | 41.25 | 167.5 | 33.5 | 200 | 25.1 | 225 | 22.5 |
| 15–30 min | 216.7 | 55 | 43 | 43 | 71.5 | 37.8 | 108.3 | 37.8 | 145.2 | 29.0 | 173.3 | 21.8 | 195 | 19.5 |
| 30–60 min | 183.3 | 45 | 36.6 | 36.6 | 60.5 | 30.3 | 91.7 | 30.3 | 122.8 | 24.6 | 146.7 | 18.4 | 165 | 16.5 |
| 6–90 min | 166.7 | 25 | 33.3 | 33.3 | 55 | 27.5 | 83.3 | 27.5 | 111.7 | 22 | 133.3 | 16.8 | 150 | 15 |
| Propofol McFarlan | Ketamine | 1 : 1 | 2 : 1 | 3 : 1 | 5 : 1 | 6.7 : 1 | 10 : 1 | |
|---|---|---|---|---|---|---|---|---|
| Infusion duration | Time to P50 | |||||||
| 30 min infusion | 5 min | 27 min | 17 | 17 | 24 | 22 | 20 | 16 |
| 30 min infusion without rate reduction | 3 h | 2 h | 1 h 25 min | 45 min | 35 min | 20 min | ||
| 90 min infusion | 8 min | 70 min | 63 min | 50 min | 60 min | 50 min | 47 min | 40 min |
| 90 min infusion without rate reduction | 4 h 35 min | 3 h 15 min | 2 h 25 min | 1 h 35 min | 1 h 10 min | 50 min | ||
| Propofol McFarlan | Ketamine | 1 : 1 | 2 : 1 | 3 : 1 | 5 : 1 | 6.7 : 1 | 10 : 1 | |
|---|---|---|---|---|---|---|---|---|
| Infusion duration | Propofol concentration > 0.343 mg·l−1 | |||||||
| 30 min infusion | 5 min | – | 15 min | 43 min | 66 min | 83 min | 95 min | 110 min |
| 90 min infusion | 8 min | – | 22 min | 50 min | 90 min | 135 min | 175 min | 210 min |
| Ketamine concentration >0.2 mg·l−1 | ||||||||
| 30 min infusion | – | 180 | 150 min | 120 min | 110 min | 83 min | 70 min | 30 min |
| 90 min infusion | 210 | 200 min | 180 min | 170 min | 150 min | 130 min | 90 min | |
Bispectral index values for anesthesia using ratios of 1 : 1. 5 : 1 and 10 : 1 are shown in Figure 4. Higher BIS scores are predicted as the proportion of ketamine is increased relative to propofol infused.

Discussion
Both ketamine and propofol are drugs with half-lives that are context sensitive. Consequently, single bolus dose ketofol use for procedural sedation and analgesia remains popular because drug proportions are not as important as during infusion. Simply adding ketamine to a propofol infusion and using the manual rates suggested by McFarlan 29 results in unacceptable recovery duration times if infusion duration is longer than 20 min. The context-sensitive half-life of ketamine is known to increase dramatically after 30 min 13. Infusion rates should be reduced as the proportion of ketamine is increased for these longer duration infusions. When infusion rate is reduced, then all mixes resulted in a P50 after 90 min that ranged from 40 to 63 min, with quicker emergence for lower ratios. The ‘best’ ratio will depend both on clinical factors—analgesia requirement, anesthesia duration, and local postanesthesia care unit (PACU) practices—and on the results of further investigation into the incidence of respiratory compromise and postoperative nausea and vomiting with different ketamine to propofol ratios.
Predictions for infusion rates of ketamine alone are similar to those reported by Singh and colleagues 30 in spontaneous breathing children (age 1 day–14 years, n = 107) undergoing interventional cardiac procedures. The procedure duration was 68 (sd 11) min and children were kept in PACU for 65 (sd 5) min, consistent with our predictions. Ketofol simulations are based on the premise that the pharmacodynamic responses in a child (5 years) are similar to that in an adult 31. We suggest that a propofol:ketamine mix of 10 : 1 be used for cardiac catheterization where patient stimulation after cannulation is minimal. This will result in the lower P50 of 40 min after a 90-min procedure. A number of other different infusions rates have been suggested, but these commonly require individual syringe pumps 5, 7. Burn dressing changes are more painful, and propofol : ketamine ratios of 2 : 1 and 1 : 1 have been used 8, 11. These ratios were associated with high incidence of psychotropic effects (10% unpleasant dreams) and ratios of 4 : 1 to 6.7 : 1 proved just as effective 32. We would suggest that ratios of 5 : 1 or 6.7 : 1 be used depending on treatment duration.
Nausea and vomiting following ketamine use occurs with an incidence of 7–8% in children and 5–15% in adults, peaking in early adolescence after IV administration 33, 34. Less nausea and vomiting are reported when propofol is employed with ketamine for sedation. Children receiving ketamine with propofol had less vomiting than those given ketamine alone (2% vs 12%) after orthopedic procedures 35. Propofol has an antiemetic effect with an EC50 0.343 mg·l−1. The time that this ‘antiemetic threshold’ exceeds the analgesic threshold for ketamine (0.2 mg·l−1) changes with both the dose ratio and duration of infusion. This rationale further supports that upper ratios of 5 : 1 or 6.7 : 1 be used depending on treatment duration. During infusion, concentrations of ketamine were above 0.6 mg·l−1 and these concentrations are associated with satisfactory analgesia 36.
Anesthesia may require the use of neuromuscular blocking drugs. It may be advantageous to use a modified electroencephalographic signal monitor on these occasions. However, ketamine has minimal effect on bispectral index (BIS) 37. Consequently, BIS values are >80 when the ketofol 1 : 1 ratio is used. We suggest that a BIS of 60, rather than 50 is targeted when using ratios of 5 : 1 or 6.7 : 1 (Figure 4).
Disclosures
Ethical approval was not required for this simulation study. Brian Anderson is a Section Editor for the journal Pediatric Anesthesia. This research was funded by departmental sources. J Hannam was supported by a PhD scholarship from the Green Lane Research and Educational Fund Board, Auckland, New Zealand.
Funding
This research was carried out without funding.
Conflict of interest
No conflicts of interest declared.




