Middle Pleistocene revelations: unravelling taphonomic processes in mammals including Mesotherium cristatum (Mesotheriidae, Notoungulata), Corralito Site, Córdoba Province, Argentina
Editor. Mary Silcox
Abstract
Taphonomic studies of Cenozoic mammals are scarce. We report a study of the taphonomy of the Corralito site (Middle Pleistocene to Holocene), Córdoba Province, Argentina, which documents the last population of the South American native ungulate typotherid Mesotherium cristatum. We discovered two specimens of M. cristatum (a hemimandible and postcranial remains) with numerous traces, along with one indeterminate camelid metapodial. Extensive and detailed analysis of these traces using macroscopic and confocal laser scanning microscopy has allowed us to identify various taphonomic agents: carnivoran bite traces, rodent gnawing, trampling, and root etching. We document the ichnotaxa Machichnus and Nihilichus and describe Corralitoichnus conicetensis gen. et sp. nov., which are attributed to Ctenomys incisors, along with Katagmichnus myelus gen. et sp. nov., associated with deep transverse traces on long bones diaphysis linked to bone breakage and marrow consumption by a medium–large carnivoran. This represents the first evidence of such behaviour in South America during the Cenozoic. Furthermore, the taphonomic time sequence of each recognized biological agent was reconstructed using a comprehensive understanding of the different biological processes that affected the specimens from post-mortem to post-burial. This study offers direct evidence of distinct biological agents from the Middle Pleistocene, particularly in the western Pampean region, focusing on one of South America's most iconic mammals (M. cristatum). It establishes a solid foundation for future taphonomic research on fossil bones, especially on predation or scavenging traces (Family Machichnidae), a relatively understudied area in South American native ungulates and the continent as a whole.
The Argentine Pampean region has been the focus of extensive palaeontological study since the 18th century (Cuvier 1796; Owen 1840; Burmeister 1867, 1879; Ameghino 1875, 1881, 1888, 1889; Doering 1881, 1907; Roth 1888). Numerous subsequent studies have focused on its extinct, mostly Pleistocene, fauna (Rusconi 1936; Bond 1999; Cione & Tonni 1999; Fernicola et al. 2009; Tomassini et al. 2010; Cione et al. 2015; Deschamps & Tomassini 2016; Prates & Perez 2021). It is also noteworthy that many of the Pampean fossils currently form part of the historical collections of the most important natural history museums in the country: the Museo de Ciencias Naturales ‘Bernardino Rivadavia’ (MACN) in Buenos Aires, and Museo de La Plata (MLP) in the homonymous city, even the historical collections of museums from the Old Continent (Roth 1898; San Gil 1964; Franco & Scotton 1989; Podgorny 2001; Vera et al. 2015; Carrillo & Püschel 2023; Toledo 2023). This has influenced numerous ecological evolutionary studies including the effect of the Great American Biotic Interchange (GABI) mainly focused on the Pampean region (Tonni et al. 1992; Alberdi et al. 1995; Cione et al. 2015; Carrillo et al. 2020; Prado et al. 2021), thus making it one of the best-studied areas in this regard, in contrast to other regions in South America and Argentina in particular. The Pampean region has long been a key area for evolutionary and palaeontological studies (see Parodi Bustos 1944; Bond 1998; Tonni et al. 1999a; Bond et al. 2001; Farinati et al. 2010; Sánchez-Villagra et al. 2023). Moreover, this region represents a significant habitat where the iconic megafauna (>44 kg), became extinct during the Pleistocene–Holocene transition (Prado et al. 2015; Prates & Perez 2021; Prates et al. 2022; Vizcaíno et al. 2023); these animals hold immense palaeontological significance and captivate the collective imagination of researchers and the general public alike.
In addition, the Pampean region has undergone extensive studies across various chronostratigraphic units (ages), mostly in the Pleistocene and Holocene (e.g. Marplatan, Ensenadan, Bonaerian, Lujanian ages; Pascual et al. 1996; Cione & Tonni 1999, 2005; Cione et al. 2015), and also the numerous biostratigraphic units (biozones) that have been established (see Cione & Tonni 1999, 2005; see also Deschamps 2005). Recently, Fernández-Monescillo et al. (2023a) formulated a new chronostratigraphic and biostratigraphic model based on the presence of the taxon M. cristatum in sediments from the Bonaerian Age.
Studies on taphonomy or evidence related to carnivore/scavenger traces on fossilized bones of South American native ungulates (SANUs) are notably scarce. After analysing the available literature, we can report only two references for the order Notoungulata; one for the suborder Toxodontia (Toxodontid indet., Chichkoyan et al. 2017a), and another for suborder Typotheria (Monte Hermoso locality, Pseudotypotherium exiguum [= Typotherium maendrum], Boscá 1923; see Fernández-Monescillo et al. (2022a, 2023b) for taxonomic details about Pseudotypotherium). On the other hand, there are numerous taphonomic fossil studies on carnivoran traces on other native lineages throughout South America (Peru, mylodontine indet., Pujos & Salas-Gismondi 2020; Brazil, megatheriidae, Eremotheirum laurillardi, mylodontine, Glossotherium, Araújo-Júnior et al. 2011, 2013, da Costa et al. 2023), although they are the predominantly based in the Pampean region for the Pliocene (e.g. Cingulata, Glyptodontidae, Eosclerocalyptus, De Los Reyes et al. 2013) and Pleistocene–Holocene time range (e.g. Glossotherium robustum, Chichkoyan et al. 2017a; Pilosa, Folivora, Mylodon darwini, Martín 2018; Proboscidea, Haplomastodon waringi, Dominato et al. 2011, Labarca et al. 2014; camelid Lama guanice, cervid Ozoteros bezoarticus, rhea Rhea americana, euphractine Eutatus seguini, Rafuse 2017). Taphonomic studies of tooth traces have also been carried out in other South American regions, such as in the Pleistocene of northern Brazil (Araújo-Júnior et al. 2011). Several works document the presence of lithic artefacts alongside South American faunal remains (Megatherium americanum, Politis et al. 2016, Chichkoyan et al. 2017b; Doedicurus, Chichkoyan et al. 2017b) or even direct cutting traces on bones (Lestodon, Domínguez-Rodrigo et al. 2021; Mylodon, Toledo 2016, 2021, Tauber et al. 2023; or notoungulate toxodontids Toxodon sp., Scheifler & Messineo 2016; Toxodon platensis, Toledo 2023) or the presence of tools or projectiles alongside such faunas (Castellanos 1933; Martínez 2001). Another type of study involving notoungulates, specifically typotheres, reports evidence of root traces in the mandibles of Paedotherium minor (Notoungulata, Hegetotheriidae; see Montalvo 2002; Tomassini et al. 2017).
Gnawing and biting traces on vertebrate bones represent a common component of the fossil record. Their presence is very important from a palaeobiological viewpoint, as they provide evidence of predator–prey relationships and of scavengers (Haynes 1980a, 1980b, 1982, 1983a, 1983b, 2018), and are useful for interpreting the behaviour of ancient vertebrates, as well as for reconstructing palaeoenvironmental and palaeoecological scenarios. The size and shape of traces, in combination with modern analogues, can sometimes help to identify the originator of the trace. Bite traces are a product of feeding behaviour of vertebrates and can be generated during events of predation or even scavenging on a thanatocoenosis (Pobiner 2008). Thus, these biological signs are useful for interpreting the behaviour of ancient vertebrates, and play a prominent role as evidence of direct biological interaction in vertebrate palaeontology.
- The taxon lends its name to the Suborder Typotheria Zittel 1893 suborder (based on the incorrect binomial nomenclature combination of Typotherium cristatum coined by Gervais (1867), and predominant during the late 19th and early 20th centuries; see Simpson (1940), and Fernández-Monescillo et al. (2022b) for details).
- It has a traditionally been recognized as the guide fossil of the Ensenadan Age (1.98–0.4 Ma; Tonni et al. 1999b; Cione & Tonni 2005; see Fernández-Monescillo et al. (2023a) for details).
- It forms the basis of the homonymous biozone (formerly referred to as the ‘Tolypeutes pampaeus – Daedicuroides Zone’, Cione & Tonni (1995b), later renamed it the ‘Tolypeutes pampaeus Biozone’ (Cione & Tonni 1999), and finally as the ‘Mesotherium cristatum Biozone’ (Cione & Tonni 2005)).
- Mesotherium cristatum stands as the final representative of the Typotheria suborder (a clade with a survival span of 53 myr, encompassing the Eocene to the Pleistocene; Patterson & Pascual 1968; Croft et al. 2020).
It is important to note that recent dating analysis (Fernández-Monescillo et al. 2023a) conducted on in situ fossils of the taxon M. cristatum, within the study area of this study (Corralito site, between the south of Departamento de Santa María and north of Departamento de Tercero Arriba, Córdoba Province, Argentina), indicate the presence of this species in post-Ensenadan sediments (220 ± 13 ka), as was previously hypothesized (Tauber 2008). Consequently, it is imperative to recognize that there is no justification for maintaining M. cristatum as the most important and exclusive representative of the Ensenadan Age sediments. The biostratigraphic units can only be established with reference to their fossiliferous content and the temporal limits cannot be extended without fossil evidence to the temporal limits of chronostratigraphic units (see Murphy & Salvador 1999). However, chronostratigraphic unit boundaries may be established based on the temporal limits of biozones (Murphy & Salvador 1999), a criterion that does not align with the known fossils of M. cristatum found in Ensenadan Age sediments (Tonni et al. 1999a; Soibelzon et al. 2008a), and is even less applicable when considering the evidence of M. cristatum in post-Ensenadan sediments, such as the Corralito site, where apparently it coexists with Megatherium americanum (see Fernández-Monescillo et al. 2023a).
- To conduct a taphonomic analysis of the multiple traces found on remains belonging to the last population of the Quaternary mesotheriine and typotheriid Mesotherium cristatum, and the fragmented metacarpals/metatarsals of a camelid indet.
- To investigate and decipher the potential biological agents responsible for creating these traces, specifically considering the taxa identified as sympatric faunas of M. cristatum and the camelid specimen at the Corralito site.
- For each identified fossil specimen of M. cristatum (hemimandible fragment and postcranial), to identify the temporal sequence of each recognized biological agent prior to and during final burial. This will reveal hitherto unknown aspects of the palaeoecology of the western Pampean region (in particular at the Corralito site) and more specifically of the Middle Pleistocene.
GEOLOGICAL SETTING
History, location & stratigraphy
Corralito is a fossil vertebrate site discovered after torrential rains in 1979–80s (see Argüello et al. 2006 for details), where numerous studies, stratigraphic and sedimentary analyses have been conducted (see Argüello et al. 2006; Frechen et al. 2009; Sanabria & Argüello 1999, 2011; Sanabria et al. 2013; Rouzaut et al. 2015; Rouzaut & Orgeira 2017). In addition, it is one of the palaeontological sites in Argentina with the highest number of dates (c. 15) obtained on its loess levels (see Frechen et al. 2003, 2009; Sanabria et al. 2006; Rouzaut & Orgeira 2017; Rouzaut et al. 2019; Fernández-Monescillo et al. 2023a), indicating an age range from the Middle Pleistocene to the Holocene (ranged from 220 ± 13 ka to 311/474 calibrated years before present (BP); or from Chibanian to Greenlandian; sensu Cohen et al. 2013 (v2023/06); marine isotope stage (MIS) 7-1).
The Corralito site is located 3 km northeast of Corralito village between the south of Departamento de Santa María and the north of Departamento de Tercero Arriba, Córdoba Province, Argentina (Fig. 1). Corralito site is a gully, c. 20 km long and oriented east–west, partly formed by anthropogenic factors such as extensive peanut and soybean cultivation and the type of plow used, combined with increased rainfall during the hydrologic year 1979–80 (see Argüello et al. 2006). It lies between the Xanaes (Segundo) and Ctalamuchita (Tercero) rivers. Geomorphologically, this area is known as the ‘Plataforma Basculada’ (Tilted Platform; see Capitanelli 1979).
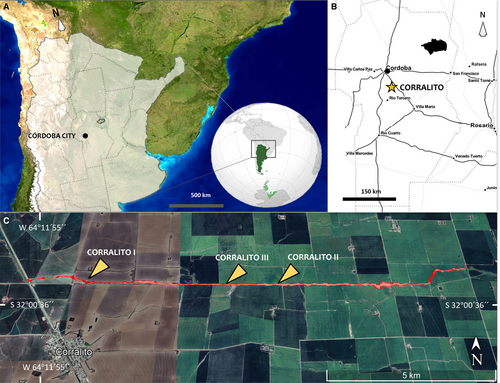
The stratigraphy of the Corralito gully reveals massive loess walls ranging from 4 to 22 m high, interspersed with fossiliferous layers (Frechen et al. 2009; Rouzaut et al. 2015; Fernández-Monescillo et al. 2023a). In the western part of the gully, different levels of cross-bedding have been identified, including troughs with large clasts (20 cm diameter) and planar cross-stratification; indicating various fluvial levels (Tauber et al. 2014). Additionally, some sections feature crotovines (mammal burrows) with circular or elliptical cross-sections of varying length, width and orientation (Tauber et al. 2012). The most noticeable stratigraphic layer throughout the 20 km gully is the palaeosol (PS-2), with a variable thickness of 70 ± 30 cm, distinguishable by its colour and texture in the loess walls (Fernández-Monescillo et al. 2023a).
Previous studies of dating & temporal distribution of the faunal community
Three sections have been previously identified in Corralito: Corralito I (S 32°0′7″ W 64°11′8″, Rouzaut et al. 2015, feldspar luminescence dating (IRSL): 115 ± 21 to 13.8 ± 18.8 ka, thermoluminescence dating (TL): 99.8 ± 17.5 to 11.8 ± 1.8 ka, see Frechen et al. 2009); Corralito II (S 32°0′16″ W 64°7′21″, Rouzaut et al. 2015, IRSL: 43.3 ± 3.3 to 10.6 ± 1 ka, see Rouzaut & Orgeira 2017); and Corralito III (S 32°0′16.15″ W 64°8′16.93″, IRSL: 220 ± 13 ka, Fernández-Monescillo et al. 2023a). The exposed loessic sediments of the Corralito gully, along with the associated faunal assemblage, span from the Middle Pleistocene to the Holocene (Chibanian–Greenlandian; sensu Cohen et al. 2013; MIS 7-1) (Fig. 1C). Meanwhile that of the mesoterine (M. cristatum) stratigraphic level is currently identified in the Bonaerian Age in what has so far been identified as the most basal loess layer at Corralito site, designated as L-1 (see Fernández-Monescillo et al. 2023a, fig. 2).
MATERIAL & METHOD
Material
Four fossil specimens corresponding to two individuals of the mesotheriine Mesotherium cristatum were analysed. Specifically, the right hemimandible fragment (CORD-PZ 4456) with m1–3 was identified based on the morphological characteristics outlined by Fernández-Monescillo et al. (2022b) and Fernández-Monescillo & Tauber (2024).
We examined the remains of the appendicular skeleton of another specimen of M. cristatum, with identified taphonomic traces, from Corralito site, primarily focusing on the forelimb, which included a fragmented right humerus (stylopod), ulna + radius (zeugopod) in anatomical position (MRFA 0836-1; elbow flexion; c. 38° of flexion between humerus and ulna; and c. 25° humerus–radius). This specimen also includes hind limb remains with identified taphonomic traces, encompassing a left proximal femur fragment (MRFA 0836-2) and a left tibia (MRFA 0836-3), all of them of the same individual. The taxonomic identification of both fore and hind elements as belonging to M. cristatum is based on the morphological characteristics identified and detailed in previous works (Serres 1867; Gervais 1867; Cattoi 1943; Fernández-Monescillo et al. 2018).
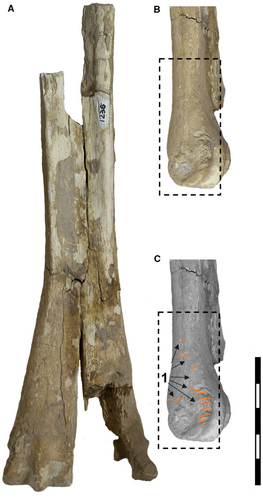
Furthermore, we incorporated into the analysis distal fragments of metacarpals of an indeterminate camelid (MRFA PV 1236). Based on the presence of camelids in the Pampean region in the Middle Pleistocene and Holocene (Ensenadan–Bonaerian–Lujanian Age; Chibanian–Greenlandian) we consider that these remains could belong to various camelid taxa such as Lama guanicoe or Hemiauchenia paradoxa, previously identified in the western Pampean region (Castellanos 1944; Menegáz 2000; Cruz et al. 2012; Tauber et al. 2014); the latter also identified at Corralito (Tauber et al. 2014).
Methods
We acknowledge that in the majority of studies concerning the taphonomy of structures, whether abiotic or biotic in nature, present on the surface of current or fossilized bones, the term ‘marks’ is commonly employed. However, in accordance with Vallon (2015) and other authors (Bertling et al. 2006, 2022; Mikuláš et al. 2013), we consider the term ‘trace’ to be more appropriate. This preference is grounded in the specific definition of trace as a morphologically recurrent structure resulting from the life activity of an individual organism (or homotypic organisms) modifying the substrate, encompassing dwelling traces, feeding traces, and bite traces (see Bertling et al. 2006, p. 266).
A taphonomic analysis was conducted on the superficial traces observed on a fragment of the right hemimandible of Mesotherium cristatum (CORD-PZ 4456). These traces were analysed using confocal laser scanning microscopy (CLSM), at millimetre or nanometre scale. This procedure was performed at the Laboratorio de Análisis de Materiales by Espectrometría de Rayos X (LAMARX) of the Facultad de Matemáticas, Astronomía, Física y Computación (FaMAF) of the Universidad Nacional de Córdoba (UNC), Argentina. Macroscopic traces were measured in length and width using a digital calliper to the nearest 0.01 mm, for comparison with previous studies recognized in the Argentinian Pampean region for the Pleistocene–Holocene periods to identify productors (e.g. Chichkoyan et al. 2017a). Moreover, the fossil material here analysed was photographed in the institutions where the material is housed (MRFA and Museo de Paleontología, UNC) with a Canon 550D camera, with a fixed focal length lens of 50 mm.
Taphonomic trace identifications & modification stages
We followed the methodology described in Fernández-Jalvo & Andrews (2016) to identify the traces of the different specimens analysed. The classification of different bone modification stages caused by carnivory or scavenging that have affected the distinct bones studied herein (i.e. hemimandible, humerus, radius, ulna, femur and tibia) is based on that of Sala et al. (2014) and Sala & Arsuaga (2018) (i.e. low, moderate, heavy modifications) and modified to account for the absence of soft tissue in fossils, using the classification of Haynes (1980a, 1980b, 1981) for extant mammals.
Institutional abbreviations
CORD-PZ, Museo de Paleontología, Facultad de Ciencias Exactas, Físicas y Naturales, Universidad Nacional de Córdoba, Córdoba, Argentina (PZ, indicating Palaeozoology Collection); MRFA, Museo Regional Florentino Ameghino, Casa de La Cultura, Río Tercero, Córdoba Province, Argentina (PV indicating, Paleovertebrate collection).
Anatomical description & muscle location
The anatomical descriptions follow the terminology of Schaller (2007) using primarily the terms of the Nomina Anatomica Veterinaria (Waibl et al. 2017). We use the following anatomical directional terms of direction for craniomandibular material: rostral, caudal, medial, lateral, dorsal and ventral; and these other for appendicular remains: medial, lateral, cranial, caudal, proximal and distal.
The identification of the various muscles in the different regions associated with carnivory or scavenging is primarily based on the works of Sosa & García López (2018) and Fernández García (2018) for the hemimandible. Given the high degree of osteological morphology conservation for the Mesotheriidae family (Trachytheriinae + Mesotheriinae; Shockey et al. 2007), we infer the muscular arrangement based on the reconstruction of the late Miocene taxon Plesiotypotherium achirense (see Fernández-Monescillo et al. 2018; Fernández García 2018).
Biostratigraphic & chronostratigraphic context
Mesotherium cristatum is the final representative of the Typotheria suborder and the only taxon of this clade to reach the Quaternary. It is the only mesotherine taxon found at the Corralito site, thus becoming the only palaeontological site with the presence of this taxon in the Bonaerian Age (see Tauber 1991, 2008; Fernández-Monescillo et al. 2023a; Fernández-Monescillo & Tauber 2024). This contrasts with the historical and traditional consideration of identifying it as a taxon from the older Ensenadan Age (Rusconi 1936; Bond et al. 1995; Cione & Tonni 1995a, 1995b, 1999, 2005; Bond 1999; Pomi 2008; Soibelzon et al. 2008a, 2008b, 2009, 2019; Cione et al. 2015). Krapovickas & Tauber (2016) and Krapovickas et al. (2017, p. 232) identified possible latest faunal records at several Upper Pleistocene (Lujanian Age) sites in the Sierras Pampeanas of Córdoba (e.g. Atos Pampa, Atum Pampa, Copina Bosque Alegre, Pampa de Olaen, Pampa Vaca Corral). This suggests that this area could serve as an important faunal reservoir compared to other areas of the Pampean region and the Chaco-Pampa plains.
RESULTS
Macroscopic & microscopic biological identification of taphonomic traces
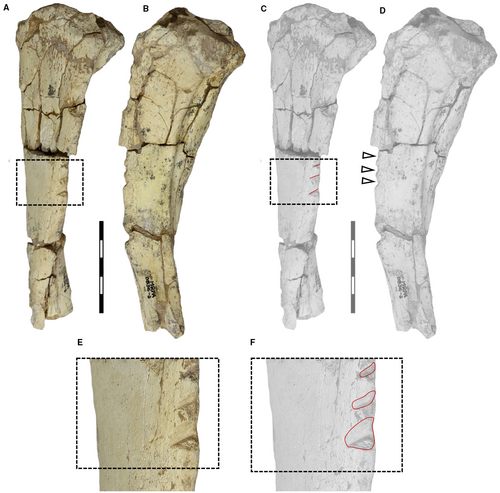
The most identifiable traces on the ventral border of the hemimandible of M. cristatum (CORD-PZ 4456) are macroscopically observable, particularly in lateral (Fig. 2A–C) and medial view (Fig. 2D–G). Both pseudo-parallel trace patterns present in ventral view a longitudinal symmetry axis marked on the ventral hemimandibular edge (Fig. 2G, H).
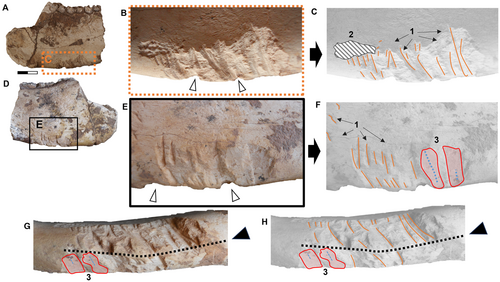
On the lateral side of the hemimandible (CORD-PZ 4456), we identified several traces. Using CLSM, we were able to distinguish pseudo-parallel and linear traces exhibiting a U-shaped cross-section (identified as carnivore traces, Haynes 1980b, 1982; Fiorillo 1984) without internal striations (Fig. 3A–E). This pattern is characteristic of traces made by premolars/molars and is indicative of carnivoran feeding behaviour (see Fernández-Jalvo & Andrews 2016, p. 32). Besides these, we also identified canine puncture traces recognized by their deep, rounded cone shapes (two traces visible in lateral view; Fig. 3G, H). Additionally, there are deep root etching traces in two distinct areas also analysed using CLSM (Fig. 3F, I, J), and other slightly marked or irregular coarse and elongated traces recognized as the result of trampling (Fig. 3K, L).
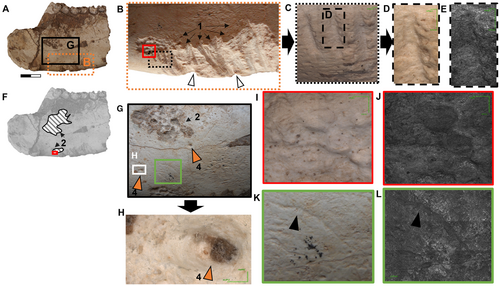
In addition to the carnivory traces indicated above (Fig. 2E, F), the medial side of CORD-PZ 4456 was also analysed using CLSM (Fig. 4A–D), revealing an evident root trace largely situated ventral to the molar line (m1–3) (Fig. 4E). Towards the caudal end of the ventral carnivoran traces, two flat impressions with distinct ridges characterized by parallel and straight edges are visible, and perpendicular to the longitudinal hemimandible axis, that we interpret as rodent gnawing traces (see Pokines et al. 2016; Indra et al. 2022; Figs 2E–G, 4B, F, G). Additionally, within the medial area of these markings, there seems to be a convergence axis between the upper and lower incisors (Fig. 4H, dotted black lines; without being able to know whether a/a′ or b/b′ are produced by an upper or lower incisor, see Fig. 4H). Particularly noteworthy is the presence of a discernible structure resembling a small central ridge in the central part (Fig. 4H, yellow dotted lines), interpreted as the diastema between the right and left incisors.
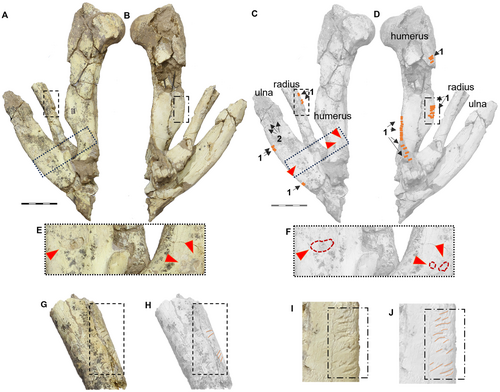
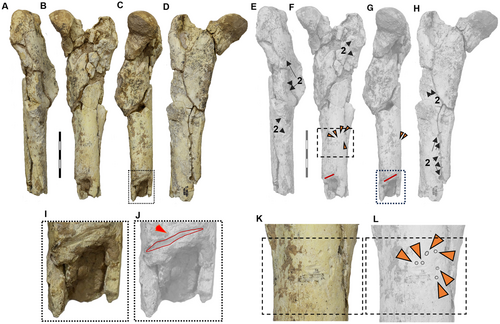
In the appendicular skeleton, we observed numerous traces. For example, on the right limb in anatomical position (humerus, ulna and radius), there are small traces similar to those found on the hemimandible. Traces are present on the caudal edge of the ulna, the proximal part of the radius, and the proximolateral edge (epicondylar crest) of the humerus (Fig. 5). Additionally, deep traces, probably caused by large carnivorans, appear on the proximal part of both the ulna and the humerus (Fig. 5E, F). In the case of the left femur fragment (MRFA 0836-2), we found small but numerous traces on the cranial part of the diaphysis, with the most surprising being a deep and transverse bite trace (Fig. 6C, G, I, J). On the tibia (MRFA 0836-3), we observed three deep and transverse traces on the cranial surface (Fig. 7). Finally, on the metacarpal specimen of a camelid (Hemiauchenia?/Paleolama?; MRFA-PV 1236), we found small longitudinal traces on the distal part (Fig. 8), similar to those found on the hemimandible and parts of the humerus, ulna and radius of the appendicular skeleton of M. cristatum.
SYSTEMATIC ICHNOLOGY
Family MACHICHNIDAE Wisshak et al. 2019
Type genus
Machichnus Mikuláš et al. 2006.
Diagnosis
Includes punctures and grooves in bone, both somewhat irregular in outline, often in sets.
Remarks
Wisshak et al. (2019) included members of a total of five genera (Knethichnus Jacobsen & Bromley 2009; Linichnus Jacobsen & Bromley 2009; Machichnus Mikuláš et al. 2006; Mandaodonites Cruickshank 1986; Nihilichnus Mikuláš et al. 2006). Which results in a total of 16 taxa: Machichnus regularis, Ma. bohemicus, Ma. multilineatus (all Mikuláš et al. 2006); Ma. normani, Ma. harlandi, Ma. jeansi (all Chumakov et al. 2013); Ma. fatimae Araújo-Júnior et al. 2017; Nihilichnus nihilicus Mikuláš et al. 2006; N. covichi Rasser et al. 2016; N. sulcatus Trifilio et al. 2023; N. hastarius, N. sicarius, N. clavus (all LaBarge & Njau 2024); N. quadripertirus Mazuch et al. 2024; and Mandaodonites coxi Cruickshank 1986 (senior synonym of Heterodontochnites hunit Rinehart et al. 2006; see Wisshak et al. 2019) to which we add two new ichnotaxa: Corralitoichnus conicetensis and Katagmichnus myelus.
In the definition of the family Machichnidae, the ethological category known as Praedichnia is included, encompassing traces of predation or scavenging. We believe it would be more appropriate to specify as a characteristic of this family the traces made by vertebrate teeth. These would therefore be traces resulting from contact between vertebrate teeth and typically hard biological remains, such as bones, antlers, horns, ossicones, turtle shells and similar vertebrate structures, as well as invertebrate shells (e.g. gastropods, ammonites). In this context, similar behaviours would be included, such as those observed in rodents, when they wear down their incisors not only on bones (i.e. Ma. regularis and Ma. multilineatus), but also on hard substrates like plant shells; accordingly this would also encompass the ichnospecies Glirotremmorpha entecta (Collison & Hooker 2000). Regarding the taxon Mandaodonites coxi, it is important to highlight the numerous revisions and considerations found in the literature. On one hand, it has been noted that its definition is overly specific, making subsequent identification challenging (see Jacobsen & Bromley 2009). On the other hand, some authors have argued that it cannot be considered an ichnogenus because the marks are not produced by biological agents (Zonneveld 2022); this argument is similar to that applied to the genus Brutalichnus (nomen dubium; see Wisshak et al. 2019).
Genus Machichnus Mikuláš et al. 2006
Type species
Machichnus bohemicus Mikuláš et al. 2006
Machichnus bohemicus Mikuláš et al. 2006
Figures 2A–H, 3A–E; 4A–D, 5A–D, G–J, 8A–C; Table 1
Description
Shallow, thin, discrete, parallel to subparallel smooth-bottomed scratches; scratches occur in small groups or series.
Remarks
The morphological comparison of various traces found on specimens from Corralito identifies them as Ma. bohemicus. These traces appear on both Mesotherium cristatum specimens (hemimandible: CORD-PZ 4456; ulna, radius, humerus: MRFA 1836-1) and the camelid Hemiauchenia?/Paleolama? (metacarpal: MRFA PV 1236). Machichnus bohemicus was first described from early Miocene specimens in the Czech Republic (Mikuláš et al. 2006) and from the Early Pleistocene (see Fejfar 1957), as well as from the Late Pleistocene and Early Holocene of Brazil (Araújo-Júnior et al. 2017). Given the U-shaped morphology at its base, we infer that Ma. bohemicus is an ichnotaxon produced by the cusps of carnivoran molars or premolars scraping or dragging over the substrate (see Discussion for details).
Genus Nihilichnus Mikuláš et al. 2006
Type species
Nihilichnus nihilicus Mikuláš et al. 2006
Nihilichnus clavus LaBarge & Njau 2024
Figures 3G–H; 6B, C, F–G, K–L; Table 1
Description
Rounded pits with deformed cortical bone, sloping to a centre point; surrounding border forms fairly irregular to near-perfect circle (see Njau & Gilbert 2016; LaBarge & Njau 2024).
Remarks
The variation in shape, size and depth of this ichnotaxon associated with puncture traces can be quite diverse, depending on the intensity of the bite, the hardness of the substrate, the specific producer (carnivoran, reptile, etc.) or their body mass, and the size and shape of the crown of the tooth or teeth (Njau & Gilbert 2016; LaBarge & Njau 2024).
Nihilichnus nihilicus Mikuláš et al. 2006
Figure 5A, C, E, F; Table 1
Description
Circular, subcircular, ovoid, triangular, crescent-shaped holes, or external punctures in cortical or cancellous bone, these traces are often found on long bones of mammals, including the calcaneus, astragalus, and phalanges. The diameter varies from 2 to 12 mm.
Remarks
One of the characteristics of this ichnotaxon is the depression of the cortical bone, which appears as the base of the generated trace. The observed traces measured 5.59 mm wide and 11.95 mm long on the ulna, and 3.47 × 5.78 mm (caudal trace) and 2.95 × 3.39 mm (cranial trace) on the humerus (see Table 1).
| Specimen | Fossil identification | Trace type | Ichnotaxon (illustrations) | Length (mm) | Width (mm) | Number of traces (n) | Possible biotic agent |
|---|---|---|---|---|---|---|---|
| CORD-PZ 4456 | M. cristatum mandible fragment | Short and straight with U-shaped bottom | Machichnus bohemicus (Fig. 2) | 3.13–14.53 | 0.40–0.70 | 40 | Small carnivore |
| CORD-PZ 4456 | M. cristatum mandible fragment | Flat bottom, straight, parallel edges | Corralitoichnus conicetensis gen. et sp. nov. (Figs 2E–H, 4B, F–H) | 10.36 (a+a′)* 9.41 (b+b′)* |
5.69 (a/a′) 4.00 (b/b′) |
2 | Ctenomys sp. (Rodent) |
| CORD-PZ 4456 | M. cristatum mandible fragment | Little punctures, cone shaped | Nihilichnus clavus (Fig. 3G, H) | 1.80 | 1.06 | 2 | Small carnivore |
| MRFA 0836-1 | M. cristatum right humerus | Short and straight with U-shaped bottom | Machichnus bohemicus (Fig. 5B, D, I, J) | 2.6–6.85 | 0.4–0.8 | 18 | Small carnivore |
| MRFA 0836-1 | M. cristatum right humerus | Completely eaten epicondylar ridge | Machichnus bohemicus (Fig. 5B, D) | – | – | – | Small carnivore |
| MRFA 0836-1 | M. cristatum right humerus | Big puncture | Nihilichnus nihilicus (Fig. 5E, F) | 3.39–5.78 | 2.95–3.47 | 2 | Large carnivore |
| MRFA 0836-1 | M. cristatum right ulna | Big puncture | Nihilichnus nihilicus (Fig. 5E, F) | 11.95 | 5.59 | 1 | Large carnivore |
| MRFA 0836-1 | M. cristatum right ulna | Short and straight with U-shaped bottom | Machichnus bohemicus (Fig. 5A, C) | 3.08–5.87 | 0.4–0.8 | 3 | Small carnivore |
| MRFA 0836-1 | M. cristatum right radius | Short and straight with U-shaped bottom | Machichnus bohemicus (Fig. 5A, C, G, H) | 3.02–6.02 | 0.5–0.7 | 4 | Small carnivore |
| MRFA 0836-2 | M. cristatum prox. left femur | Deep and elongated traces | Katagmichnus myelus gen. et sp. nov. (Fig. 6I, J) | 17.5 | 2 | 1 | Medium–large carnivore |
| MRFA 0836-2 | M. cristatum prox. left femur | Little punctures, cone shaped | Nihilichnus clavus (Fig. 6F, K, L) | – | – | 6 | Small/large carnivore? |
| MRFA 0836-3 | M. cristatum prox. left tibia | Flat and on edges resulting in notches | Katagmichnus myelus gen. nov. sp. nov. (Fig. 7A–F) | 5.10–6.13 | 2.62–4.06 | 3 | Medium–large carnivore |
| MRFA PV 1236 | Camelid indet. dist. metapod | Short and straight with U-shape bottom | Machichnus bohemicus (Fig. 8B, C) | 5.2–7.2 | 0.45–1.1 | 12 | Small carnivore |
- * Total length of the upper and lower incisor of rodent traces.
Genus Corralitoichnus nov.
LSID
https://zoobank.org/NomenclaturalActs/efacd216-c163-41e4-9642-817309ed261c
Derivation of name
The prefix Corralito refers to the Argentine locality where a fossil of a hemimandibular fragment of Mesotherium cristatum was found, which in turn serves as substrate for this ichnogenus, and the suffix -ichnus, from the Greek ‘ἴχνος’, meaning ‘trace’.
Diagnosis
As for type and only species.
Corralitoichnus conicetensis sp. nov.
Figures 2D–H, 4B, E–H; Table 1
LSID
https://zoobank.org/NomenclaturalActs/ed408a6f-88a9-4809-8cc9-9931122ef3d9
Derivation of name
The specific epithet conicetensis refers to CONICET (National Council for Scientific and Technical Research of Argentina), the national government agency dedicated to research and science in Argentina. This species is dedicated to CONICET in gratitude for its support in the training and professional development of many of the authors of this manuscript. We acknowledge its essential role in our education and research funding, particularly during a time of severe defunding and institutional vulnerability. This context resonates symbolically with the extinct status of the taxon described herein.
Holotype
Bite traces located on the ventral to medial side of the fragmented right hemimandible of Mesotherium cristatum (CORD-PZ 4456; Figs 2D–G, 4E–H).
Diagnosis
Flat surface traces with parallel or subparallel edges, approximately twice as high as they are wide. A slight longitudinal crest of the diastema (between right and left I1s or i1s) may be visible in the vertical plane, along with a transverse contact line between upper and lower incisors.
Locality & horizon
Corralito site is an east–west oriented gully located 3 km northeast of the village of Corralito between the north of Departamento Tercero Arriba and the south of Departamento de Santa María, Córdoba Province, Argentina. The only date associated with fossil (M. cristatum) remains at this site is documented at 220 ± 13 ka (Middle Pleistocene, Bonaeran Age; see Fernández-Monescillo et al. 2023a for details); there are other reports of dates (Frechen et al. 2009; Rouzaut et al. 2015) or other characteristics of this exceptional site (Argüello et al. 2006; Sanabria et al. 2006; Tauber 2008; Tauber et al. 2014).
Remarks
A detailed bibliographic comparison allows us to identify these flat traces in combination with their size (see height = 9.41–10.36 mm width; width = 4.00–5.69 mm; see Table 1) as a distinct new ichnotaxon. Corralitoichnus differs from Machichnus in three aspects: (1) the height-to-width ratio of the bite traces (nearly twice as tall as they are wide); (2) the shape of the traces (flat); and (3) the repeatability of the traces (there are two). However, some of the traces of Machichnus, produced by rodents (Ma. regularis; Ma. multilineatus) generally occur in multiple series and are notably narrow (in terms of their height-to-width ratio), allowing them to be described as grooves.
Genus Katagmichnus nov.
LSID
https://zoobank.org/NomenclaturalActs/2e7cd4b3-b652-4428-850a-f2910592ea1b
Derivation of name
From the Greek katagma (κάταγμα), meaning ‘fracture’, and -ichnus (ἴχνος), meaning ‘trace’ or ‘mark’, referring to the traces left by teeth biting and fracturing bone.
Diagnosis
As for type and only species.
Katagmichnus myelus sp. nov.
Figures 6B, C, F, G, I–J; 7A–F; Table 1
LSID
https://zoobank.org/NomenclaturalActs/98f637cd-30f2-48ed-b16a-89af5b788f81
Derivation of name
The specific epithet comes from the Greek myelos (μυελός), meaning ‘bone marrow’, indicating that the traces are associated with exposure or the bone marrow due to the bone fracture or attempted fracture of the bone.
Holotype
Bite traces are located on the diaphyseal region (distal third) of the cranial side of the left femur (MRFA 0836-2) of the Quaternary typotherid mesotheriine Mesotherium cristatum.
Paratype
Bite traces are located on the diaphyseal region of the left tibia (MRFA 0836-3) of the Quaternary typotherid mesotheriine Mesotherium cristatum.
Diagnosis
Longitudinal bite traces with broad, nearly flat cutting planes, oriented transversely to the long axis of the diaphysis in long bones, which may or may not lead to transverse bone fractures.
Description
An elongated trace, similar to those identified as Machichnus bohemicus, but larger (17.5 mm long) and much deeper, often penetrating the cortical bone to the point of causing peripheral bone fractures that grant access to the bone marrow.
Locality & horizon
An east–west oriented gully located 3 km northeast of the village of Corralito between the north of Departamento Tercero Arriba and the south of the Departamento de Santa María, Provincia de Córdoba, Argentina; middle Pleistocene, Bonaeran Age. For more detail see Corralitoichnus conicetensis above.
DISCUSSION
Identification of biotic taphonomic agents & bone modifications stages in fossil bones from the Corralito site
The hemimandible fragment (CORD-PZ 4456) is the most remarkable fossil due to the abundance of traces identified on its surface. The most characteristic and abundant traces are those of carnivoran teeth, identified both macroscopically and microscopically. The affected area is possibly linked to the area of the powerful masseter muscle (m. masseter) in mesotheriids (Fernández García 2018; Sosa & García López 2018), whose insertion is located caudally to the predominant area in ventral edge of the hemimandible. These suggest the active acquisition of meat or carrion presumably by mammalian carnivorans, leaving bite traces from premolars or molars imprinted on the cortical layer of the bone (Fig. 2G). At the time when these traces were made, the bone still retained its associated muscular tissue, indicating its freshness (Haynes 1980a; Cáceres et al. 2002) and suggesting the recent demise of the specimen, in this case M. cristatum.
Among the carnivorans discovered in Corralito, some are notably large, like Smilodon populator (220–360 kg; Christiansen & Harris 2005; Chimento et al. 2019), which we ruled out as a likely producer of the traces here analysed here due to their small size. Potential candidates for the producers of the traces on CORD-PZ 4456 include fossils identified in Corralito such as canid Cerdocyon sp. (MRFA 0899) (5–8 kg, Berta 1982) and the mustelids Galictis sp. (1.2–3.8 kg, Yensen & Tarifa 2003) and the mephitid Conepatus sp. (MRFA 0735) (1.1–4.5 kg, Dragoo & Sheffield 2009). The distance between the two small punctures seen in the lateral view of the mandible, here inferred to be the upper canines (Fig. 3A, G) is 16.2 mm, a measurement comparable to the alveolar distance between the upper canines of the lesser grison Galictis sp. (13–19 mm, see Yensen & Tarifa 2003, p. 1, or 16.75 ± 1.41 mm, see Bornhold et al. 2013, table 4), but also to that of Conepatus sp. (16–24 mm; see Dragoo & Sheffield 2009), and Cerdocyon sp. These punctures traces are likely to have been made during movement or transport of the hemimandible. While we cannot definitively determine the specific mammal responsible for creating the carnivoran bite traces, or conclusively assert whether they are exclusively attributable to a single taxon (there are at least two of different trace sizes; see Table 1), the morphology and shape enable us to confidently eliminate the involvement of other scavengers, such as reptiles, raptors or vultures, which are also well-known for their scavenging behaviours (see Naves-Alegre et al. 2020). Even so, of the small to medium carnivorans present at the site (the mephitid Conepatus sp., the mustelid Galictis sp. and the canid Cerdocyon sp.) it is probable that scavenging activity was primarily undertaken by the former (see Prugh & Sivy 2020). Analysis of the scavenging traces of the extant lesser grison G. cuja (<2 mm in length the bone specimens analysed, and most of them appear to have been previously digested; see Gutiérrez et al. 2021 for details) shows a large size difference compared with the traces identified here; this taxon is therefore ruled out as the producer. Conepatus sp. is also a small taxon for which, to our knowledge, there are no studies on possible tooth traces on bones with which to compare. However, there is current evidence of scavenging on carcasses (Castillo & Schiaffini 2019), including instances of kleptoparasitism on larger carnivorans (see Valenzuela & Leichtle 2015). None of the taphonomic traces found on the hemimandible of the M. cristatum specimen allow us to ascertain the cause of death of that specimen.
We identified two bite traces on the ventral portion of the hemimandible fragment CORD-PZ 4456 (Fig. 4H), characterized by parallel lateral edges and a predominantly flat base, not particularly deep (Fig. 4F, G). These features suggest that they are likely to have been produced by rodents (see Bunn 1981; Shipman & Rose 1983; Fernández-Jalvo & Andrews 2016; Pokines et al. 2016; Indra et al. 2022). Rodent gnawing traces not related to the location of muscle insertions in mesotheriines (see Sosa & García López 2018; Fernández García 2018) are very commonly positioned on the ventral border of mandibles (Fernández-Jalvo & Andrews 2016). Furthermore, the presence of a small longitudinal ridge in the middle of each bite trace (a–a′) and (b–b′) is notable (Fig. 4H, dotted yellow lines), which we infer to be the incisive (I1 right – I1 left) diastema. Additionally, by observing the ‘upper’ (a and b) and ‘lower’ (a′ and b′) shape of the traces we can infer the transversal location point of the bite in complete occlusion between ‘upper/lower’ incisors (Fig. 4H, transverse black dotted lines; however, due to the similar width of the ‘upper’ and ‘lower’ counterparts (see Table 1), we cannot determine which trace corresponds to which of the first incisors). The bite traces identified on the hemimandible are 4–5.69 mm wide (Table 1), which is consistent with the buccolingual width of either the I1 or i1 of specimens recognized as Ctenomys (MRFA PV 0567, MRFA PV 0018) from Corralito, but exclude Lagostomus as a producer due to its larger incisive width. There is little evidence of this type of bite trace on bones produced by rodents of the genus Ctenomys, but it has been suggested as the potential source of such traces on the bones of camelids (archaeological sites: KCH56, Iroco Region, Departamento de Oruro, Bolivia; Capriles & Tripcevich 2016). The potential causes of rodent bite traces on bones can be attributed to nutritional requirements, such as the intake of minerals (phosphate), or the necessity to gnaw in order to maintain the proper length of continuously growing incisors (Froberg-Fejko 2014).
Additionally, irregular surface traces have been observed on the hemimandible specimen (CORD-PZ 4456) identified using CLSM analysis as indicative of trampling (Fig. 3K, L). This suggests that the mandible had previously been broken, becoming a hemimandible fragment with the lateral side facing upward, before being stepped on by fauna sympatric to Mesotherium cristatum.
The other Mesotherium cristatum remains analysed belong to a single individual preserving postcranial elements from both the right forelimb (MRFA 0836-1, humerus–ulna and radius in anatomical position and in flexion) and the left hind limb (MRFA 0836-2, femur fragment, and MRFA 0836-3, tibia fragment). We identified the traces of small and medium–large carnivorans, and root etchings, but no evidence of trampling or rodent bite traces.
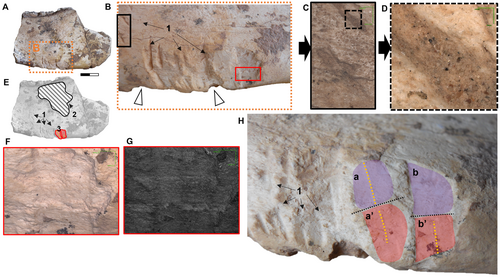
The right humerus maintains its anatomical position alongside the radius and ulna, and displays a series of elongated and fine traces, similar to those previously found in two regions on the hemimandible. Specifically, on the distal side of the lesser tubercle that is anterolaterally placed to the head of the humerus, we observed traces (n = 4) arranged in a transverse pattern, close to the insertion zone of the deltoid crest where the m. deltoideus pars spinalis, pars scapularis, and the m. brachioradialis are located (see Fernández-Monescillo et al. (2018) for a myological forelimb reconstruction of the late Miocene mesotheriine Plesiotypotherium achirense; see Fernández-Monescillo et al. (2019) for dating). In addition, the caudal edge is completely bitten (along a length of c. 9 cm) resulting in the complete loss of the bony edge of the crista supracondylaris lateralis (epicondylar crest) where the m. brachioradialis (m. supinator longus), digital extensor muscles (m. extensor digitorum lateralis, m. extensor digitorum communis, and m. extensor digitorum radialis) are inserted (see Fernández-Monescillo et al. 2018, fig. 4) (Fig. 5). On the cranial edge midway along the diaphysis, there is a parallel series of narrow linear traces (n = 10) which we also infer, from their similarity in size and shape to those identified on the hemimandible, to be produced by small carnivorans (Fig. 5C, D; Table 1). In addition, we observed small traces (n = 4) on the medial aspect of the radius in the middle part of the diaphysis (Fig. 5C; Table 1). In the distal region, there are two oval punctures (3.39 and 5.78 mm in maximum transverse length, and 2.95 and 3.47 mm in perpendicular width, respectively) probably caused by a large carnivorans (Fig. 5E, F; Table 1).
In the ulna we identified small longitudinal traces (n = 4) on the caudal edge of the medial side at the mid-height of the diaphysis and also on the same caudal edge, though more proximally located (n = 2) (Fig. 5C). In the proximal first third of the ulna, on the medial aspect, we can observe an oval-shaped and large-sized trace (11.95 mm maximum transverse length, 5.59 mm of perpendicular width; Fig. 5E, F; Table 1) that possibly corresponds to the canine of a large carnivoran, as the previous canine punctures identified close together on the humerus (Fig. 5E, F). In addition, in the distal portion of the ulna we observed a large area on which deep root etchings are traced (Fig. 5C); these are not present on the radius or humerus, despite being close to these elements. The identified modification of these forelimb bones (humerus, ulna and radius) is low to moderate based on the classification proposed by Sala et al. (2014), and Sala & Arsuaga (2018). Damage to the humerus is moderate; the whole of the lateral deltoid crest and crista supracondylaris are affected, as well as the medial entepicondyle (epicondylus medialis) (Fig. 5I, J). We can even suggest some carnivoran activity in the areas of the greater trochanter and the entepicondyle, which are completely missing and the resulting fractures filled with sediment. The ulna lacks its distal epiphysis, and the most distal part of the olecranon. However, we cannot ascertain whether the absence of these parts is a result of scavenging activity by carnivorans or for other reasons. The same uncertainty applies in the distal part of the radius (Fig. 5G, H). There is no evidence of the radial sesamoid located between the ulna and the humerus, previously recognized in different taxa of the family Mesotheriidae (Trachytheriinae + Mesotheriinae; Sydow 1988; Shockey et al. 2007; Fernández García 2018).
In the left fragment of the femur (MRFA 0836-2), we have identified abundant and deep root etching traces, some of which exhibit oval or rounded shapes (Fig. 6A–H). On the cranial side, small rounded to oval traces (n = 6) can be observed, probably caused by tooth contact from scavengers (Fig. 6F, K, L; Table 1). In size, these resemble those observed on the hemimandible (CORD-PZ 4456) discussed above (Fig. 3G, H). This suggests that both sets of punctures were made by small carnivorans (e.g. Galictis sp., Cerdocyon sp., Conepatus sp.), recognized by fossil evidence at the site. In the medial half portion of the femur, a pronounced longitudinal and transverse trace (17.5 mm) can be observed, extending deeply (2–3 mm) from the superficial surface of the femur (Fig. 6I, J). This is direct evidence of femoral shaft fracture caused by a large carnivoran bite, as described in the prey of extant lion (Panthera leo; Domínguez-Rodrigo et al. 2021), or other scavengers (Diedrich 2012, figs 8, 7b). There are two other transverse traces distally, spanning the entire cortical width (c. 4 mm), probably also caused by premolar/molar teeth during attempts to break the bone and access the bone marrow. The femoral diaphysis, MRFA 0836-2, was undoubtedly cracked by a large carnivoran, to gain access to the bone marrow (see Blumenschine 1986). Among the large carnivorans present in the Pampean region at that time (post-Ensenadan Age) were Canis nehringi at 30–38 kg (Prevosti & Vizcaíno 2006; Lujanian Age), Aenocyon (= Canis) dirus at 30–70 kg (Prevosti & Forasiepi 2018; Prevosti 2023), Lujanian Age, and Arctotherium sp. (Cruz et al. 2012; Soibelzon et al. 2014). Although so far, to the best of our knowledge, no direct evidence of diaphyseal cross-bite breakage of a medium–large mammal (megafauna; >44 kg; Cione et al. 2009) has been reported from for the Pampean region. Accordingly, it is important to note that the femur diaphysis crack documented here (Fig. 6I, J) or the attempt to crack the tibia (Fig. 7A–F) represents the first direct evidence of bone-cracking feeding behaviour in the Quaternary of South America in native South American lineage (Notoungulata, Typotheria). A potential scavenger that may have had the strength to break a femur of M. cristatum, and was present in the Middle Pleistocene of the Pampean region, is the bear Arctotherium sp. (Soibelzon et al. 2014; Cruz et al. 2012), for which the possibility of bone-breaking has been suggested (Soibelzon et al. 2014) (Fig. 6I, J). We consider that Smilodon populator, with its highly specialized biting action focusing on the soft neck region of its prey, would avoid another feeding action such as tooth–bone contact to break the diaphysis (see Turner et al. 2011). Based on the classification scheme of Sala & Arsuaga (2018, table 2) the femur shows moderate to heavy modification.
Regarding the proximal fragment of the left tibia (MRFA 0836-3) of M. cristatum (see Cattoi 1943; Fernández García 2018), only deep traces (n = 3; see Table 1) possibly made by premolars/molars were found located on the cranial edge of the diaphysis (Fig. 7A–F). This signifies a distinctive pattern indicative of activity carried out by carnivorans (Fernández-Jalvo & Andrews 2016). This taphonomic scenario occurs when individuals perish in close proximity to the location where they are scavenged, preserving soft tissues (e.g. ligaments) that remain intact. No evidence of abrasion by transport (e.g. wear traces) or weathering (e.g. desiccation traces) has been observed in the specimens (see Behrensmeyer 1978, 1991; Shipman 1981).
Sequence of taphonomic processes in hemimandible CORD-PZ 4456
- Death of the specimen of Mesotherium cristatum, which, based on the height of the mandible at the level of m2 (58.68 mm) and comparisons with the same measurement in other adult specimens of this species from Corralito (45.29–60.85 mm; Fernández-Monescillo & Tauber 2024), we infer as being ontogenetically adult. We do not know the exact cause of death of the specimen.
- Carnivorous or scavenging activity is evident on the bones, at least on the mandible. It is likely that the carnivoran activity/scavenging (Fig. 2) occurred when the complete mandible of M. cristatum was still intact and shortly after its death, indicating that the bone was still fresh (see Johnson 1985).
- Once exposed, the complete mandible of M. cristatum fragmented, with evidence of trampling on the medial side (Fig. 3K, L), indicating prior breakage to make this surface accessible. Rodent bite traces are also present (Fig. 4B, E, F–H). Figure 9A reconstructs this scenario. The shape and dimensions of the rodent bite traces (Table 1) are consistent with attribution to the genus Ctenomys sp., remains of which are found at the site, and probably occurred when the bone was in a dry state (see Johnson 1985; Klippel & Synstelien 2007; Pokines et al. 2016). At this taphonomic stage, we cannot determine whether the trampling or the rodent traces occurred first.
- Finally, the M. cristatum hemimandible was buried, and its surface was affected by roots. These roots are more pronounced and deeper on the lateral side (Fig. 3F; even overlapping with previous carnivory traces, see Fig. 3B), and less profound yet more extensive on the medial side (Fig. 4E).
Taphonomic trace analysis of Mesotherium cristatum postcranial remains
The evidence suggests that the skeletal remains of a single individual of M. cristatum (MRFA 0836-1, 0836-2, 0836-3) underwent distinct stages of scavenging activity. Firstly, an extensive scavenging phase was carried out by small-sized carnivorans (e.g. Cerdocyon sp., Conepatus sp.) A later phase of carcass consumption entailed the fracture of the femur bone diaphysis followed by the consumption of its medulla (see Blumenschine 1986). We unequivocally identified a large–medium carnivoran as the originator scavenger of these bone-cracker traces (Arctotherium?, Protocyon?) (Fig. 5). The fact that some of the appendicular remains of the forelimb (humerus, radius and ulna) still retain their anatomical connection suggests that the carnivory/scavenging event occurred shortly after death.
Taphonomic trace identification & vertebrate tooth ichnotaxonomy
From an ichnological perspective, we found several specimens at the Corralito site of ichnotaxa Machichnus bohemicus, Nihilichnus clavus and N. nihilicus (see Mikuláš et al. 2006; LaBarge & Njau 2024). We also describe two new ichnotaxa: Corralitoichnus conicetensis and Katagmichnus myelus. Corralitoichnus conicetensis is inferred to be produced by the upper and lower incisors of rodents (probably Ctenomys; Fig. 9A; Table 1); Katagmichnus myelus comprises massive bite traces (probably made by medium–large carnivorans) causing diaphysis bone breakage to access the bone narrow in large bones (Fig. 9B).
We are aware that ichnotaxonomic classification is mainly based on trace morphology and therefore often lacks biological (taxonomic) and phylogenetic support, which complicates the biological, palaeobiogeographical or temporal tracking of such classifications (e.g. Ma. bohemicus is identified in the Pleistocene of Brazil, Miocene of the Czech Republic, Middle Pleistocene of the Pampean region of Argentina, and in the present day; see Haynes 1983a, 1983b, Mikuláš et al. 2006; Araújo-Júnior et al. 2017). However, it is still useful to characterize a type of trace based on a common or similar biological function or behaviour of the producers, to provide direct evidence of an interaction between the two taxa (‘producer’ and ‘substrate’) (see Zonneveld et al. 2022). Nevertheless, we can still categorize these palaeontological ichnotaxa based on their resultant shape, size and mode of production. We acknowledge that different recognized ichnotaxa may represent a wide range of behaviours exhibited by a single carnivoran taxon, for example including cutting, catching, nibbling and cracking, depending on the specific function of the carnivore tooth (see Diedrich 2012) or function/behaviour (Cassini et al. 2021) involved. These actions (such as biting to feed on surrounding muscle tissue, transporting bone and associated flesh, or breaking bone to access marrow) can collectively indicate a single scavenging event.
The significance of taphonomic studies lies in their ability to deepen our understanding of ecological interactions between the trace producers and other sympatric taxa within shared environments. This study reveals previously unknown biological, faunal and ecological interactions among sympatric species during the Early Pleistocene, specifically in the western Pampean region near the Sierras Pampeanas de Córdoba.
CONCLUSION
Most of the analysed tooth traces from the Pleistocene of South America, are found on megafaunal specimens (>44 kg) (toxodonts (Toxodon), ground sloths (Megatherium, Eremotherium, Glossotherium), gliptodonts (Glyptotherium)) and considered to be implemented by large carnivorans.
In our detailed taphonomic study, we discovered multiple lines of evidence of biological interaction for one of the most iconic taxa within South American native faunas, the notoungulate typotherid mesotheriine Mesotherium cristatum. The last representative of the suborder Typotheria, and also for the last known population of this taxon at the Corralito site of post-Ensenadan Age (Middle Pleistocene).
In comparison to the traces analysed in the Pampean region, and caused by extant and extinct carnivorans, the patterns of damage observed in the two individuals of Mesotherium cristatum (CORD-PZ 4456; and MRFA 0836-1, 0836-2, 0836-3) are exceptional. Notably, these traces appear unprecedented, representing some of the first evidence of carnivory or scavenging in a notoungulate.
- Initially, the death of the M. cristatum individual occurred due to unknown causes.
- Subsequent traces of scavenging or carnivory traces were observed in one part of the hemimandible fragment, probably made by small carnivorans (Cerdocyon sp.)
- Trampling traces were identified, inflicted once the fragment was parallel to the ground.
- Rodent bite traces attributed to the rodent Ctenomys sp. were produced once the bone of M. cristatum had dried.
- Finally, burial allowed root growth direct contact.
The scavenging traces observed on additional specimens of M. cristatum from the same individual, such as those identified on the right arm (MRFA 0836-1), indicate that they probably occurred shortly after the animal's demise. This inference is supported by the fact that, despite the extensive scavenging activity that the bones of M. cristatum underwent, the humerus, ulna and radius remained connected anatomically.
The results of the analysis of the other remains from the same M. cristatum specimen (femur, MRFA 0836-2; tibia, MRFA 0836-3) are exceptional, particularly due to the numerous instances of a single individual bearing traces of carnivory or scavenging. This reveals different processes, including initial, extensive damage probably caused by small carnivorans (inferred from the size and type of traces analysed). Subsequently, the femoral shaft/diaphysis was broken, presumably to access the bone marrow, thought to be induced by large carnivorans (among those suggested for their presence in the Pampean region at that time: Canis nehringi, Aenocyon dirus and Arctotherium sp.), as suggested by the transverse bite traces that could lead to femur fractures. Notably, these traces appear to be unprecedented, representing the first well-documented reported evidence of bone-cracking in South America evidenced in the Middle Pleistocene (Bonaerian Age; Chibanian-Meghalayan Stage), for the last known typotherid population of the mesotheriine taxon, Mesotherium cristatum.
We documented various vertebrate bite traces, including previously recognized ichnotaxa such as Machichnus bohemicus, Nihilichnus clavus and N. nihilicus. Additionally, we introduce two new ichnotaxa: Corralitoichnus conicetensis, that we attribute to rodent incisor traces (possibly Ctenomys), and Katagmichnus myelus, characterized by significant bite damage leading to diaphysis fracture of long bones for marrow extraction. These two new ichnotaxa are assigned to the Family Machichnidae, used to unify and classify the traces produced by carnivory or scavenging. Previously described ichnotaxa associated with rodent bite traces (Ma. multilineatus and Ma. regularis) are also classified within the same family. This family definition should encompass not only carnivory and scavenging but also the activity of rodents wearing down their continuously growing incisor crowns by gnawing on hard substrates, such as bones.
In conclusion, taphonomic studies offer valuable insights into the ecological dynamics between producers and their environments, shedding light on interactions that shape fossil assemblages. This research reveals new biological, faunal, and ecological relationships from the Early Pleistocene, particularly in the Pampean region, enriching our understanding of the complex ecosystems of this period.
Acknowledgements
The authors acknowledge the technical support of S.E. García from the High-Resolution Microscopy Lab (LAMARX) at the Facultad de Matemática, Astronomía, Física y Computación (FaMAF), Universidad Nacional de Córdoba (UNC), for conducting CLSM analysis to examine the traces. Furthermore, the main author (MF-M) extends sincere gratitude to J. Ochoa, a dedicated palaeontologist, for his invaluable assistance in the preservation of Argentina's significant fossils, particularly in Córdoba, Argentina. J. Ochoa's unwavering dedication substantially facilitated this research by providing access to collections and ensuring its success. We thank Dr Sally Thomas for her invaluable assistance throughout the editorial process of this manuscript, as well as the two anonymous reviewers, and Dr H.E. Anderson for their thoughtful and constructive comments. In particular, we appreciate the reviewer who provided detailed feedback to include the ichnotaxa section, which significantly enhanced the quality and scope of this work. We also wish to acknowledge the editorial work of Dr Robert Sansom and Dr Mary Silcox, editors of Palaeontology, for their professional support in refining the manuscript. This study was funded by the Comunidad de Madrid, Spain through the 2023 call for research talent attraction grants ‘César Nombela' (2023-T1/ECO-29191), as part of the research project ‘PANPERISSOEVO: Evolutionary Analysis of South American and Iberian Panperissodactyla’ to MF-M. This study is a production of the Quaternary Ecosystem Research Group (Acronym ECOCUA) 970827, UCM, Madrid, Spain (https://www.ucm.es/grupos/grupo/827).
Author contributions
Conceptualization Marcos Fernández-Monescillo (MF-M), Eugenia Romero-Lebrón (ER-L); Formal Analysis MF-M; Funding acquisition MF-M; Methodology MF-M, María D. Pesquero (MDP); Writing – Original Draft Preparation, MF-M, MDP; Investigation MF-M, ER-L, MDP, Augusto Haro (AH), Pablo E. Rodríguez (PER), Jerónimo Krapovickas (JK), Adan A. Tauber (AAT), Writing – Review & Editing MF-M, ER-L, MDP, AH, PER, JK, AAT.
Data archiving statement
This published work and the nomenclatural acts it contains, have been registered in ZooBank: https://zoobank.org/References/757bc60b-b457-4d2a-b93e-796d69ce7027.




