Dietary adaptations and palaeoecology of Lophialetidae (Mammalia, Tapiroidea) from the Eocene of the Erlian Basin, China: combined evidence from mesowear and stable isotope analyses
Data archiving statement
Supplementary data (Appendix A, B) for this study are available in the Dryad Digital Repository: https://doi.org/10.5061/dryad.j77qp27
Abstract
Lophialetidae is an extinct group of endemic Asiatic tapiroids that are widely distributed in the Eocene sediments of Asia. Schlosseria magister and Lophialetes expeditus are the most abundant species in this family. However, their dietary and ecological characteristics are largely unknown. For the first time, we reconstruct the palaeodiet and habitat of these two lophialetids using a combination of mesowear and stable carbon isotope analysis of fossil teeth excavated from the Erlian Basin, China. Mesowear analysis (n = 141) suggests that the dietary structure of S. magister and L. expeditus shifted from less to more abrasive diets from ~52 to ~42 Ma. Stable carbon isotope analysis (n = 137) suggests that the habitats of S. magister and L. expeditus became drier and/or more open through time. The dietary shifts of the two lophialetids are consistent with evident changes in habitat. The changes in the diet and habitat were probably related to global climate change during that time period. The gradual drop in global temperatures during the early–middle Eocene led to a drier and more open terrestrial ecosystem in the Erlian Basin, probably resulting in changes in floral composition of the environment inhabited by S. magister and L. expeditus. Hence, herbivores highly susceptible to vegetation modification had to develop new resource exploitation strategies to adapt to these changes. Schlosseria magister, considered to be the sister-group of L. expeditus and with a low level of ecological flexibility, was unable to adapt to the habitat changes finally becoming extinct at ~45 Ma.
Reconstructing the palaeodiet of extinct animals is a significant aspect in the study of vertebrate palaeontology. It aids in understanding the ecology of a certain geological time period, and identifying the habitat of extinct species. Meanwhile, it can reveal the dietary adaptation and foraging behaviour of fossil groups in the process of evolution, thus providing significant information for elucidating the relationship between the evolution of terrestrial mammals and palaeoenvironmental changes (Fortelius & Solounias 2000; Kaiser et al. 2000; Schulz et al. 2007; Solounias et al. 2010; DeMiguel et al. 2011; Strani et al. 2018). In order to obtain palaeodiet information for various fossil groups, a variety of dietary analysis methods have been proposed and applied, including dental wear analysis (e.g. Fortelius & Solounias 2000; Kaiser et al. 2000; Solounias & Semprebon 2002), stable carbon and oxygen isotope analyses (e.g. Cerling et al. 1997; Wang et al. 2008a; Zazzo et al. 2010;), hypsodonty index analysis (e.g. Janis 1988; Jernvall & Fortelius 2002; Damuth & Janis 2011), premaxillary shape analysis (e.g. Solounias et al. 1988; Solounias & Moelleken 1994; Dompierre & Churcher 1996), masticatory morphology analysis (e.g. Solounias et al. 1995; Solounias & Moelleken 1999) and dental calculus analysis (e.g. Power et al. 2015; Chen et al. 2018). In general, multi-proxy approaches (using two or three analytical techniques) are frequently used to reconstruct the palaeodiet of a species, to obtain more reliable and detailed dietary information. For specific research materials (fossil teeth), multi-proxy approaches combining dental wear (including microwear and mesowear) analysis and stable isotope analysis has been used widely in the past few years (e.g. Rivals et al. 2014; Kubo et al. 2015; Jones & Desantis 2017).
The Mongolian Plateau has attracted the interest of many palaeontologists and geologists, as one of the most significant regions in the world for Palaeogene vertebrate palaeontological and stratigraphical research (Wang et al. 2010). The Palaeogene Asian Land Mammal Ages (ALMA) are based mainly on the mammalian faunas discovered in this region (particularly the Erlian Basin), because of well-exposed Palaeogene strata and abundant mammalian fossils (Wang et al. 2010, 2019). The Eocene mammalian faunas of the Erlian Basin are dominated by diverse perissodactyls (Tapiroidea, Rhinocerotoidea, Brontotheriidae and Chalicotherioidea) (Tong et al. 1995; Wang et al. 2007; Bai et al. 2018). A large number of academic papers and monographs based on the abundant fossils from the Erlian Basin have contributed to our knowledge of the perissodactyls (Matthew & Granger 1926; Radinsky 1965; Qi 1987; Wang et al. 2008b, 2010; Bai et al. 2017, 2018). However, most previous research has concentrated on their morphology and biostratigraphy, and there has been a lack of studies related to palaeodiet, palaeoecology and palaeoclimate in the area. Based on analyses of the fossil mammal assemblages in the eastern Erlian Basin and other regions, Wang et al. (2010) suggested that the mammalian evolutionary pattern in the early Palaeogene appeared to be directly influenced by global environmental changes during that time. It is widely accepted that mammalian evolution on the Mongolian Plateau corresponded to and coevolved with global environmental changes represented by the mammalian fauna turnover (Meng & McKenna 1998; Wang et al. 2010). Global climate changes forced a turnover of the terrestrial flora, resulting in changes in faunal habitat. However, given the poor conditions of preservation, there is no palaeoecological evidence (e.g. palynology or palaeobotany) to test whether or not there was a floral turnover caused by global or regional environmental change during that period, and if that turnover impacted mammalian faunas.
Lophialetidae are endemic Asiatic tapiroids that were distributed widely in Asia during the Eocene (Matthew & Granger 1926; Radinsky 1965). Two extinct species, Schlosseria magister and Lophialetes expeditus, are the most abundant in this family with holotypes discovered in the Erlian Basin (Matthew & Granger 1926; Radinsky 1965). Our recent stratigraphic investigation confirms that the presence of S. magister at Huheboerhe is restricted to the Arshanto Formation (AS), and L. expeditus is present only in the Irdin Manha Formation (IM), suggesting that they can be used as index fossils (Li 2009; Li & Wang 2010). Traditionally, the two species are considered to be browsers on the basis of their brachydont cheek teeth, and were thought to inhabit dense forest. However, there is no convincing evidence to support this hypothesis.
In this study, we aim to reconstruct the diets and habitats of the two most abundant lophialetids S. magister and L. expeditus uncovered in the Huheboerhe area of the Erlian Basin, Inner Mongolia. We combined dental mesowear analysis and stable carbon isotope analysis to reconstruct and compare the diets and habitats of these two species and to address the following questions: (1) what are the dietary traits and habitats of S. magister and L. expeditus; (2) how did S. magister and L. expeditus adapt to the changing environment during the early–middle Eocene; and (3) what was the impact of global climate change on the diets and ecology of S. magister and L. expeditus?
Geological setting
The Erlian Basin (42°–44°N, 110°–114°E) is located in central Inner Mongolia, near the border of China and Mongolia (Fig. 1) (Jiang 1983; Wang et al. 2012a). Palaeogene deposits are well exposed in the Erlian Basin. Recently, Wang et al. (2010, 2012a, 2019) provided improved chronological data for several formations based on comprehensive investigations and comparisons between the new data and previous studies and archives. In the eastern part of the Erlian Basin, exposures of the lower Palaeogene are subdivided into three lithological units in ascending order: the Nomogen Formation (NM) mainly consisting of sandstones, muddy sandstones and sandy mudstones; the Arshanto Formation (AS) composed of red mudstones and siltstones; and the Irdin Manha Formation (IM) consisting of white sandy mudstones, sandstones and conglomerates (Meng et al. 2007; Wang et al. 2010). In the Huheboerhe area, these formations have been well-documented in a composite section that is based on four sections measured in the area (Fig. 2) (Meng et al. 2007; Wang et al. 2012a). Moreover, at least 12 mammal-bearing horizons are identified, including 4 in the Nomogen Formation (NM-1 to NM-4), six in the Arshanto Formation (AS-1 to AS-6), and two in the Irdin Manha Formation (IM-1 and IM-2) (Fig. 2) (Wang et al. 2010, 2012a), which are correlated to four Asian Palaeogene Land Mammal Ages (Gashatan, Bumbanian, Arshantan and Irdinmanhan) (Wang et al. 2010). Wang et al. (2010, 2019) deduced the time frame for several formations including the temporal ranges of related land mammal ages according to the recent palaeomagnetic results in the Huheboerhe area and other related areas. Estimated numerical ages for the base of Irdin Manha Formation and Arshanto Formation are ~45 and ~52 Ma respectively (Fig. 2). The Arshanto Formation is early Eocene and middle Eocene, and the Irdin Manha Formation is middle Eocene in age.
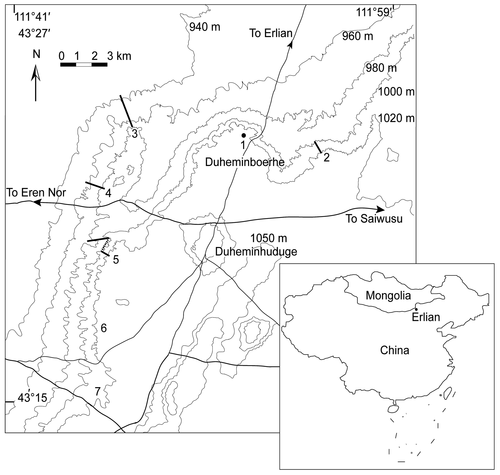
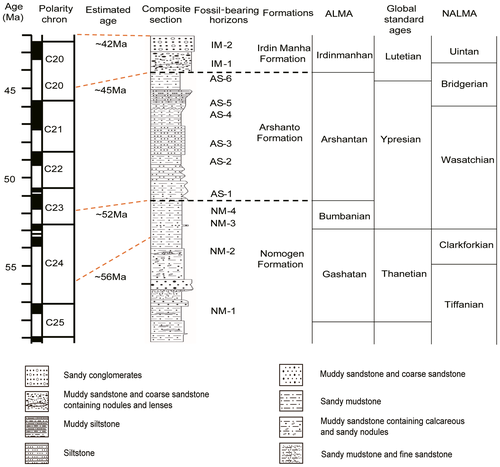
Material and method
The Palaeogene deposits in the Huheboerhe area are rich in fossils of mammals, and a large number of specimens have been collected with detailed stratigraphical record during the field investigation in recent decades. The materials analysed in this study are fossil teeth of S. magister and L. expeditus unearthed from the Huheboerhe area of the Erlian Basin, Inner Mongolia. Unbroken upper second molars were selected for mesowear analysis. Identifiable tooth fragments and some complete molars were selected for stable isotope analysis. Premolars and third molars were examined whenever possible to reduce the effects of pre-weaning diet on the δ13C of the tooth enamel. Additional teeth from the same individual were excluded to the greatest possible extent to ensure that every tooth represents a different individual. The teeth covered by sandstone or mudstone were cleaned in a laboratory at the Institute of Vertebrate Palaeontology and Palaeoanthropology (IVPP), Chinese Academy of Sciences.
Tooth mesowear analysis
Mesowear analysis is a quantitative, robust and convenient method to reconstruct palaeodiet (Fortelius & Solounias 2000; Kaiser & Solounias 2003). It is based on the physical properties of ungulate foods as reflected in the relative amounts of attritive and abrasive wear that they cause on occlusal surface enamel (Fortelius & Solounias 2000; Rivals et al. 2007). Mesowear is observed and scored from the buccal side of upper molars, preferably the paracone of M2 (Fortelius & Solounias 2000; Rivals & Lister 2016). Herbivorous mammals with low abrasion (high attrition) diets (e.g. browsers) usually maintain high relief and sharpened apices. Mammals with high abrasion (low attrition) diets (e.g. grazers) result in low relief occlusal surfaces, and blunted cusp apices. The diet of grazers consists of grasses containing more abrasive biogenic silica in the form of phytoliths as compared to tree leaves, resulting in heavy wear on a tooth (Fortelius & Solounias 2000; Saarinen et al. 2015; Cammidge 2017). It is important to note that low statured plants, or the parts of plants close to the ground, also are highly abrasive because exogeneous grits/sediments are included more often in the diet of mammals consuming these plants (Saarinen et al. 2015; Cammidge 2017). Moreover, dust and grit are more likely to adhere to plants growing in open and/or dry environments than those growing in closed and/or wet environments. Grazers and/or species in open habitats may ingest more abrasive matter than browsers and/or species in closed habitats. Teeth of mixed feeders having diets between grazers and browsers often exhibit moderate relief and rounded buccal cusp apices. Compared with dental microwear, which relates only to the last few meals, mesowear is more likely to reflect diet over an extended period of the individual's life (Danowitz et al. 2016). The time period can range approximately from weeks to years and is related to the overall rate of dental wear (Damuth & Janis 2011). When selecting the materials that could reflect the real diet of extinct species, as far as possible, unworn teeth (very young individuals), extremely worn teeth (very old individuals) and those with broken or damaged cusp apices should be excluded from mesowear analysis (Fortelius & Solounias 2000; Rivals & Lister 2016). Cusp sharpness is sensitive to ontogenetic age among young individuals (who have not yet developed substantial wear facets) and among dentally senescent individuals (Rivals & Lister 2016). However, for intermediate age groups which typically include the majority of individuals in a fossil collection, mesowear has been found to be less sensitive to age and more strongly related to diet (Rivals et al. 2007, Rivals & Lister 2016). Therefore, this method can offer a quantitative, robust and rapid estimation of the physical properties of ungulate foods, and has been applied widely to fossil teeth for palaeodietary reconstruction, and for palaeoecological and palaeoenvironmental analysis (Mihlbachler et al. 2011; Gong 2017).
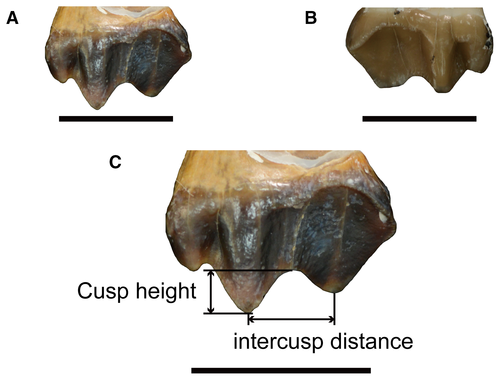
We used the standard method introduced by Fortelius & Solounias (2000) to measure and evaluate the cusp shape and cusp relief (occlusal relief). Cusp shape is categorized as sharp (Fig. 3A), round (Fig. 3B), or blunt according to the referenced model. Cusp relief (Fig. 3C) is classified as high or low according to the cut off value provided by Fortelius & Solounias (2000). We used the following method to calculate mesowear score (Croft & Weinstein 2008): (1) 0 for an individual with high occlusal relief and sharp cusps; (2) 1 for an individual with high occlusal relief and round cusps; (3) 2 for an individual with low occlusal relief and round cusp; (4) 2.5 for an individual with low occlusal relief and sharp cusps; (5) 3 for an individual with high or low occlusal relief and blunt cusp. The study by Fortelius & Solounias (2000) suggested that the ideal sample size for mesowear analysis is between 10 and 30. However, the available sample size from the first and sixth fossil horizons of the Arshanto Formation is less than 10. Ultimately, we analysed 141 upper M2 for mesowear (S. magister from AS2, n = 40; S. magister from AS3, n = 13; S. magister from AS4, n = 10; S. magister from AS5, n = 31; L. expeditus, n = 47). The detailed mesowear data of individual sample are presented in Gong et al. (2019, appendix A).
Stable carbon isotope analysis
Stable carbon isotope analysis of fossil tooth enamel has been established as a valuable and robust tool for reconstructing palaeodiet of extinct ungulates and inferring palaeoecological information in terrestrial ecosystems (e.g. Koch 1998; Kohn & Cerling 2002; Wang et al. 2012b; Stacklyn et al. 2017; Bowman et al. 2017; Sun et al. 2019). Tooth enamel consists of apatite crystals which are densely packed and more resistant to diagenetic alteration. Therefore, it is considered to be the most suitable material for palaeodietary and palaeoecological reconstruction by stable isotopic analysis (Kolodny et al. 1983; Shemesh et al. 1988; Quade et al. 1992; Wang & Cerling 1994; Lecuyer et al.1999). According to the different photosynthetic pathways for carbon fixation (e.g. C3 pathway and C4 pathway) of terrestrial plants, most plants are typically categorized into C3 plants (trees, forbs, most shrubs and cool season grasses) and C4 plants (mostly warm-season grasses). Plants that utilize these two different photosynthetic pathways have very different stable carbon isotopic compositions. Modern C3 plants have δ13C values of −20‰ to −35‰, with an average of −27‰; and C4 plants have δ13C values ranging from −9‰ to −17‰, averaging −13‰ (O'Leary 1988; Farquhar et al. 1989; Cerling et al. 1997, 2004). The carbon isotope difference between C3 plants and C4 plants will be transferred into the tissues (e.g. bone and tooth enamel) of animals with further isotopic fractionation through the food chain (Wang et al. 2008c). Previous studies of modern mammals with known diet suggested that tooth enamel (structural carbonate in hydroxyapatite) is enriched in the heavy carbon isotope 13C by ~13–14‰ relative to the diet (Lee-Thorp & van der Merwe 1987; Lee-Thorp et al. 1989; Wang et al. 1994; Cerling et al. 1997; Cerling & Harris 1999). Large ruminant mammals have a higher enrichment factor (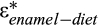 = 14.1 ± 0.5‰) (Cerling & Harris, 1999; Stacklyn et al. 2017). Non-ruminant mammals have lower enrichment factors of ~13‰, which is probably due to physiological differences between ruminant and non-ruminant (Passey et al. 2005; Stacklyn et al. 2017). In the modern world, mammals feeding on C3 vegetation typically have enamel-δ13C values of about −13‰. Mammals consuming C4 vegetation have δ13C values of about + 1‰, and mixed feeders (feeding on both C3 and C4 vegetation) have δ13C values between these two extremes (Lee-Thorp et al. 1989; Cerling et al. 1997; Wang et al. 2008a). Therefore, the dietary information and niche partitioning of herbivores can be revealed through comparison of the δ13C values of structural carbonate in their tooth enamel. The carbon isotopic composition of tooth enamel also can provide valuable insights into the mammal's habitat (e.g. Farquhar et al. 1989; Cerling & Harris 1999; Codron et al. 2005). Plants growing under water-stressed conditions or low atmospheric partial pressure of CO2 (pCO2), or in relatively open environments where more evaporation occurs, have higher δ13C values (above the average value of −27‰). Plants growing under closed canopies or in dense forests have more negative δ13C values resulting from the influence of soil respiration and light limitation (Schleser & Jayasekera 1985; Sternberg et al. 1989; van der Merwe & Medina 1989). Furthermore, the δ13C values of atmospheric CO2 (the carbon source for terrestrial plants) has changed over time (Tipple et al. 2010), affecting the δ13C values of plants and their consumers. The past changes in the carbon isotope composition of atmospheric CO2 must be accounted for in order to compare the reconstructed palaeodiet δ13C values with the δ13C values of modern plants (Bowman et al. 2017).
= 14.1 ± 0.5‰) (Cerling & Harris, 1999; Stacklyn et al. 2017). Non-ruminant mammals have lower enrichment factors of ~13‰, which is probably due to physiological differences between ruminant and non-ruminant (Passey et al. 2005; Stacklyn et al. 2017). In the modern world, mammals feeding on C3 vegetation typically have enamel-δ13C values of about −13‰. Mammals consuming C4 vegetation have δ13C values of about + 1‰, and mixed feeders (feeding on both C3 and C4 vegetation) have δ13C values between these two extremes (Lee-Thorp et al. 1989; Cerling et al. 1997; Wang et al. 2008a). Therefore, the dietary information and niche partitioning of herbivores can be revealed through comparison of the δ13C values of structural carbonate in their tooth enamel. The carbon isotopic composition of tooth enamel also can provide valuable insights into the mammal's habitat (e.g. Farquhar et al. 1989; Cerling & Harris 1999; Codron et al. 2005). Plants growing under water-stressed conditions or low atmospheric partial pressure of CO2 (pCO2), or in relatively open environments where more evaporation occurs, have higher δ13C values (above the average value of −27‰). Plants growing under closed canopies or in dense forests have more negative δ13C values resulting from the influence of soil respiration and light limitation (Schleser & Jayasekera 1985; Sternberg et al. 1989; van der Merwe & Medina 1989). Furthermore, the δ13C values of atmospheric CO2 (the carbon source for terrestrial plants) has changed over time (Tipple et al. 2010), affecting the δ13C values of plants and their consumers. The past changes in the carbon isotope composition of atmospheric CO2 must be accounted for in order to compare the reconstructed palaeodiet δ13C values with the δ13C values of modern plants (Bowman et al. 2017).
A total of 137 molars (including a few tooth fragments) were selected for stable carbon analysis in this study (S. magister from AS1, n = 16; S. magister from AS2, n = 14; S. magister from AS3, n = 26; S. magister from AS4, n = 25; S. magister from AS5, n = 23; S. magister from AS6, n = 5; L. expeditus from IM, n = 28). The results of the stable carbon isotope analyses of the samples are presented in Gong et al. (2019, appendix B). We took a bulk enamel sample from every individual by drilling along the growth axis of the tooth. No serial enamel samples were taken in this study because the teeth of S. magister and L. expeditus are small with a very thin enamel layer, and are thus not suitable for serial sampling (by drilling).
The enamel samples then were prepared following a treatment procedure described by Wang & Deng (2005). The enamel carbonate powders were soaked in 5% reagent sodium hypochlorite (NaClO) overnight at room temperature to remove any possible organic contaminants. After that, the samples were cleaned with distilled water four or five times using centrifugation to remove the remaining NaClO. The powder was then treated with 1 M acetic acid overnight to remove non-structural carbonate, and then cleaned with distilled water and freeze-dried (Wang & Deng 2005). Then, ~2–3 mg of the enamel powder was weighed into a reaction vial and baked for a few hours in a drying oven set to ~60°C to remove moisture absorbed onto the powder. Caps and septa were screwed onto the vials immediately. After flushing the vials with pure He, the enamel samples were reacted with 100% phosphoric acid at 25°C for about 72 h to produce CO2, and the carbon isotopic ratios of the CO2 were measured using a Gas Bench II Auto-Carbonate device connected to a Thermo-Finnigan MAT Delta Plus XP stable isotope ratio mass spectrometer (IRMS) at Florida State University. Three standards were run for every 15 samples. Results were calibrated (two-point calibration method) by different carbonate standards and reported in standard delta (δ) notation as δ13C value in reference to the international carbonate standard VPDB (Vienna Pee Dee Belemnite). The analytical precision (based on repeated analyses of standards including NBS-19 and our lab standards MERK, Roy-cc and MB-cc, which were processed with each batch of samples) is ±0.1‰ (1σ) or better.
Results
Mesowear analysis results
We obtained the original measurement data of every individual of S. magister and L. expeditus (Gong et al. 2019, appendix A). The ratio of cusp height to intercusp distance was greater than 0.1 for all teeth. Therefore, the occlusal relief of every tooth is categorized as high according to the standard introduced by Fortelius & Solounias (2000). Table 1 compiles summary mesowear data including average mesowear scores, percentages of individuals with different cusp types (sharp and blunt), and high occlusal relief. As a comparative dataset for dietary classification, we use 27 ‘typical’ extant species which have been shown to provide reliable dietary data without controversy in both mesowear and microwear analyses (Solounias & Semprebon 2002).
| Species | N | MS | %sharp | %blunt | %high | Diet |
|---|---|---|---|---|---|---|
| Aepyceros melampus | 17 | 0.6 | 35.2 | 0 | 100 | M |
| Alcelaphus buselaphus | 76 | 1.5 | 3.2 | 28 | 57 | G |
| Alces alces | 30 | 0 | 100 | 0 | 100 | B |
| Bison bison | 15 | 2.7 | 0 | 73.3 | 0 | G |
| Carpicornis sumatraensis | 22 | 0.7 | 45.4 | 4.5 | 100 | M |
| Ceratotherium simum | 26 | 2.3 | 0 | 28 | 0 | G |
| Cervus canadensis | 19 | 0.5 | 47.3 | 0 | 100 | M |
| Connochaetes taurinus | 52 | 1.5 | 15.3 | 28.8 | 55 | G |
| Damaliscus lunatus | 5 | 2 | 20 | 20 | 20 | G |
| Dicerorhinus sumatrensis | 5 | 0.2 | 80 | 0 | 100 | B |
| Diceros bicornis | 34 | 0.1 | 94.1 | 0 | 100 | B |
| Equus burchelli | 122 | 2.3 | 27 | 33.6 | 0 | G |
| Equus grevyi | 29 | 2.2 | 34.4 | 24.1 | 0 | G |
| Eudorcas thomsonii | 146 | 0.6 | 55.4 | 1.3 | 88 | M |
| Giraffa camelopardalis | 61 | 0.3 | 73.7 | 0 | 94 | B |
| Hippotragus equinus | 26 | 1.1 | 3.8 | 0 | 85 | G |
| Hippotragus niger | 20 | 1.3 | 0 | 15 | 85 | G |
| Kobus ellipsiprymnus | 22 | 1 | 0 | 0 | 96 | G |
| Nanger granti | 18 | 0.6 | 50 | 0 | 88 | M |
| Odocoileus hemionus | 33 | 0.5 | 72.7 | 0 | 100 | B |
| Odocoileus virginianus | 18 | 0.1 | 88.8 | 0 | 100 | B |
| Okapia johnstoni | 8 | 0.1 | 87.5 | 0 | 100 | B |
| Ovibos moschatus | 52 | 0.8 | 57.6 | 0 | 81 | M |
| Redunca redunca | 77 | 1.1 | 6.4 | 2.5 | 91 | G |
| Rhinoceros sondaicus | 5 | 0 | 100 | 0 | 100 | B |
| Taurotragus oryx | 14 | 0.3 | 50 | 0 | 100 | M |
| Tragelaphus scriptus | 47 | 0.5 | 51 | 0 | 100 | M |
| Schlosseria magister (AS2) | 40 | 0.3 | 67.5 | 0 | 100 | |
| Schlosseria magister (AS3) | 13 | 0.4 | 61.5 | 0 | 100 | |
| Schlosseria magister (AS4) | 10 | 0.5 | 50 | 0 | 100 | |
| Schlosseria magister (AS5) | 31 | 0.6 | 41.9 | 0 | 100 | |
| Lophialetes expeditus (IM) | 47 | 1.2 | 32.6 | 26.1 | 100 |
- Data of 27 modern ungulates from Croft & Weinstein (2008).
- N, sample size; MS, mesowear score; % sharp, the percentage of individuals with sharp cusps; %blunt, the percentage of individuals with blunt cusps; %H, the percentage of individuals with high cusp relief; M, mixed feeder; G, grazer; B, browser.
For the 27 typical modern ungulates, there are range separations of mesowear scores according to different type of diet (Fig. 4). The browsers have the mesowear scores of 0–0.5, with an average of 0.2. The mixed feeders have mesowear scores of 0.3–0.8, with an average of 0.6, and the grazers have mesowear scores of 1.0–2.7, with an average of 1.7. A one-way ANOVA was significant (p < 0.0001).

The scores of S. magister from AS2, AS3 and AS4 (0.3, 0.4 and 0.5) fall into the overlapping area between browsers and mixed feeders. It suggests that S. magister from AS2, AS3 and AS4 were either browsers or mixed feeders. The scores of S. magister from AS5 (0.6) are all within the range of mixed feeders while scores of L. expeditus (1.2) fall into the range of grazers. It suggests that S. magister from AS5 were typical mixed feeders, and L. expeditus from IM were grazers.
The discriminant function analyses (DFA) of the 27 typical modern ungulates dataset resulted in two canonical discriminant functions. The first function was highly significant (p > 0.001, Wilks’ lambda) and responsible for 99.2% of the original variable information. The variable ‘percentage of individuals with sharp cusps’ had the greatest influence on this function (loading = 0.746). The other three variables had relatively lower loadings (high loading = 0.221; blunt loading = −0.188; mesowear score loading = −0.412). A plot of these two functions (Fig. 5) illustrates good separation of modern taxa along the x-axis (function 1) and little separation along the y-axis (function 2).
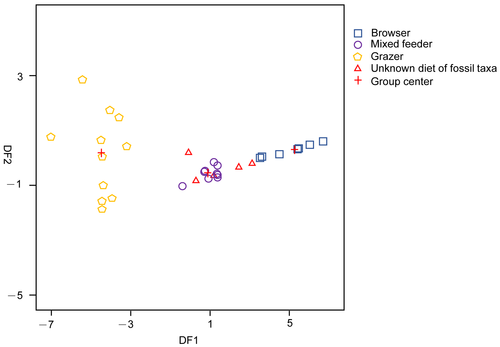
Schlosseria magister specimens from AS2 are classified as browsers, and the S. magister specimens from other fossil horizons (AS3, AS4 and AS5) and L. expeditus are classified as mixed feeders. The posterior classification probabilities are mostly high (0.95 for S. magister from AS3; 1 for S. magister from AS4, AS5; 1 for L. expeditus), except for specimens of S. magister from AS2 (0.54). Conditional probabilities ranged widely from 0.09 (S. magister from AS2) to 0.95 (S. magister from AS4). The scores on discriminant function 1 (DF1) for S. magister decrease progressively from AS2 to AS5. Lophialetes expeditus had the lowest score on discriminant function 1 that plotted to the left of the mixed feeders range (Fig. 5).
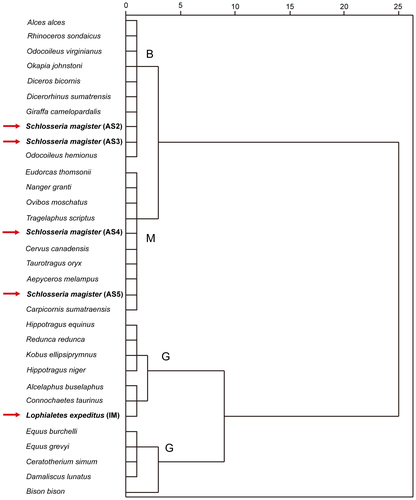
Hierarchical cluster analysis (HCA) of the typical ungulate dataset produced three main clusters: two of grazers and one of mixed feeders and browsers (Fig. 6). The non-grazer cluster includes two subclusters: one of browsers; and one of mixed feeders. The mixed feeder subcluster includes all mixed feeder species among the 27 modern ungulates of known diet, and the browser subcluster includes all of the browser species among the 27 modern ungulates. Among the 27 typical modern ungulates, Schlosseria magister from AS2 and AS3 clustered with browser subcluster, and S. magister from AS4 and AS5 clustered with the mixed feeder subcluster. And Lophialetes expeditus from IM clustered with grazer cluster.
Stable carbon isotope analysis results
The results of carbon stable isotope composition are summarized in Table 2.
| Species | Fossil-bearing horizon | Average δ13C (‰ vs VPDB) | Range of diet-δ13C (‰ vs VPDB) | Average diet-δ13C (‰ vs VPDB) | Standard deviation (±1σ) | No. of samples/individuals |
|---|---|---|---|---|---|---|
| S. magister | AS1 | −10.8 | −22.0 to −25.9 | −23.8 | 1.0 | 16 |
| S. magister | AS2 | −10.7 | −20.9 to −25.4 | −23.7 | 1.2 | 14 |
| S. magister | AS3 | −10.3 | −20.5 to −26.0 | −23.3 | 1.2 | 26 |
| S. magister | AS4 | −9.6 | −20.7 to −24.1 | −22.6 | 0.8 | 25 |
| S. magister | AS5 | −9.7 | −21.1 to −24.0 | −22.7 | 1.0 | 23 |
| S. magister | AS6 | −9.8 | −21.5 to −24.8 | −22.8 | 1.7 | 5 |
| L. expeditus | IM | −8.6 | −20.5 to −23.8 | −21.6 | 0.7 | 28 |
Enamel δ13C values of S. magister from the Huheboerhe area range from −7.5 to −13‰, with a mean of −10.1 ± 1.1‰ (n = 109), and enamel δ13C values of L. expeditus range from −7.5 to −10.8‰, with an average of −8.6 ± 0.7‰ (n = 28). The isotopic variations in S. magister and L. expeditus reflect differences in their diets and habitats. Using an enrichment factor (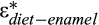 ) value of 13‰ for both S. magister and L. expeditus (non-ruminants) and the enamel δ13C values, we reconstructed the diet-δ13C values of these two Eocene species from the fossil-bearing horizons as follows.
) value of 13‰ for both S. magister and L. expeditus (non-ruminants) and the enamel δ13C values, we reconstructed the diet-δ13C values of these two Eocene species from the fossil-bearing horizons as follows.
For S. magister from AS1, the δ13C values of enamel samples range from −9.0 to −12.9‰, with an average of −10.8 ± 1.0‰. This gives a reconstructed diet-δ13C of −23.8 ± 1.0‰, ranging from −22.0 to −25.9‰. For S. magister from AS2, the δ13C values of enamel samples range from −7.9 to −12.4‰, with an average of −10.7 ± 1.2‰. This yields a reconstructed diet-δ13C of −23.7 ± 1.2‰, ranging from −20.9 to −25.4‰. For S. magister from AS3, the δ13C values of enamel samples range from −7.5 to −13.0‰, with an average of −10.3 ± 1.2‰. This gives a reconstructed diet-δ13C of −23.3 ± 1.2‰, ranging from −20.5 to −26.0‰. For S. magister from AS4, the δ13C values of enamel samples range from −7.7 to −11.1‰, with an average of −9.6 ± 0.8‰. This gives a reconstructed diet-δ13C of −22.6 ± 0.8‰, ranging from −20.7 to −24.1‰. For S. magister from AS5, the δ13C values of enamel samples range from −8.1 to −11.0‰, with an average of −9.7 ± 1.0‰. This gives a reconstructed diet-δ13C of −22.7 ± 1.0‰, ranging from −21.1 to −24.0‰. For S. magister from AS6, the δ13C values of enamel samples range from −8.5 to −11.8‰, with an average of −9.8 ± 1.7‰. This gives a reconstructed diet-δ13C of −22.8 ± 1.7‰, ranging from −21.5 to −24.8‰. For L. expeditus from IM, the δ13C values of enamel samples range from −7.5 to −10.8‰, with an average of −8.6 ± 0.7‰. This gives a reconstructed diet-δ13C of −21.6 ± 0.7‰, ranging from −20.5 to −23.8‰.
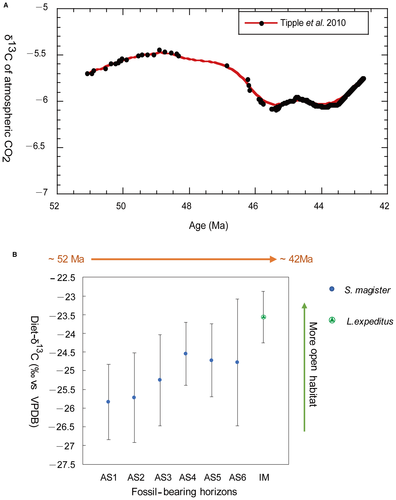
Geochemical evidence indicates that the δ13C values of atmospheric CO2 have changed significantly over geological time (e.g. Tipple et al. 2010), affecting the δ13C values of plants and ultimately tooth enamel of herbivores (e.g. Bowman et al. 2017). Proxy records suggest that the δ13C of atmospheric CO2 during the early–middle Eocene time period represented by our samples ranged from −5.45‰ to −6.09‰, with an average of −6.0 ± 0.1‰ (Fig. 7A; Tipple et al. 2010). That was about 2‰ higher than the present-day atmospheric CO2 δ13C value of −8‰ (Kohn 2010; Tipple et al. 2010). Thus, a correction factor of −2‰ needs to be added to the above reconstructed diet δ13C values to make them directly comparable to modern plants or diet δ13C values.
Discussion
Mesowear features and dietary traits
The DFA and HCA of the tooth mesowear data on S. magister from AS2 to AS5 and L. expeditus from IM presented here provide the first evidence for the dietary habits of lophialetids from the Eocene of Erlian Basin, Inner Mongolia, China. The results from these statistical analyses show dietary traits with a clear tendency toward more abrasive from S. magister (AS) to L. expeditus (IM).
By comparing the mesowear scores, both DFA and HCA suggest browsing diets or browse-dominated mixed feeding dietary traits for S. magister from AS2 and AS3, typical mixed feeding dietary traits for S. magister from AS4 to AS5, and grazing diet or graze-dominated mixed feeding dietary traits for L. expeditus from IM. It is important to note that the dietary types of fossils are more complicated than that of extant mammals because we know very little about the palaeoecological and palaeoclimatic context of the extinct animals. Therefore, when interpreting the mesowear data, other factors also should be considered. S. magister from AS2 have low mesowear scores (0.3) and a high percentage of individuals with sharp cusps (67.5%) consistent with the features of typical extant browsers. DFA assigns the S. magister from AS2 to the browsing group, and HCA allocates it to the mixed feeder cluster. In addition, S. magister has low-crowned molars with shearing crests, suggesting a possible browsing diet. Taking the above factors into account, S. magister specimens from AS2 were most likely to have been browsers feeding on soft leaves, tender twigs, and/or other less abrasive plant materials. For S. magister from AS3, DFA indicates a mixed feeding diet, and HCA indicates a browsing diet. Moreover, the mesowear score of S. magister from AS3 falls into the overlapping area between browsers and mixed feeders. Taken together, the above data suggest that S. magister individuals from AS3 were probably browse-dominated mixed feeders that consumed a higher percentage of less abrasive plant material, such as soft leaves. For S. magister fossils from AS4 and AS5, both DFA and HCA show that they were mixed feeders. Given the general understanding of mixed feeders, they were herbivores eating both leaves and grasses. However, mesowear analysis only determines whether the level of abrasion and tooth wear features on the occlusal surface of molars are similar to those of extant mixed feeders. In other words, the mixed feeders are those that consume both soft plants parts (e.g. leaves, fruits, tender twigs and tender grasses) and more abrasive items (e.g. stiff branches, harsh grasses, fruit with hard covers or seeds, soil and grit). The mesowear analysis suggests that the dietary composition of S. magister gradually changed from AS2 to AS5, with increasing amounts of abrasive items consumed by S. magister over time. As there was no significant change in the body size of S. magister over that time, we assume that the quantity of food consumed by individuals from different fossil horizons was very similar. Thus, a change in the dietary composition of S. magister is the most plausible explanation for the differences in the mesowear data. As noted above, the mesowear data indicate that S. magister fossils from AS4 and AS5 have typical mixed feeding dietary traits, with more abrasive items in their diet than S. magister specimens from AS2 and AS3. The mesower features of L. expeditus teeth from IM, which include a high mesowear score (1.2), a low percentage of individuals with sharp cusps (32.6%) and high percentage of individuals with blunt cusps (26.1%), are consistent with those of typical extant grazers. DFA assigns the L. expeditus fossils to the mixed feeders group, and HCA places that species in the grazer cluster. Moreover, dental morphological analysis reveals larger sized and much higher lophodont teeth in Lophialetes than in Schlosseria, as well as a greater degree of molarization of the premolars. This molarization indicates that Lophialetes had a greater ability to consume a more abrasive diet than that of Schlosseria. Based on the above analysis, it is most likely that L. expeditus was a grazer, consuming harsh vegetation or other abrasive items.
Stable carbon isotopes, diet and habitat change
Enamel samples from S. magister and L. expeditus exhibit significant stable carbon isotopic variations (Table 2). The reconstructed diet-δ13C values range from −20.5‰ to −26‰ with the highest δ13C value found in both L. expeditus and S. magister from AS3 and the lowest in S. magister teeth from AS3. Considering that the δ13C value of atmospheric CO2 during the early–middle Eocene represented by our samples was about 2‰ higher than that of the modern atmosphere (Tipple et al. 2010), 2‰ should be deducted from the reconstructed diet-δ13C value of S. magister and L. expeditus to make it directly comparable to a diet-δ13C value found in modern terrestrial-plant ecosystems. That is, the reconstructed diet-δ13C values range from −20.5‰ to −26‰ for S. magister and L. expeditus individuals should be equivalent to a modern diet-δ13C values of ~−22.5‰ to −28‰, which is within the range observed for modern C3 plants. This indicates that these early–middle Eocene lophialetids ate pure C3 vegetation and lived in a habitat dominated by C3 plants. The S. magister specimens from AS1 to AS3 have a large range of δ13C variation (with Δδ13C value 3.9‰, 4.5‰ and 5.5‰ respectively) and S. magister and L. expeditus teeth from other fossil horizons have a relatively small range of δ13C variation (about 3‰). This variation implies that the S. magister individuals from AS1 to AS3 had wider dietary range and more dietary flexibility, and S. magister and L. expeditus from other fossil horizons had a more specialized diet.
Previous research indicated that modern C3 vegetation has a considerable range in δ13C values (−20‰ to −35‰). Compared to the average δ13C value of about −27‰, water-stressed ecosystems have higher δ13C (as high as −20‰), and plants growing in the understory of the closed canopies of dense forests have more negative δ13C values (as low as −35‰) (Farquhar et al. 1989; van der Merwe & Medina 1989; Ehleringer et al. 1991; Ehleringer & Monson 1993; Cerling et al. 1997). The reconstructed average diet-δ13C values for S. magister and L. expeditus range from −21.6‰ to −23.8‰, equivalent to modern diet-δ13C values of ~−24‰ to −26‰, which are significantly higher than −27‰. This result indicates that S. magister and L. expeditus did not live in a dense forest with a closed canopy. On the contrary, water-stressed ecosystems or relatively open environments where more evaporation occurs were most likely to be the habitats of S. magister and L. expeditus during the early–middle Eocene. We found that the reconstructed mean diet-δ13C values of S. magister and L. expeditus displayed an overall increasing trend from AS1 to IM (Fig. 7B). Only the diet-δ13C values of S. magister fossils from AS5 and AS6 deviate slightly (by ~0.1‰) from this overall trend, probably reflecting the slight decrease in the δ13C of atmospheric CO2 at around 44–45 Ma (Tipple et al. 2010). This general increasing trend, which is opposite to the trend in the reconstructed δ13C of atmospheric CO2 for the same time interval (Fig. 7A; Tipple et al. 2010), indicates that the habitats of S. magister and L. expeditus became more open and/or drier as a whole over time. Moreover, the diet-δ13C value of L. expeditus was 1.2‰ higher than that of S. magister from AS6, and at least 1‰ higher than that of S. magister from the other older fossil horizons. These data suggest that the habitat of L. expeditus had become significantly drier and more open than that of its earlier relatives. However, Ni et al. (2007, 2010) reported a new omomyid euprimate (Baataromomys ulaanus) from Bumbanian strata at Wulanboerhe and a new primate (Tarkops mckennai) from the Irdin Manha Formation in the Erlian Basin. The occurrence of these primates suggests that forested habitats must have been present, at least sporadically, in the Erlian Basin during the early–middle Eocene. The large range of variation in the reconstructed diet-δ13C values (from −20.5‰ to −26‰, equivalent to modern values of −22.5‰ to −28‰) for S. magister and L. expeditus individuals suggest that the basin had diverse habitats ranging from forest (perhaps riparian forest) to more open and drier habitats. Therefore, the S. magister and L. expeditus individuals probably lived in edge zone of a forest or a relatively open area where the forest (or woodland) canopies were broken.
Implications for palaeoecology and palaeoclimate
Deep-sea oxygen isotope records (Zachos et al. 2001) reveal a trend of decreasing global temperatures (from ~12 to ~8°C) during the period when the S. magister and L. expeditus lived (~52 to ~42 Ma). A significant but complicated question is whether the global temperature changes had an effect on the habitats and diet of S. magister and L. expeditus during the early–middle Eocene of the Erlian Basin. Here, we compare the temporal changes of dietary composition and diet-δ13C values with the deep-sea temperature record as shown in Figure 8 in order to address this question.
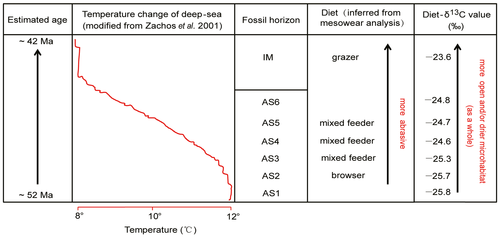
From the underlying Arshanto Formation to the upper Irdin Manha Formation, our mesowear data show that S. magister and L. expeditus changed from browser to grazer as their diet became more abrasive, and the diet-δ13C values increased from −23.8‰ to −21.6‰, which cannot be explained by changes in the δ13C values of atmospheric CO2 (Fig. 7A), indicating a shift to a more open and/or drier habitat. The drying trend (a process tending to drier conditions but not necessarily to an arid climate type) from ~52 to ~42 Ma in the study area as indicated by the increasing diet-δ13C values corresponds to a decreasing trend in global temperatures (Fig. 8). This suggests that the change in diet and habitat of the two lophialetids was most likely to have been caused by global climate change during this period as documented by deep-sea sediment δ18O records (Zachos et al. 2001). Therefore, global climate change probably had an important impact on mammalian evolution. Our mesowear and stable carbon isotope data suggest that the gradual drop in global temperatures following the Early Eocene Climatic Optimum (from ~52 to ~42 Ma) led to a progressively drier and/or more open terrestrial ecosystem, with related changes in floral composition and density in the Huheboerhe area of the Erlian Basin. In a relatively open environment like a woodland (or a low-density forest) with plenty of sunlight and limited shade, lophialetids had to adjust their diet by consuming more abrasive items and different plants in order to adapt to the ecological/environmental change. That change is because more exogenous hard materials like soil and grit were ingested with plant materials in more open habitats, resulting in higher incidences of abrasive mesowear features (Kaiser & Schulz 2006). Thus, the more abrasive diet of lophialetids was probably a response to change in habitat or ecology.
Fossils of S. magister and L. expeditus are only known from the Arshanto and Irdin Manha formations, respectively. Previous studies (e.g. Matthew & Granger 1926; Radinsky 1965) suggested that S. magister was probably the ancestral form of L. expeditus. The change in habitat probably was the significant driving force in the evolution from S. magister to L. expeditus. Mesowear documents the dietary shifts of lophialetids during a drying climate. There were three distinct dietary shifts. One happened between S. magister from AS2 (browser) and S. magister from AS3 (browse-dominated mixed feeder). Another shift happened between S. magister from AS3 (browse-dominated mixed feeder) and S. magister from AS4 (typical mixed feeder); and the last happened between S. magister from AS5 (typical mixed feeder) and L. expeditus from IM (grazer). Moreover, there are two conspicuous variations in mean diet-δ13C values determined by two-tailed t-tests (Table 3) that happened between AS3 and AS4 (Δδ13C = 0.7‰, p < 0.05), and between AS6 and IM (Δδ13C = 1.2‰, p < 0.05). Assuming that same species living in the same area would have a similar diet under similar ecological conditions, the very close average diet-δ13C values between AS1 (δ13C = −23.8‰) and AS2 (δ13C = −23.7‰) indicates that S. magister from these fossil horizons probably had similar diets and habitats. The reconstructed diet-δ13C values also suggest that the diets and habitats of S. magister from AS5 (δ13C = −22.7‰) and AS6 (δ13C = −22.8‰) were very similar. Therefore, we consider the S. magister individuals from AS1 as browsers, and S. magister fossils from AS6 as mixed feeders. The dietary shifts of the two lophialetids inferred from mesowear are consistent with the changes in habitat as documented by the stable carbon isotope data (Fig. 8; Table 3). This implies that the changes in habitat were probably the driving factor in the dietary shifts of these two lophialetids. With the habitat getting drier and/or more open, S. magister was unable to adapt to such a dramatic change in ecological environment and became extinct after AS1 (~45 Ma). Meanwhile, L. expeditus, with a stronger dietary adaptability, evolved from S. magister and was able to survive in a drier and more open habitat.
| Sample | Mean difference (‰) | df | t | p | Significant difference at 95% confidence interval? |
|---|---|---|---|---|---|
| S. magister (AS1) vs S. magister (AS2) | 0.1 | 28 | −0.275 | 0.786 | No |
| S. magister (AS2) vs S. magister (AS3) | 0.4 | 38 | −1.153 | 0.256 | No |
| S. magister (AS3) vs S. magister (AS4) | 0.7 | 49 | −2.394 | 0.021 | Yes |
| S. magister (AS4) vs S. magister (AS5) | 0.1 | 46 | 0.646 | 0.521 | No |
| S. magister (AS5) vs S. magister (AS6) | 0.1 | 26 | 0.069 | 0.946 | No |
| S. magister (AS6) vs L. expeditus (IM) | 1.2 | 31 | −2.740 | 0.010 | Yes |
Conclusions
Combining both stable isotope analysis and mesowear analysis, we have drawn the following conclusions and inferences:
- Schlosseria magister individuals from AS2 were browsers and S. magister fossils from AS3 were probably browse-dominated mixed feeders. S. magister specimens from AS4 and AS5 were typical mixed feeders. S. magister fossils from AS1 had a diet/habitat similar to their counterparts from AS2, and were probably browsers. S. magister fossils from AS6 had a diet/habitat similar to those from AS5, probably mixed feeders. Lophialetes expeditus individuals from IM were grazers. The food ingested by S. magister and L. expeditus became more abrasive from AS1 to IM.
- All of the reconstructed diet-δ13C values fall within the range for C3 plants, indicating that both S. magister and L. expeditus ate C3 vegetation and lived in an environment dominated by C3 plants. This result is consistent with previous studies (Cerling et al. 1993, 1997) suggesting that the expansion of C4 plants happened in the late Miocene and terrestrial ecosystems were dominated by C3 plants prior to the late Miocene. No substantial evidence so far demonstrate that C4 plants existed in Erlian Basin in the early–middle Eocene. Our data show that the habitats of S. magister and L. expeditus progressively became more open and/or drier from AS1 (˜52 Ma) to IM (˜42 Ma). Taking the occurrence of early–middle Eocene primate fossils in the Huheboerhe area into account, S. magister and L. expeditus probably inhabited a relatively open forest environment like a woodland (or a low-density forest).
- It appears that global climate change (global deep-sea temperature declining from ˜52 to ˜42 Ma) was a major driving factor for the changes in diet and habitat of S. magister and L. expeditus. In response to declining global temperature from ˜52 to ˜42 Ma, local ecosystems in the Huheboerhe area in the Erlian Basin became more open and drier. S. magister and L. expeditus, adapted to the changing ecosystem (habitat) through changes their diet (by consuming more abrasive items). The dietary shifts of the two lophialetids inferred from mesowear are consistent with the evident changes in habitat revealed by the enamel carbon isotope data. Therefore, the dietary shift was probably related to the palaeoecological change during the early–middle Eocene in the Huheboerhe area, Erlian Basin. Lophialetes expeditus, which was the sister-group of S. magister and had strong dietary adaptability, was able to survive in a drier and more open ecological environment. S. magister was unable to adapt to such a changing habitat and became extinct after AS1 (˜45 Ma).
Acknowledgements
We thank Wei Zhou, Shijie Li, Qi Li, Yongxing Wang, Yongfu Wang, Liqun Shi, Xijun Ni, Tianyu Wang, K Christopher Beard, Daniel L. Gebo, Chengkai Sun, Jian Wang, Qiang Cao, Qiang Li and Wei Gao for field work; Shijie Li (IVPP) for preparation of specimens; Chance Hannold, Fajun Sun and Burt Wolff (all FSU) for laboratory assistance and academic discussion; Thomas Stidham (IVPP) for assistance with the English text. We also thank the editor Lionel Hautier, and Sally Thomas and two anonymous reviewers for their careful reading of our manuscript and their many insightful comments and suggestions. This study is supported by the Strategic Priority Research Program of Chinese Academy of Sciences, Grant No. XDB26000000; the National Natural Science Foundation of China, Grant No. 41572021, 41672014; the ‘Joint PhD Training Program’ in UCAS. Stable isotope analyses were performed in Stable Isotope Lab within Geochemistry Program at the National High Magnetic Field Laboratory, which is supported by National Science Foundation Cooperative Agreement No. DMR-1644779 and the State of Florida.




